Abstract
Extracellular ATP and adenosine have immunoregulatory roles during inflammation. Elevated extracellular ATP is known to exacerbate GVHD, and the pharmacologic activation of the adenosine A2A receptor is protective. However, the role of endogenous adenosine is unknown. We used gene-targeted mice and a pharmacologic inhibitor to test the role of adenosine generated by CD73/ecto-5′-nucleotidase in GVHD. In allogeneic transplants, both donor and recipient CD73 were protective, with recipient CD73 playing the dominant role. CD73 deficiency led to enhanced T-cell expansion and IFN-γ and IL-6 production, and the migratory capacity of Cd73−/− T cells in vitro was increased. However, the number of regulatory T cells and expression of costimulatory molecules on antigen-presenting cells were unchanged. A2A receptor deficiency led to increased numbers of allogeneic T cells, suggesting that signaling through the A2A receptor via CD73-generated adenosine is a significant part of the mechanism by which CD73 limits the severity of GVHD. Pharmacologic blockade of CD73 also enhanced graft-versus-tumor activity. These data have clinical implications, as both the severity of GVHD and the strength of an alloimmune antitumor response could be manipulated by enhancing or blocking CD73 activity or adenosine receptor signaling depending on the clinical indication.
Introduction
Patients with hematologic malignancies that are refractory to conventional chemotherapy have a chance of cure by allogeneic hematopoietic stem cell transplantation (allo-HCT).1 However, the success of this treatment is limited by GVHD.2 We have recently shown that extracellular ATP, which is released from dying or stressed cells and serves as an endogenous danger signal to evoke systemic inflammatory responses, enhances GVHD by activation of the purinergic receptor P2X7R.3,4 The abundance of extracellular ATP is regulated by ecto-nucleotidases, such as CD39, which dephosphorylates ATP to ADP and AMP. Extracellular AMP is dephosphorylated to adenosine via the action of CD73, a glycosyl phosphatidylinositol-anchored glycoprotein with ecto-5′-nucleotidase enzyme activity.5-7 CD73 is expressed on many cell types, including subsets of T lymphocytes, endothelial cells, and epithelial cells.7,8 It is also present as a secreted form lacking a glycosyl phosphatidylinositol anchor.9 CD73-generated adenosine can activate any of 4 G-protein-coupled 7-transmembrane-spanning adenosine receptors (ARs; A1, A2A, A2B and A3) and can act as either a pro- or anti-inflammatory mediator depending on the physiologic setting and the type of AR engaged.10-12 In most circumstances, A1 and A3 receptor triggering is proinflammatory, whereas activation of A2A and A2B receptors is anti-inflammatory or tolerogenic.13,14
The importance of CD73 in producing adenosine for AR signaling has been revealed through studies with CD73-deficient mice. For example, CD73-generated adenosine reduces inflammation and fibrosis in lungs of bleomycin-treated mice15 and is tolerogenic for cardiac and airway allografts.16,17 CD73-dependent A2BAR signaling protects mice during renal ischemia,18 inhibits systemic vascular leakage during hypoxia,19,20 and is also required for cardioprotection as a result of ischemic preconditioning.21 Extracellular adenosine inhibits platelet activation and leukocyte adhesion to the vascular endothelium22 and limits lymphocyte migration into draining lymph nodes after an inflammatory stimulus via a CD73-dependent mechanism.23 CD73 also inhibits antitumor immune responses.24-27
There are several additional model systems where AR signaling has been shown to play a role in immune regulation, but the importance of CD73 in producing adenosine to trigger the receptors has not been studied. For example, immune regulation via the activation of A2A and/or A2B AR in T cells and dendritic cells (DCs) has been documented.28,29 In addition, a specific A2AAR agonist has been shown to inhibit allogeneic immune activation in vitro via actions on both T cells and antigen-presenting cells and to delay skin allograft rejection in vivo.14 Another A2AAR agonist was shown to attenuate acute GVHD after allo-HCT.13 These findings support the concept that extracellular adenosine counteracts ATP-triggered immune activation as a negative feedback mechanism to prevent uncontrolled tissue destruction because of excessive inflammation.
The goal of this study was to delineate the role of CD73 in acute GVHD and graft-versus-leukemia (GVL) responses. We used in vivo bioluminescence imaging (BLI) based T cell and tumor cell tracking methodologies30 in combination with mice genetically deficient for CD73. Our data clearly indicate that CD73 plays a tolerogenic role after allo-HCT and has an impact on GVL effects that could be exploited in the clinical situation using a CD73 inhibitor.
Methods
Mice
C57BL/6 (H-2Kb, CD45.2), B6-SJL/Ly5.1 (H-2Kb, CD45.1), FVB/N (H-2Kq), and BALB/c (H-2Kd, CD45.2) mice were purchased from the local stock of the animal facility at Freiburg University and bred there or obtained from The Jackson Laboratory and bred at the Oklahoma Medical Research Foundation (OMRF). All mice were raised under specific pathogen-free conditions. CD73-deficient (Cd73−/−) mice were developed at OMRF as described previously19 and back-crossed on to the C57BL/6 (H-2Kb) and BALB/c (H-2Kd) backgrounds for 14 generations. A2AAR−/− mice on the C57BL/6 background were kindly provided by Dr Jiang-Fan Chen (Boston University, Boston, MA). The luciferase-expressing (luc+) transgenic FVB/N L2G85 line has been previously described.31 Genotypes were confirmed by PCR. Mice were used between 6 and 12 weeks of age. All animal protocols were approved by the University Committee on the Use and Care of Laboratory Animals at Albert-Ludwigs University, Freiburg or the Institutional Animal Care and Use Committee of OMRF.
BMT model and induction of GVHD
Bone marrow transplantation (BMT) experiments were performed as previously described.32 Briefly, in Freiburg, recipients received myeloablative total body irradiation (10 Gy in 2 equal split doses, 4 hours apart), followed by intravenous injection of 5 × 106 BM cells. For induction of acute GVHD, CD4+ and CD8+ splenic T cells were enriched with CD4/CD8 MACS Micro Beads and LS columns (Miltenyi Biotec). The number of injected T cells varied depending on the transplant model: C57BL/6 → BALB/c (3 × 105 T cells), FVB/N → C57BL/6 (1 × 106 T cells), and BALB/c → C57BL/6 (2 × 105 T cells). At OMRF, C57BL/6 mice received myeloablative doses of total body irradiation (13 Gy in 2 equal split doses, 3 hours apart) followed by intravenous injection of 5 × 106 BM cells and 10 to 20 × 106 splenocytes from BALB/c mice. After BMT, Baytril was given in the drinking water at 0.5 mg/mL for 3 to 4 weeks to prevent infection. Any mice with weight loss more than 30% of the original body weight or with clinical33 GVHD scores more than 6 were killed. GVHD was also induced by transplantation of C57BL/6 cells into BALB/c mice using 10 Gy in 2 equal split doses, 3 hours apart, 5 × 106 BM cells plus 5 to 10 × 106 C57BL/6 splenocytes, and loss of body weight more than 35% as a criterion for death.
Histopathology scoring of acute GVHD
Sections of liver and small and large intestine collected 10 days after BMT were stained with hematoxylin and eosin and scored by an experienced pathologist (U.V.G.) blinded to the treatment groups as described by Kaplan et al.34 Intestinal GVHD was scored on the basis of crypt apoptosis (0 indicates rare to none; 1, occasional apoptotic bodies per 10 crypts; 2, few apoptotic bodies per 10 crypts; 3, the majority of crypts contain an apoptotic body; and 4, the majority of crypts contain > 1 apoptotic body) and inflammation (0 indicates none; 1, mild; 2, moderate; 3, severe, without ulceration; and 4, severe, with ulceration).
