Abstract
Amyloidoses are characterized by organ deposition of misfolded proteins. This study evaluated immunohistochemistry as a diagnostic tool for the differentiation of amyloid subentities, which is warranted for accurate treatment. A total of 117 patients were systematically investigated by clinical examination, laboratory tests, genotyping, and immunohistochemistry on biopsy specimens. Immunohistochemistry enabled the classification in 94% of the cases. For subsequent analysis, the patient population was divided into 2 groups. The first group included all patients whose diagnosis could be verified by typical clinical signs or an inherited amyloidogenic mutation. In this group, immunohistochemical subtyping was successful in 49 of 51 cases and proved accurate in each of the 49 cases, corresponding to a sensitivity of 96% and a specificity of 100%. The second group included patients with systemic light chain amyloidosis without typical signs, senile transthyretin, or hereditary amyloidosis with a concomitant monoclonal gammopathy. Immunohistochemistry allowed to define the subentities in 61 of 66 (92%) of these cases. Immunohistochemistry performed by a highly specialized pathologist combined with clinical examination and genotyping leads to a high accuracy of amyloidosis classification and is the standard in our center. However, new techniques, such as mass spectroscopy-based proteomics, were recently developed to classify inconclusive cases.
Introduction
Amyloid is defined as the deposition of insoluble protein fibrils, forming histologically a homogeneous, eosinophilic mass, which stains positive for the Congo red dye and displays green birefringence under polarized light because of its β-pleated sheet conformation.1 Amyloidosis constitutes a heterogeneous group of distinct diseases, which differ in their pathogenesis and clinical course.2 The most frequent amyloid disorder in the Western world is immunoglobulin light chain–derived (AL) amyloidosis, which is caused by the deposition of light chains in the setting of a monoclonal plasma cell dyscrasia or a lymphoproliferative disorder. The acute-phase reactant serum amyloid A–derived (AA) amyloidosis is the result of chronic inflammation. Hereditary variants are attributable to amyloidogenic mutations in genes encoding normally soluble proteins, such as transthyretin (ATTR), fibrinogen (AFib), apolipoprotein A1 (AApo A1), and lysozyme (ALys). In the setting of senile amyloidosis, wild-type transthyretin forms amyloid deposits mostly in the heart and vessels. Accurate and reliable differentiation of the amyloid subentity is of paramount importance, given the widely differing therapies, ranging from chemotherapy for AL amyloidosis to liver transplantation for some hereditary forms.
Several strategies are applied to differentiate the distinct amyloid subentities. Clinically, the organ involvement pattern is sometimes suggestive of the amyloid subentity, given the selective organ tropism of the amyloidogenic protein.3 Periorbital bleeding and macroglossia are considered typical symptoms of AL amyloidosis.4 As for laboratory testing, the detection of a monoclonal gammopathy is also suggestive of AL amyloidosis. Screening for a monoclonal gammopathy has been refined in recent years since the introduction of the serum-free light chain assay in addition to immunofixation techniques to identify and quantify even very small light chain-producing B-cell clones. Molecular testing allows the identification of mutations underlying hereditary amyloidoses.
Another strategy, the immunohistochemical classification of amyloid on formalin-fixed and paraffin-embedded tissue sections, has been applied and refined in the last 20 years,5–8 using commercial and noncommercial antibodies. However, rather disappointing results have been reported in some instances.9 The technical difficulties of immunohistochemistry have triggered the development of new diagnostic strategies, such as immunoelectron microscopy,10 proteomic analysis after laser microdissection and mass spectrometry,11,12 or amino acid sequencing.13 However, these techniques are only established in a few specialized centers, are difficult to apply in small tissue biopsies with minute amounts of amyloid, are rather sophisticated, and have not been evaluated in large and controlled studies.
In this study, we reassessed the sensitivity and specificity of immunohistochemistry in a prospective and blinded manner. Although a couple of previous studies have addressed immunohistochemistry as a diagnostic tool for amyloid subtyping,5,8,14–17 this is, to our knowledge, the first comprehensive study, validating immunohistochemistry in combination with clinical, laboratory, and genetic results in the context of a routine clinical setting in more than 100 patients.