In vivo inhibition of CD73 and antagonism of ARs
APCP [adenosine 5′-(α, β-methylene) diphosphate; Sigma-Aldrich] or an equal volume of PBS was administered intraperitoneally at 50 mg/kg per day from days 0 to 6 after allogeneic BMT in the GVHD model and from days 0 to 10 in the B-cell leukemia model. This dose of APCP has been found to be nontoxic in mice and to induce phenotypes similar to those displayed by Cd73−/− mice.12 Mice received the general AR antagonist caffeine (Sigma-Aldrich) at 10 mg/kg per day or PBS by intraperitoneal injection 6 times in the first week after BMT and 3 to 5 times in the second week and later. This dose of caffeine has been previously documented to antagonize ARs.35
B-cell leukemia model
To generate luc+ A20 B-cell leukemia cells, a retrovirus encoding a luciferase-aminoglycoside phosphotransferase fusion gene was constructed from the luciferase gene of pUHC 13-3 and the neomycin resistance gene from pcDNA 3.1 (Invitrogen), using L1_eGFP_IRES as a backbone.36 The transduction of A20 cells (ATCC, #TIB-208) using a VSV-G pseudotyped retrovirus (BD Clontech) was performed according to the manufacturer's instructions. To induce leukemia, BALB/c mice were lethally irradiated and transplanted with 5 × 106 C57BL/6 BM cells and 5 × 104 wild-type (WT) or luc+ A20 cells (BALB/c background). After 2 days to allow the A20 cells to home to the BM and to establish leukemia, 1 to 3 × 105 CD4+/CD8+ T cells from a C57BL/6 donor were given to induce GVL. Tumor expansion was quantified by in vivo BLI.
In vivo BLI
For in vivo BLI, 10 minutes after intraperitoneal injection with 150 μg/g body weight luciferin [D-Luciferin 1-(4,5-dimethoxy-2-nitrophenyl) ethyl ester; Biosynth], mice were imaged using an IVIS100 charge-coupled device imaging system (Xenogen) with an exposure time of 5 minutes.37 The signal from luc+ transgenic FVB/N T cells or A20 leukemia cells was quantified in photons/s/mouse. Imaging data were analyzed and quantified with Living Image Version 3.2 software (Calipers).
Cytokine measurements in serum of mice with GVHD
IL-6 and IFN-γ were measured in serum collected from transplant recipients 6 to 7 days after BMT using the Ready-Set-Go ELISA kit (eBioscience).
Generation of BMDCs
Bone marrow-derived dendritic cells (BMDCs) were prepared as described,38 except that IL-4 was not added, and were used on days 7 to 9 of culture.
In vitro and in vivo T-cell proliferation assays
Splenic CD4+ T cells were isolated using CD4 MACS Microbeads and LS Columns (Miltenyi Biotec) and labeled with 1μM Vybrant carboxyfluorescein diacetate succinimidyl ester (Invitrogen) as previously described.39 T cells (2 × 105) were cocultured with 2 × 104 or 2 × 105 allogeneic BMDCs in a 96-well round-bottom plate in RPMI 1640, supplemented with 10% FCS, l-glutamine, 100 U/mL penicillin, 0.1 mg/mL streptomycin, and 25μM 2-mercaptoethanol (Invitrogen). After 5 days, T cells were harvested, stained for CD4, and analyzed by flow cytometry for carboxyfluorescein diacetate succinimidyl ester intensity.
Irradiated C57BL/6 mice were transplanted with BM cells and CFSE (5μM; Invitrogen)–labeled BALB/c splenocytes (10 × 106). On day 3, division of donor T cells was assessed by CFSE intensity by flow cytometry. Proportions of donor T cells in spleens of recipient mice were also determined 6 to 7 days after BMT by flow cytometry for the expression of CD4, CD8, and H-2Kd and H-2Kb.
T-cell migration assay
A total of 600 μL of RPMI supplemented with 2% FCS was added into the bottom of a 24-well transwell plate with or without 100 ng/mL of CXCL12 (R&D Systems). Then, inserts (membrane pore size 5.0 μm) were placed in the transwells, and 100 μL of cell suspension (CD4+/CD8+ C57BL/6 T cells at 5 × 106 cells/mL in RPMI 1640 supplemented with 2% FCS) was added. After incubation for 3 hours at 37°C and 5% CO2, the numbers of cells migrating into the bottom of the transwells and remaining in the insert were quantified by flow cytometry for 40 seconds at high pressure.
Flow cytometry and mAbs
In Freiburg, flow cytometric analysis was performed with a CyanADP flow cytometer (Beckman Coulter) and FlowJo Version 7/8 software (TreeStar). AlexaFluor-647–, allophycocyanin (APC)–, FITC-, PE- or eFluor450-conjugated mAbs (CD4, GK 1.5; CD8, 53-6.7; CD11c, N418; CXCR4, TG12; H-2Kb, AF6-88.5; CD25, PC61; Foxp3, FJK-16) were purchased from BioLegend and eBioscience.
At OMRF, flow cytometric analysis was performed with an LSR-II (BD Biosciences) and FlowJo 7.5 software. Propidium iodide was used to exclude dead cells from analysis. CountBright Absolute Counting Beads (Invitrogen) were used to count cells. For intracellular staining of Foxp3, cells were fixed with 2% paraformaldehyde and permeabilized with PBS containing 0.5% saponin and 3% FCS after cell surface staining. Sorting of T cells was performed with a Moflo cell sorter (Beckman Coulter). mAbs against the following mouse cell surface molecules were purchased: FITC-CD4 (RM4-5), CD8a (53.6-7), CD45.2 (104), CD86 (GL1), PE-CD11c (HL3), APC-Cy7-B220 (RA3-6B2), CD25 (PC61), unconjugated CD16/CD32 (2.4G2), and CD19 (1D3) from BD Biosciences; PE-H-2Kd (SF1-1.1), PE-Cy7-CD8a (53.6-7), CD19 (6D5), APC-H-2Kb (AF6-88.5), CD45.1 (A20), and APC-Cy7-CD4 (RM4-5) from BioLegend; APC-CD11b (M1/70.15) and CD8a (CT-CD8a) from Caltag Laboratories; and APC-Foxp3 (FJK-16s) from eBioscience. Nonspecific binding to surface Fc receptors was blocked by preincubation of cells with anti-CD16/CD32 mAb.
Characterization of intestinal lymphocytes during GVHD
Lymphocytes were isolated from the colons of mice with GVHD as described by Ivanov et al40 and stained for CD4, CD8, H-2Kb, and CXCR4 and also for CD4, CD25, H-2Kb, and Foxp3 to determine the numbers of Tregs.
Quantitative real-time PCR
To assess AR mRNA expression, total RNA was isolated from sorted T cells or intestinal tissues with TRIzol (Invitrogen) and then reverse-transcribed to cDNA using SuperScript III First-Strand Synthesis System (Invitrogen). Quantitative PCR was performed according to standard methods on an ABI750 sequence detection system (Applied Biosystems). The reaction mixture consisted of cDNA, SYBR Green super mix (Bio-Rad), and 0.5μM primers.23 Relative expression of AR to β-actin or β2-microglobulin was determined in duplicate samples according to the method of Pfaffl,41 which corrects for slightly different efficiencies of the 2 PCRs. A melt curve performed at the end of each run and agarose gel electrophoresis verified a specific single amplification product of the target genes.
In vivo CTL activity
In vivo cytotoxic T lymphocyte (CTL) assays were performed as previously described by Zheng et al42 with modifications. B cells were prepared by positive selection of splenocytes from Cd73−/− C57BL/6 and Cd73−/− BALB/c mice with the MACS cell separation system (Miltenyi Biotec) using anti-CD19 (1D3) mAb and goat anti–rat IgG MicroBeads. B-cell purity was more than 95% as assessed by CD19 expression (data not shown). After labeling with CFSE, equal numbers of C57BL/6 and BALB/c B cells (1-2 × 106 cells each) were intravenously transferred as target cells into C57BL/6 recipients of BALB/c BM plus splenocytes on days 6 to 7 after BMT. Three hours later, recipient mice were killed, and surviving target cells in the spleen were assessed by flow cytometry for the expression of H-2Kd and H-2Kb. In vivo anti-C57BL/6 CTL activity was indicated by a decrease (ie, < 50%) in the percentage of C57BL/6 B cells in the total surviving CFSE-labeled target cells (C57BL/6 B cells plus BALB/c B cells).
Irradiation-induced DC activation
Twenty-four hours after receiving lethal irradiation, the expression of CD86 on CD11c+CD11b+B220− C57BL/6 splenic DCs was analyzed by flow cytometry.
Competitive engraftment
Competitive engraftment assays were performed as previously described by Esplin et al43 with modifications. Irradiated Cd73+/+ or Cd73−/− BALB/c mice (H-2Kd, CD45.2, A2AAR+/+) received intravenous injection of CD45.1+CD45.2+ BM cells (5 × 106) from (C57BL/6 × B6-SJL/Ly5.1) F1 mice (H-2Kb, A2AAR+/+) together with equal numbers (2.5 × 106 each) of splenocytes prepared from B6-SJL/Ly5.1 mice (H-2Kb, CD45.1, A2AAR+/+) and from C57BL/6 A2AAR−/− or A2AAR+/+ mice (H-2Kb, CD45.2; supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). On day 8, the degree of chimerism of donor CD45.1−CD45.2+ cells in total H-2Kd-negative CD4+ and CD8+ splenocytes was analyzed by flow cytometry for the expression of CD4, CD8, H-2Kd, CD45.1, and CD45.2. The change in chimerism (fold increase) after BMT was compared with that of preinjection donor cells (day 0).
Statistical analysis
Statistical analyses were performed with GraphPad Prism Version 4/5 software. Data are reported as mean ± SEM or SD. Kaplan-Meier survival curves were analyzed by log-rank tests. Comparisons among groups in other experiments were performed with a 2-tailed unpaired Student t test. P values < .05 were considered to be statistically significant.
Results
CD73 deficiency leads to enhanced GVHD severity
To study the role of CD73 in acute GVHD, different MHC class I and II mismatch BMT models were used. Transfer of allogeneic Cd73−/− BM and T cells (or splenocytes) into Cd73−/− recipients enhanced GVHD-related mortality compared with the transfer of allogeneic WT (Cd73+/+) BM and T cells (or splenocytes) into WT recipients in both C57BL/6 → BALB/c and BALB/c → C57BL/6 transplants (Figure 1A-B; P < .0001). To determine the relative contribution of CD73 on donor versus recipient cells in dampening the severity of GVHD, 3 types of transplantations were performed. First, purified T cells from either WT or Cd73−/− C57BL/6 mice were transplanted into WT BALB/c recipients (Figure 1C; P = .0045). Second, purified T cells from WT BALB/c mice were transplanted into either WT or Cd73−/− C57BL/6 recipients (Figure 1D; P = .0019). Third, purified T cells from WT FVB/N mice were transplanted into either WT or Cd73−/− C57BL/6 recipients (Figures 1E; P = .0052). Deficiency of CD73 in both the donor and the recipient contributed to increased GVHD severity (Figure 1C-E). Enhancement of GVHD was more intense when the recipient was CD73-deficient (Figure 1D-E) in the BALB/c → C57BL/6 and the FVB/N → C57BL/6 models compared with the situation when the donor was CD73-deficient (Figure 1C). This finding could be explained by the higher abundance of the enzyme in the numerous recipient cells compared with the relatively small number of CD73+ hematopoietic cells infused during transplantation. In addition, CD73 deficiency on nonhematopoietic cells could play a more important role in determining the severity of GVHD. To verify that the effects were the result of CD73's ecto-5′-nucleotidase enzyme activity, allo-HCT recipients were treated with a CD73 enzyme inhibitor (APCP). Pharmacologic inhibition enhanced GVHD-related mortality (Figure 1F; P = .0022), suggesting that CD73-generated adenosine plays a central role in tolerance after allo-HCT. These data indicate that the enzymatic function of CD73 is critical to reduce the severity of GVHD. Other postulated functions of CD73, such as signal transduction7 or cell adhesion,44 would not be impacted by this pharmacologic intervention.
CD73 deficiency causes enhanced GVHD mortality. All BMTs were performed on lethally irradiated mice as described in “BMT model and induction of GVHD,” and survival was monitored for the indicated times. (A) Cd73+/+ (n = 12) and Cd73−/− (n = 13) BALB/c mice were transplanted with 5 × 106 BM cells with or without 10 × 106 splenocytes from Cd73+/+ or Cd73−/− C57BL/6 mice, respectively (P < .0001). (B) Cd73+/+ and Cd73−/− C57BL/6 mice were transplanted with 5 × 106 BM cells with or without 15 × 106 splenocytes from Cd73+/+ or Cd73−/− BALB/c mice, respectively (n = 10/group). P < .0001. (A-B) Results are representative of 3 independent experiments. (C) Cd73+/+ BALB/c mice were transplanted with 5 × 106 BM cells with or without 3 × 105 CD4+/CD8+ splenic T cells from Cd73+/+ or Cd73−/− C57BL/6 mice (n = 22/group; data pooled from 4 experiments). P = .0045. (D) Cd73+/+ and Cd73−/− C57BL/6 mice were transplanted with 5 × 106 BM cells and 2 × 105 CD4+/CD8+ splenic T cells from a Cd73+/+ BALB/c donor (n = 11/group; data pooled from 2 experiments). P = .0019. (E) Cd73+/+ (n = 11) and Cd73−/− (n = 13) C57BL/6 mice were transplanted with 5 × 106 BM cells and 1 × 106 CD4+/CD8+ splenic T cells from a Cd73+/+ FVB/N donor (data pooled from 3 experiments). P = .0052. (F) Cd73+/+ C57BL/6 mice were transplanted with 5 × 106 BM cells and 2 × 105 CD4+/CD8+ splenic T cells from Cd73+/+ BALB/c mice. One group (n = 10) was treated with the CD73 inhibitor APCP as described in “In vivo inhibition of CD73 and antagonism of ARs”; the other group (n = 15) received PBS as vehicle (data pooled from 3 experiments). P = .0022. (G) Cd73+/+ and Cd73−/− C57BL/6 mice were transplanted with 5 × 106 BM cells and 1 × 106 CD4+/CD8+ splenocytes from a Cd73+/+ FVB/N donor. Ten days later, mice were killed and paraffin sections of small intestine, large intestine, and liver were stained with hematoxylin and eosin. Histopathologic scoring was performed as described in “Histopathdology scoring of acute GVHD.” Left panel: Pooled GVHD histopathology score (mean ± SEM). *P = .0058 for liver. P = .0009 for small intestine. P = .003 for colon. n = 8-10 mice/group (data pooled from 3 independent experiments). Right panel: Representative colon sections from Cd73+/+ and Cd73−/− recipients. Evaluation of the stained tissue sections was performed on an Axio Imager A2 microscope (Zeiss) at 400× magnification and 0.60 numerical aperture. Photos were obtained using a digital camera (Horn Imaging, model MC-MD1/3, HD capture software). Arrows indicate crypt abscesses.
CD73 deficiency causes enhanced GVHD mortality. All BMTs were performed on lethally irradiated mice as described in “BMT model and induction of GVHD,” and survival was monitored for the indicated times. (A) Cd73+/+ (n = 12) and Cd73−/− (n = 13) BALB/c mice were transplanted with 5 × 106 BM cells with or without 10 × 106 splenocytes from Cd73+/+ or Cd73−/− C57BL/6 mice, respectively (P < .0001). (B) Cd73+/+ and Cd73−/− C57BL/6 mice were transplanted with 5 × 106 BM cells with or without 15 × 106 splenocytes from Cd73+/+ or Cd73−/− BALB/c mice, respectively (n = 10/group). P < .0001. (A-B) Results are representative of 3 independent experiments. (C) Cd73+/+ BALB/c mice were transplanted with 5 × 106 BM cells with or without 3 × 105 CD4+/CD8+ splenic T cells from Cd73+/+ or Cd73−/− C57BL/6 mice (n = 22/group; data pooled from 4 experiments). P = .0045. (D) Cd73+/+ and Cd73−/− C57BL/6 mice were transplanted with 5 × 106 BM cells and 2 × 105 CD4+/CD8+ splenic T cells from a Cd73+/+ BALB/c donor (n = 11/group; data pooled from 2 experiments). P = .0019. (E) Cd73+/+ (n = 11) and Cd73−/− (n = 13) C57BL/6 mice were transplanted with 5 × 106 BM cells and 1 × 106 CD4+/CD8+ splenic T cells from a Cd73+/+ FVB/N donor (data pooled from 3 experiments). P = .0052. (F) Cd73+/+ C57BL/6 mice were transplanted with 5 × 106 BM cells and 2 × 105 CD4+/CD8+ splenic T cells from Cd73+/+ BALB/c mice. One group (n = 10) was treated with the CD73 inhibitor APCP as described in “In vivo inhibition of CD73 and antagonism of ARs”; the other group (n = 15) received PBS as vehicle (data pooled from 3 experiments). P = .0022. (G) Cd73+/+ and Cd73−/− C57BL/6 mice were transplanted with 5 × 106 BM cells and 1 × 106 CD4+/CD8+ splenocytes from a Cd73+/+ FVB/N donor. Ten days later, mice were killed and paraffin sections of small intestine, large intestine, and liver were stained with hematoxylin and eosin. Histopathologic scoring was performed as described in “Histopathdology scoring of acute GVHD.” Left panel: Pooled GVHD histopathology score (mean ± SEM). *P = .0058 for liver. P = .0009 for small intestine. P = .003 for colon. n = 8-10 mice/group (data pooled from 3 independent experiments). Right panel: Representative colon sections from Cd73+/+ and Cd73−/− recipients. Evaluation of the stained tissue sections was performed on an Axio Imager A2 microscope (Zeiss) at 400× magnification and 0.60 numerical aperture. Photos were obtained using a digital camera (Horn Imaging, model MC-MD1/3, HD capture software). Arrows indicate crypt abscesses.
As expected, the increased mortality observed in Cd73−/− recipients of allogeneic BM and T cells was accompanied by histopathologic evidence of increased GVHD severity. Histopathologic evaluation of liver, small intestine, and colon according to a previously published scoring system34 demonstrated significantly higher scores in Cd73−/− recipients (Figure 1G).
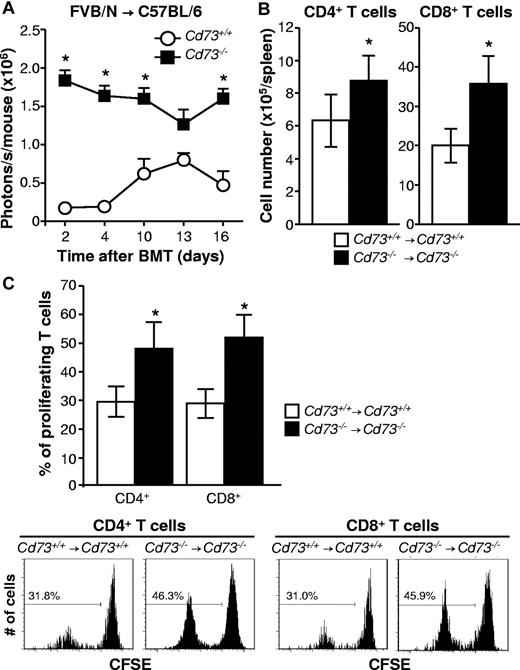
CD73 deficiency increases T-cell expansion after allo-HCT
The mechanism by which CD73 deficiency enhances GVHD could be the result of lower concentrations of extracellular adenosine, which has been shown to inhibit T-cell activation in vivo. Therefore, we assessed expansion of luc+ transgenic FVB/N T cells in C57BL/6 WT compared with CD73-deficient BMT recipients. BLI at sequential time points demonstrated significantly increased signals derived from the expanding allogeneic T cells in CD73-deficient recipients (P < .05, Figure 2A). Compatible with a more robust expansion, the numbers of donor CD4+ and CD8+ T cells were increased when the recipient (C57BL/6) and donor (BALB/c) were CD73-deficient compared with WT (P < .05, Figure 2B). BLI data were confirmed by flow cytometry showing increased percentages of proliferating CFSElo CD4+ and CD8+ T cells in Cd73−/− → Cd73−/− vs WT → WT recipients (P < .05, Figure 2C).
CD73 deficiency leads to increased expansion of allogeneic T cells after BMT. All BMTs were performed on lethally irradiated mice as described in “BMT model and induction of GVHD.” (A) Cd73+/+ and Cd73−/− C57BL/6 mice (3 each) were transplanted with 5 × 106 BM cells and 1 × 106 CD4+/CD8+ splenic T cells from a Cd73+/+luc+-transgenic FVB/N donor. In vivo expansion of luc+ T cells was quantified by BLI and is shown at sequential time points (mean ± SEM; representative results from one of 2 independent experiments). *P < .05. (B) Cd73+/+ or Cd73−/− C57BL/6 mice were transplanted with 5 × 106 BM cells and 10 × 106 spleen cells from Cd73+/+ or Cd73−/− BALB/c mice, respectively. Six to 7 days after BMT, numbers of donor CD4+ and CD8+ T cells in recipient spleens were determined by flow cytometry (mean ± SD; n = 3-5/group). *P < .05. The results are representative of 3 independent experiments. (C) Cd73+/+ or Cd73−/− C57BL/6 mice were transplanted with 5 × 106 BM cells and 10 × 106 CFSE-labeled spleen cells from Cd73+/+ or Cd73−/− BALB/c mice, respectively. Three days later, spleens were harvested and proliferating donor CD4+ and CD8+ T cells were identified by loss of CFSE intensity. The results are representative of 3 similar independent experiments (n = 3-5/group). Data are summarized as the percentage of proliferating donor CD4+ and CD8+ T cells (mean ± SD). *P < .05.
CD73 deficiency leads to increased expansion of allogeneic T cells after BMT. All BMTs were performed on lethally irradiated mice as described in “BMT model and induction of GVHD.” (A) Cd73+/+ and Cd73−/− C57BL/6 mice (3 each) were transplanted with 5 × 106 BM cells and 1 × 106 CD4+/CD8+ splenic T cells from a Cd73+/+luc+-transgenic FVB/N donor. In vivo expansion of luc+ T cells was quantified by BLI and is shown at sequential time points (mean ± SEM; representative results from one of 2 independent experiments). *P < .05. (B) Cd73+/+ or Cd73−/− C57BL/6 mice were transplanted with 5 × 106 BM cells and 10 × 106 spleen cells from Cd73+/+ or Cd73−/− BALB/c mice, respectively. Six to 7 days after BMT, numbers of donor CD4+ and CD8+ T cells in recipient spleens were determined by flow cytometry (mean ± SD; n = 3-5/group). *P < .05. The results are representative of 3 independent experiments. (C) Cd73+/+ or Cd73−/− C57BL/6 mice were transplanted with 5 × 106 BM cells and 10 × 106 CFSE-labeled spleen cells from Cd73+/+ or Cd73−/− BALB/c mice, respectively. Three days later, spleens were harvested and proliferating donor CD4+ and CD8+ T cells were identified by loss of CFSE intensity. The results are representative of 3 similar independent experiments (n = 3-5/group). Data are summarized as the percentage of proliferating donor CD4+ and CD8+ T cells (mean ± SD). *P < .05.
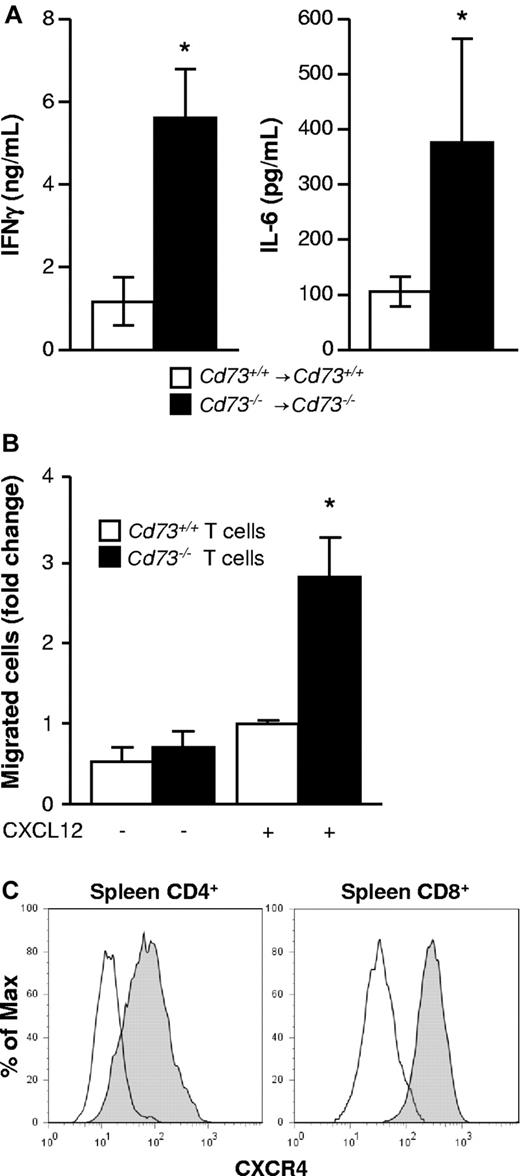
CD73 deficiency leads to increased proinflammatory IFN-γ and IL-6 serum levels and enhances T-cell migration
After allo-HCT, the proinflammatory cytokines IFN-γ and IL-6 contribute to GVHD development. Comparison of allo-HCT recipients (BALB/c → C57BL/6) revealed 4- to 5-fold higher IFN-γ and IL-6 serum levels collected 6 to 7 days after transplantation when donor and recipient were CD73-deficient (P < .05, Figure 3A). Besides proinflammatory cytokines, migration of T cells toward secondary lymphoid organs and GVHD target tissues is required for the development of GVHD. We observed enhanced T-cell migration toward a chemotactic CXCL12 gradient in vitro when the T cells were derived from Cd73−/− mice compared with WT mice (P < .05, Figure 3B). As CXCR4, the receptor for CXCL12, was abundantly expressed on both CD4+ and CD8+ T cells during GVHD (Figure 3C), these findings suggest that increased proinflammatory cytokine production and T-cell migration may contribute to the enhanced allo-immune response in the absence of CD73.
Serum proinflammatory cytokines are increased in CD73-deficient allogeneic BMT recipients, and CD73 is not required for T-cell migration in vitro. (A) Cd73+/+ or Cd73−/− C57BL/6 mice were exposed to lethal irradiation and transplanted with 5 × 106 BM cells and 10 × 106 spleen cells from Cd73+/+ or Cd73−/− BALB/c mice, respectively. Six to 7 days later, serum was collected from recipients, and the concentrations of IFN-γ and IL-6 were determined by ELISA (n = 5/group, mean ± SD). *P < .05. The data are representative of 3 similar independent experiments. (B) In vitro migration of CD4+ and CD8+ T cells purified from Cd73+/+ or Cd73−/− C57BL/6 mice along a CXCL12 gradient as described in “T-cell migration assay.” The data were normalized to the migration of Cd73+/+ T cells in the presence of CXCL12 and are expressed as mean fold increase ± SEM. *P = .0014. Pooled results are shown from 4 independent experiments performed with 3 or 4 replicates each for the CXCL12 containing wells. (C) Lethally irradiated BALB/c mice (n = 4) were transplanted with 5 × 106 BM cells and 3 × 105 CD4+/CD8+ T cells from a C57BL/6 donor. On day 8, CD4+ and CD8+ donor splenic T cells were analyzed for the expression of CXCR4 by flow cytometry. Open histograms represent isotype control staining.
Serum proinflammatory cytokines are increased in CD73-deficient allogeneic BMT recipients, and CD73 is not required for T-cell migration in vitro. (A) Cd73+/+ or Cd73−/− C57BL/6 mice were exposed to lethal irradiation and transplanted with 5 × 106 BM cells and 10 × 106 spleen cells from Cd73+/+ or Cd73−/− BALB/c mice, respectively. Six to 7 days later, serum was collected from recipients, and the concentrations of IFN-γ and IL-6 were determined by ELISA (n = 5/group, mean ± SD). *P < .05. The data are representative of 3 similar independent experiments. (B) In vitro migration of CD4+ and CD8+ T cells purified from Cd73+/+ or Cd73−/− C57BL/6 mice along a CXCL12 gradient as described in “T-cell migration assay.” The data were normalized to the migration of Cd73+/+ T cells in the presence of CXCL12 and are expressed as mean fold increase ± SEM. *P = .0014. Pooled results are shown from 4 independent experiments performed with 3 or 4 replicates each for the CXCL12 containing wells. (C) Lethally irradiated BALB/c mice (n = 4) were transplanted with 5 × 106 BM cells and 3 × 105 CD4+/CD8+ T cells from a C57BL/6 donor. On day 8, CD4+ and CD8+ donor splenic T cells were analyzed for the expression of CXCR4 by flow cytometry. Open histograms represent isotype control staining.
The consequences of CD73 deficiency are not via Tregs or costimulatory molecule expression
Based on the role of Foxp3+ regulatory T cells (Tregs) in protection from GVHD,31,32 we next evaluated whether this cell population was relevant for the observed enhanced GVHD severity in CD73-deficient mice. However, we found no differences in the numbers of donor Tregs in spleens or colons of recipients of Cd73+/+ vs Cd73−/− allo-HCT transplants (Figure 4A). We next compared the ability of WT versus Cd73−/− DCs from C57BL/6 mice to stimulate the proliferation of allogeneic WT BALB/C T cells in culture. (Figure 4B). No differences in T-cell proliferation were seen at 2 different DC/T-cell ratios, suggesting that WT and Cd73−/− DCs exhibit similar intrinsic allostimulatory activity in vitro. As previous studies had shown the relevance of the costimulatory molecules CD40, CD80, and CD86 in GVHD, we next asked whether these molecules were differentially expressed on WT compared with Cd73−/− DCs. Although LPS stimulation in vitro increased expression of the costimulatory molecules, there was no difference between WT and Cd73−/− DCs (data not shown). Similarly, there was no difference in CD86 expression of DCs in spleens of WT versus Cd73−/− mice 24 hours after lethal irradiation, suggesting that costimulatory molecule expression is not up-regulated in CD73-deficient mice, at least not as a consequence of irradiation (Figure 4C).
The exacerbation of GVHD by CD73 deficiency cannot be explained by differences in numbers of Tregs or allostimulation by DCs. (A) Lethally irradiated Cd73+/+ or Cd73−/− C57BL/6 mice were transplanted with 5 × 106 BM cells and 15 × 106 spleen cells from Cd73+/+ or Cd73−/− BALB/c mice, respectively. On day 6, the numbers of donor Treg (CD4+CD25+Foxp3+) in spleen were determined by flow cytometry (mean ± SD; n = 5). P > .05. In other experiments, lethally irradiated Cd73+/+ BALB/c mice were transplanted with 5 × 106 BM cells and 3 × 105 CD4+/CD8+ T cells from Cd73+/+ or Cd73−/− C57BL/6 mice. Lymphocytes were isolated from the colon on day 8 and analyzed for the numbers of donor Treg (mean ± SD; n = 3). P > .05. The results are representative of 2 independent experiments. (B) In vitro expansion of CFSE-labeled WT BALB/c CD4+ T cells stimulated with BMDCs derived from a Cd73+/+ or Cd73−/− C57BL/6 mouse. Left panels: Representative histograms from one of 3 independent experiments show CFSE intensity of WT CD4+ cells after 5 days at different coculture ratios. Right panel: Comparison of the percentages of proliferating WT CD4+ T cells stimulated with either Cd73+/+ or Cd73−/− BMDCs for 5 days (mean ± SEM). P > .05. (C) Twenty-four hours after lethal irradiation, spleens were isolated from Cd73+/+ and Cd73−/− C57BL/6 mice, and the expression of CD86 on CD11c+CD11b+B220− DCs was analyzed by flow cytometry. Histograms are representative of 2 independent experiments. Shaded and solid line histograms represent staining of DCs from nonirradiated mice with isotype control and anti-CD86 mAb, respectively. The dotted line histogram represents staining of DCs from irradiated mice with anti-CD86 mAb. The data are summarized as mean ± SD; mean fluorescence intensity (MFI) of CD86 staining (n = 3). P > .05.
The exacerbation of GVHD by CD73 deficiency cannot be explained by differences in numbers of Tregs or allostimulation by DCs. (A) Lethally irradiated Cd73+/+ or Cd73−/− C57BL/6 mice were transplanted with 5 × 106 BM cells and 15 × 106 spleen cells from Cd73+/+ or Cd73−/− BALB/c mice, respectively. On day 6, the numbers of donor Treg (CD4+CD25+Foxp3+) in spleen were determined by flow cytometry (mean ± SD; n = 5). P > .05. In other experiments, lethally irradiated Cd73+/+ BALB/c mice were transplanted with 5 × 106 BM cells and 3 × 105 CD4+/CD8+ T cells from Cd73+/+ or Cd73−/− C57BL/6 mice. Lymphocytes were isolated from the colon on day 8 and analyzed for the numbers of donor Treg (mean ± SD; n = 3). P > .05. The results are representative of 2 independent experiments. (B) In vitro expansion of CFSE-labeled WT BALB/c CD4+ T cells stimulated with BMDCs derived from a Cd73+/+ or Cd73−/− C57BL/6 mouse. Left panels: Representative histograms from one of 3 independent experiments show CFSE intensity of WT CD4+ cells after 5 days at different coculture ratios. Right panel: Comparison of the percentages of proliferating WT CD4+ T cells stimulated with either Cd73+/+ or Cd73−/− BMDCs for 5 days (mean ± SEM). P > .05. (C) Twenty-four hours after lethal irradiation, spleens were isolated from Cd73+/+ and Cd73−/− C57BL/6 mice, and the expression of CD86 on CD11c+CD11b+B220− DCs was analyzed by flow cytometry. Histograms are representative of 2 independent experiments. Shaded and solid line histograms represent staining of DCs from nonirradiated mice with isotype control and anti-CD86 mAb, respectively. The dotted line histogram represents staining of DCs from irradiated mice with anti-CD86 mAb. The data are summarized as mean ± SD; mean fluorescence intensity (MFI) of CD86 staining (n = 3). P > .05.
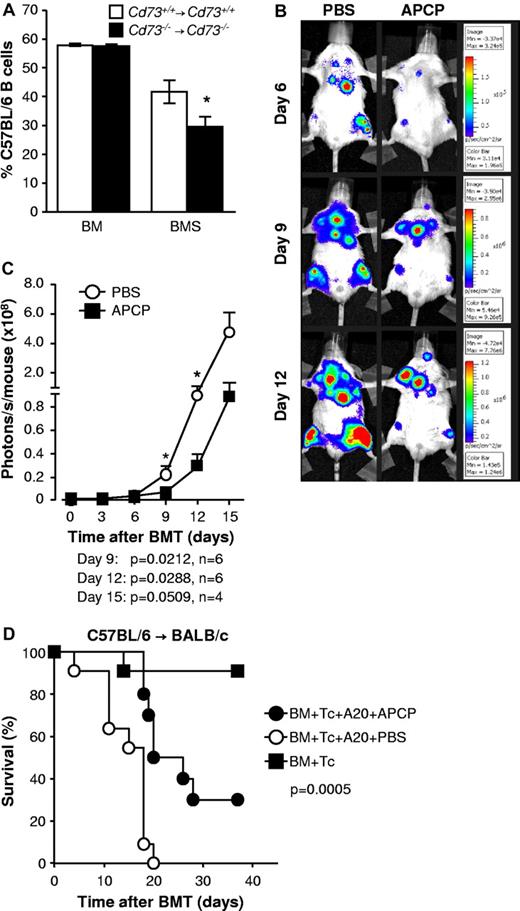
Cytotoxicity against host cells and GVL activity of donor T cells are enhanced when CD73 is deficient or inhibited
To evaluate the cytotoxic activity of donor T cells against allogeneic targets in vivo, we compared the survival of target CFSE-labeled CD19+ B cells of donor versus host origin in WT and Cd73−/− recipients of allogeneic BM plus splenocytes in the BALB/c → C57BL/6 model (WT→ WT vs Cd73−/− → Cd73−/−). When both the donor and the recipient were CD73-deficient, the proportion of CD19+ target cells that were of recipient origin 3 hours after transfer was significantly reduced, indicating a higher cytotoxic activity of the donor T cells (P < .05, Figure 5A).
Cytotoxicity against host cells and GVL activity of donor T cells are enhanced when CD73 is deficient or inhibited. (A) A total of 5 million BM cells with (BMS) or without (BM) 15 × 106 splenocytes from Cd73+/+ or Cd73−/− BALB/c mice were transplanted into irradiated Cd73+/+ or Cd73−/− C57BL/6 recipients, respectively. Six to 7 days later, equal numbers of CFSE-labeled B cells from Cd73−/− C57BL/6 and Cd73−/− BALB/c mice were transferred intravenously into the recipients as targets. Three hours later, recipient spleens were harvested and the CFSE-labeled cells were analyzed for the expression of H-2Kb and H-2Kd by flow cytometry. A decrease in the percentage of CFSE+ H-2Kb target cells is indicative of anti-host cytotoxic activity. The data are summarized as the percentage (mean ± SD) of total CFSE-labeled target B cells that are H-2Kb+ (n = 3 or 4). *P < .05. (B) Lethally irradiated BALB/c mice received 5 × 106 C57BL/6 BM cells and 5 × 104luc+ A20 cells on day 0 of allo-HCT and 3 × 105 C57BL/6 CD4+/CD8+ splenic T cells on day 2. One group (n = 6) was treated with the CD73-inhibitor APCP at a dosage of 50 mg/kg per day intraperitoneally. On days 0 to 10 after allo-HCT, the other group (n = 6) received an equal volume of PBS as vehicle. Expansion of luc+ A20 cells was monitored via BLI as described in “In vivo BLI.” Representative bioluminescent images on days 6, 9, and 12 are shown. (C) Expansion of luc+ A20 tumor cells as measured in photons over total body area at different time points with 6 mice per group is shown. Data from one of 2 independent experiments with similar results are shown (mean ± SEM). (D) Same as in panel B, except that the recipient mice received WT A20 tumor cells and only 105 CD4+/CD8+ splenic T cells. A control group of mice did not receive A20 tumor cells. Survival was monitored for 5 weeks. Data are pooled from 2 independent experiments (n = 10 or 11/group). P = .0005 (PBS vs APCP treatment).
Cytotoxicity against host cells and GVL activity of donor T cells are enhanced when CD73 is deficient or inhibited. (A) A total of 5 million BM cells with (BMS) or without (BM) 15 × 106 splenocytes from Cd73+/+ or Cd73−/− BALB/c mice were transplanted into irradiated Cd73+/+ or Cd73−/− C57BL/6 recipients, respectively. Six to 7 days later, equal numbers of CFSE-labeled B cells from Cd73−/− C57BL/6 and Cd73−/− BALB/c mice were transferred intravenously into the recipients as targets. Three hours later, recipient spleens were harvested and the CFSE-labeled cells were analyzed for the expression of H-2Kb and H-2Kd by flow cytometry. A decrease in the percentage of CFSE+ H-2Kb target cells is indicative of anti-host cytotoxic activity. The data are summarized as the percentage (mean ± SD) of total CFSE-labeled target B cells that are H-2Kb+ (n = 3 or 4). *P < .05. (B) Lethally irradiated BALB/c mice received 5 × 106 C57BL/6 BM cells and 5 × 104luc+ A20 cells on day 0 of allo-HCT and 3 × 105 C57BL/6 CD4+/CD8+ splenic T cells on day 2. One group (n = 6) was treated with the CD73-inhibitor APCP at a dosage of 50 mg/kg per day intraperitoneally. On days 0 to 10 after allo-HCT, the other group (n = 6) received an equal volume of PBS as vehicle. Expansion of luc+ A20 cells was monitored via BLI as described in “In vivo BLI.” Representative bioluminescent images on days 6, 9, and 12 are shown. (C) Expansion of luc+ A20 tumor cells as measured in photons over total body area at different time points with 6 mice per group is shown. Data from one of 2 independent experiments with similar results are shown (mean ± SEM). (D) Same as in panel B, except that the recipient mice received WT A20 tumor cells and only 105 CD4+/CD8+ splenic T cells. A control group of mice did not receive A20 tumor cells. Survival was monitored for 5 weeks. Data are pooled from 2 independent experiments (n = 10 or 11/group). P = .0005 (PBS vs APCP treatment).
As the enhanced alloreactivity seen in the Cd73−/− mice could impact GVL effects, we next evaluated antileukemic immune responses in allo-HCT recipients in the presence or absence of the CD73/ecto-5′-nucleotidase inhibitor (APCP). Lethally irradiated BALB/c mice were reconstituted with C57BL/6 BM and received 5 × 104luc+ A20 cells intravenously. Two days later, they received purified C57BL/6 T cells. APCP was administered daily from days 0 to 10 after allo-HCT. We observed significantly reduced growth of luc+ A20 cells in vivo when CD73 was blocked, as shown by quantification of photons emitted by luc+ A20 cells at sequential time points (Figure 5B-C; P < .03 on days 9 and 12). This observation is compatible with the enhanced alloreactive expansion and cytokine production of donor T cells seen in Cd73−/− recipients. APCP also increased survival of tumor-bearing mice in additional experiments where the T-cell dose was lowered to ensure that GVHD did not contribute to mortality (Figure 5D; P = .0005). Before death, tumor-bearing mice displayed hind limb paralysis, a symptom that was never observed in mice with GVHD.
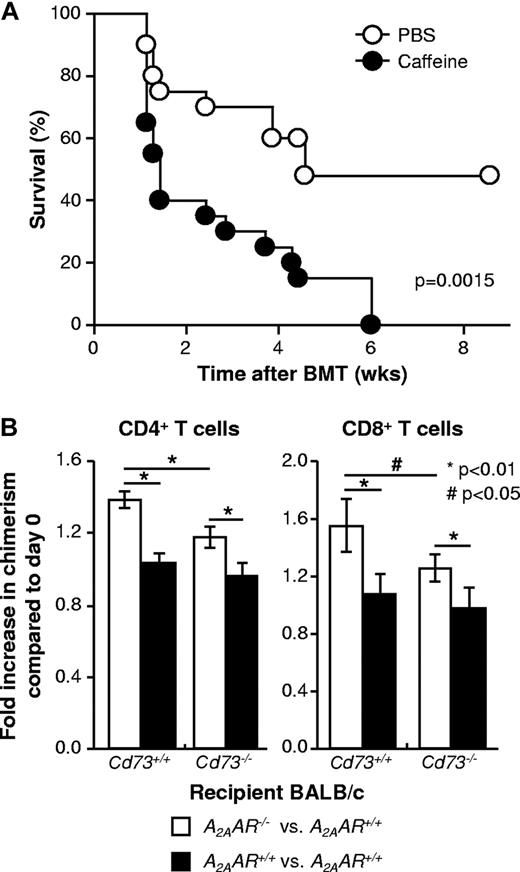
Extracellular adenosine reduces the severity of GVHD at least in part via signaling through the A2AAR on T cells
One mechanism by which CD73 deficiency could lead to increased severity of GVHD is via reduced AR signaling. To test this hypothesis, WT mice underwent allogeneic BMT and were then treated with the general AR antagonist, caffeine at a dose (10 mg/kg, intraperitoneally) previously shown to inhibit AR signaling.35 As shown in Figure 6A, mortality was increased (P = .0015) by chronic treatment with caffeine, indicating that the severity of GVHD was exacerbated and suggesting that endogenous AR signaling limits the extent of GVHD.
Extracellular adenosine reduces the severity of GVHD at least in part via signaling through the A2AAR on T cells. (A) Irradiated C57BL/6 mice (H-2Kb) received 5 × 106 BM cells and 20 × 106 spleen cells from BALB/c mice (H-2Kd) followed by IP injection of caffeine (10 mg/kg) or an equal volume of PBS 6 times in the first week after BMT and 3 to 5 times in the second week and later. The mice were monitored for survival for 9 weeks (P = .0015). The results are combined from 2 independent experiments (n = 20/group). (B) Irradiated BALB/c (CD45.2, A2AAR+/+) mice received intravenous injection of 5 × 106 BM cells from CD45.1+CD45.2+ C57BL/6 F1 congenic mice (A2AAR+/+) together with 2.5 × 106 each of splenocytes prepared from C57BL/6 A2AAR+/+ congenic mice (CD45.1) and from C57BL/6 A2AAR−/− or A2AAR+/+ congenic mice (CD45.2), as shown in supplemental Figure 1. On day 8, spleen cells were prepared from recipient mice and analyzed by flow cytometry for the expression of CD4, CD8, H-2Kd, CD45.1, and CD45.2 to determine the degree of chimerism. The bar graph represents the changes in the percentages of CD45.2+CD45.1− cells in total donor CD4+ and CD8+ T cells (ie, changes in chimerism) after allo-HCT compared with that in the preinjection donor cells (day 0): open bar represents A2AAR−/− versus A2AAR+/+; filled bar, A2AAR+/+ versus A2AAR+/+. Data are mean ± SD (n = 5). *P < .01. #P < .05. This experiment was performed using Cd73+/+ (left 2 bars) and Cd73−/− (right 2 bars) BALB/c mice as recipients. The results are representative of 2 similar independent experiments (n = 5/group).
Extracellular adenosine reduces the severity of GVHD at least in part via signaling through the A2AAR on T cells. (A) Irradiated C57BL/6 mice (H-2Kb) received 5 × 106 BM cells and 20 × 106 spleen cells from BALB/c mice (H-2Kd) followed by IP injection of caffeine (10 mg/kg) or an equal volume of PBS 6 times in the first week after BMT and 3 to 5 times in the second week and later. The mice were monitored for survival for 9 weeks (P = .0015). The results are combined from 2 independent experiments (n = 20/group). (B) Irradiated BALB/c (CD45.2, A2AAR+/+) mice received intravenous injection of 5 × 106 BM cells from CD45.1+CD45.2+ C57BL/6 F1 congenic mice (A2AAR+/+) together with 2.5 × 106 each of splenocytes prepared from C57BL/6 A2AAR+/+ congenic mice (CD45.1) and from C57BL/6 A2AAR−/− or A2AAR+/+ congenic mice (CD45.2), as shown in supplemental Figure 1. On day 8, spleen cells were prepared from recipient mice and analyzed by flow cytometry for the expression of CD4, CD8, H-2Kd, CD45.1, and CD45.2 to determine the degree of chimerism. The bar graph represents the changes in the percentages of CD45.2+CD45.1− cells in total donor CD4+ and CD8+ T cells (ie, changes in chimerism) after allo-HCT compared with that in the preinjection donor cells (day 0): open bar represents A2AAR−/− versus A2AAR+/+; filled bar, A2AAR+/+ versus A2AAR+/+. Data are mean ± SD (n = 5). *P < .01. #P < .05. This experiment was performed using Cd73+/+ (left 2 bars) and Cd73−/− (right 2 bars) BALB/c mice as recipients. The results are representative of 2 similar independent experiments (n = 5/group).
To gain insight into which AR(s) might be involved, the expression of all 4 ARs was evaluated by quantitative RT-PCR in CD4+ and CD8+ T cells isolated from control mice and mice experiencing GVHD. The A1, A2B, and A3 ARs were each expressed at low levels, and their expression did not change in a reproducible fashion during GVHD (Table 1). In contrast, the level of A2AAR expression was up-regulated 3- to 5-fold on both CD4+ and CD8+ T cells during GVHD (Table 1). To further explore the role of the A2AAR in limiting the extent of GVHD, competitive transplants were performed where WT BM from C57BL/6 mice was transplanted into either WT or Cd73−/− BALB/c mice along with a mixture of WT and A2AAR−/− splenocytes (on the C57BL/6 background) that could be distinguished based on the expression of either CD45.1 or CD45.2 (supplemental Figure 1). In control experiments, mice were transplanted with a mixture of CD45.1 and CD45.2 WT (ie, A2AAR+/+) splenocytes. Figure 6B shows that, with both WT and Cd73−/− recipients, there were significantly increased proportions of A2AAR−/− CD4+ and CD8+ T cells. These results are consistent with the idea that the proliferation of allogeneic T cells is suppressed by signaling through the A2AAR by endogenously produced adenosine. As expected, the ratio of A2AAR−/−/A2AAR+/+ T cells was less in Cd73−/− recipients, presumably because less exogenous adenosine was available to suppress the growth of T cells expressing the A2AAR. Thus, these results also confirm a significant role for CD73 in the production of extracellular adenosine for A2AAR signaling.
ARs were also expressed in GVHD target tissues, such as the liver, small intestine, and colon (Table 1). The most significant changes after the induction of GVHD were a 2.8-fold increase in the A1AR in the colon and a 2.8-fold increase in the A2BAR in the small intestine. As much less is known about the consequences of AR signaling in these tissues, additional experimentation will be required to determine whether they play a role in the less severe GVHD observed in WT compared with CD73-deficient animals.
Discussion
Tissue damage is recognized by the immune system via the release of endogenous molecules derived from the cytosol or mitochondria of stressed cells, such as damage-associated molecular patterns45 and ATP3 or via damaged extracellular matrix.4,46 Therefore, mechanisms that control the abundance of such danger signals are critically required for immune regulation. CD39 degrades ATP to AMP and CD73 further metabolizes AMP into adenosine, which can bind the A2AAR and thereby exert tolerogenic effects. Here we show that deficiency of CD73 causes enhanced GVHD severity with stronger in vivo T-cell expansion and cytotoxicity and increased concentrations of proinflammatory cytokines. Both donor and recipient CD73 were relevant for tolerogenic effects as genetic deficiency in either one of them caused enhanced GVHD; however, recipient CD73 played a more prominent role. One cell type in recipients that probably contributes to the protective effects of CD73 is the mesenchymal stem cell. Mesenchymal stem cells are characterized by high expression of CD73,47 and several groups have demonstrated their ability to dampen the severity of GVHD.48 Further experiments will be required to confirm the role of CD73 for MSC-mediated GVHD protection.
Our findings are compatible with previous studies in the cardiac allograft model showing a protective role of CD73 for organ graft survival.16 In light of these data from solid organ transplantation and our data, CD73 appears to act as a rescue mechanism along with CD39 to protect cells from the proinflammatory effects of extracellular ATP released as a consequence of tissue damage. The anti-inflammatory action of CD73 after allo-HCT is probably based on its ability to generate adenosine, as treatment of mice with an inhibitor of CD73's ecto-5′-nucleotidase enzyme activity also increased the severity of GVHD. Furthermore, CD73-generated adenosine is known to inhibit proinflammatory cytokine production,28 to decrease the expression of adhesion molecules on endothelial cells,49 and to inhibit endothelial permeability,7 actions that should reduce the severity of GVHD. Our findings extend the observations of Lappas et al13 who showed that pharmacologic activation of the A2AAR can reduce the severity of GVHD. In contrast, our results showed that endogenous levels of A2AAR signaling reduced the expansion of allogeneic T cells and demonstrated that CD73 was the source of a significant portion of the activating ligand.
Alloreactivity is certainly detrimental in the solid organ allograft setting but could be of therapeutic use in the situation of allo-HCT for hematologic malignancies. Patients with residual tumor cells or relapse after allo-HCT are often treated with donor lymphocyte infusions2 and by reducing immunosuppressive medication. Based on our findings, pharmacologic blockade of CD73 could enhance alloreactivity and thereby also antitumor effects. This is of particular interest as it was shown that tumor-induced immunosuppression is, at least in part, based on intact CD73.24-27 Compatible with these data from different tumor models, we observed that antileukemia immune effects were more potent when CD73 was inhibited. This could be exploited in the clinic when patients experience relapse or have minimal residual disease after allo-HCT and are treated with donor lymphocyte infusion. Pharmacologic blockade of CD73 may enhance the immune-mediated effects of donor lymphocyte infusion after allo-HCT. Comparable approaches combining donor lymphocyte infusion with anti–CTLA-4 antibodies have already been successfully applied in the clinic.50
In conclusion, we observed that genetic deficiency or blockade of CD73 enhanced GVHD and GVL effects. Therefore, treatment with a CD73 inhibitor (or an AR antagonist) may be a justified clinical approach when residual tumor cells are detected after allo-HCT. Conversely, in the clinical setting when allo-HCT is applied for the correction of metabolic defects, such as immunodeficiencies or hemoglobinopathies where GVHD is a main barrier, immune modulation by addition of CD73 enzyme or an AR agonist could improve the outcome.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Michelle Joachims and Paul Kincade for critical review of the manuscript, Ms Stephanie McGee for expert technical assistance, and Ms Mary Flynn for manuscript preparation.
This work was supported by the Deutsche Forschungsgemeinschaft (ID 7/4-2, M.I.; ZE 872/1-1, R.Z.; Emmy-Noether fellowship, M.I.; and Heisenberg fellowship, R.Z.) and the National Institutes of Health (grant AI18220, L.F.T.). L.F.T. holds the Putnam City Schools Distinguished Chair in Cancer Research.
National Institutes of Health
Authorship
Contribution: H.T and P.C. performed experiments, analyzed data, and wrote the manuscript; K.A., J.G., and M.F. performed experiments; U.V.G. and J.W.R. performed histopathology evaluations; A.R. performed the quantitative RT-PCR analyses for A2AAR expression; R.Z., M.I., and L.F.T. designed the studies, supervised experiments, analyzed data, and wrote the manuscript; and all authors have read and agreed to the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for H.T. is Division of Molecular Cell Biology, Department of Biomolecular Sciences, Saga University Faculty of Medicine, Saga, Japan.
Correspondence: Linda F. Thompson, Oklahoma Medical Research Foundation, 825 NE 13th St, Oklahoma City, OK 73104; e-mail: linda-thompson@omrf.org.
References
Author notes
H.T. and P.C. contributed equally to this study.
R.Z., L.F.T., and M.I. contributed equally to this study.