Methods
Patients
Between March 2006 and March 2009, 353 patients were admitted to our Amyloidosis Center with the diagnosis of amyloidosis. A total of 156 patients had no or an inconclusive immunohistochemical analysis of the diagnostic biopsy. Therefore, we asked the primary pathologists to send their biopsies for reference assessment to C.R. who consecutively investigated them by immunohistochemistry. The remaining group consisted mostly of patients with kidney biopsies, which had been analyzed by other specialized pathologists and for that reason were not included in this analysis. Inclusion criteria for our systematic retrospective analysis were as follows: patient was examined in our Amyloidosis Clinic, diagnosis of systemic disease, complete genetic screening by P.L., and reference pathology by C.R. Thirty-seven patients with a localized amyloidosis were excluded. One patient with an isolated renal amyloidosis, without monoclonal gammopathy and without typical symptoms of AL and no known amyloidogenic mutation, could not be allocated to any subtype of amyloidosis and was therefore excluded. Recently, we have sent this renal biopsy for high performance liquid chromatography and tandem mass spectrometry11 to the Mayo Clinic, Rochester, MN. The diagnosis of amyloidosis was confirmed, but the type could also not be determined. Another patient had been pretreated with high-dose steroids without gammopathy assessment. At admission in our center, we were not able to detect a monoclonal gammopathy. Immunohistochemistry was positive for AL λ and could therefore not be confirmed by our diagnostic approach, so that we also had to exclude her from this analysis.
Patient characteristics of the remaining 117 patients are shown in Table 1. We screened all patients for organ involvement as previously described.18 The presence of a monoclonal gammopathy was investigated using immunofixation of serum and urine and free light chain measurement. Bone marrow was examined cytologically and additionally by FISH analysis of CD138+-sorted plasma cells.19 Periorbital bleeding and macroglossia were counted as typical symptoms of AL amyloidosis.20 A positive family history was assumed if at least 1 family member had the clinical suspicion of amyloidosis.
The diagnosis of amyloidosis was always based on histologic confirmation. It was often reached by external pathologists, and only Congo red-positive, amyloid-bearing tissue samples were then forwarded to the study pathologist (C.R.) for further immunohistochemical analyses. Blood samples were taken in the Amyloidosis Center Heidelberg and screened for amyloidogenic mutations. Both the pathologist (C.R.) and the geneticist (P.L.) were blinded with regard to clinical and laboratory data. Approval was obtained from the Ethics Committee of the University of Heidelberg, and the patients gave their informed consent in accordance with the Declaration of Helsinki.
Histology and immunohistochemistry
All tissue samples were fixed in formalin and embedded in paraffin. Serial sections were stained with hematoxylin and eosin. Amyloid was detected in Congo red-stained sections viewed under cross-polarized light. Immunohistochemistry was performed with commercially available monoclonal antibodies directed against AA amyloid (1:600) and polyclonal antibodies against amyloid P-component (1:5000), fibrinogen (1:2000), lysozyme (1:3000), transthyretin (1:4000), λ-light chain (1:160 000), κ-light chain (1:160 000; all Dako Denmark), as well as with noncommercially available polyclonal antibodies directed against apolipoprotein AI (1:1000),21 λ-light chain-derived amyloid proteins (AL1, 1:3000),22 and λ-light chain-peptide antibodies (AL3, 1:250; AL7, 1:500, supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Immunostaining was done with the BenchMark XT immunostainer, using the ultraView Universal Alkaline Phosphatase Red Detection Kit (both Ventana Medical Systems) or the NOVADetect DAB-Substrat Kit (Dianova). Before the incubation with primary antibodies, sections were pretreated with Cell Conditioning 1 according to the manufacturer's instructions (CC1; Ventana; amyloid P-component, l-light chain, AL7, k-light chain, transthyretin) or with sodium citrate (4 times, 5 minutes, 600 W, microwave oven ApoAI). The specificity of the immunostaining was verified using specimens containing known classes of amyloid (AA amyloid, transthyretin, λ-light chain) or using positive controls recommended by the manufacturers (remaining antibodies). In addition, a tissue microarray containing 16 spots of tissue samples with liver, AA, ATTR, and ALλ amyloid was used as an on-slide positive control and negative control on each staining round during the entire study period. Omission of the primary antibody served as a further negative control. All antibodies used in the present study had been validated extensively in the past and were shown to immunoreact with the amyloid proteins of the respective type.23
Classification of amyloid was carried out as described in detail elsewhere24 : Strong and even immunostaining of the entire amyloid deposit by 1 non–anti-AL antibody was categorized as proof of the non–AL-fibril protein (eg, AA-, AApoAI-, ALys-, and ATTR-amyloid). An exception to this rule was renal AFib amyloidosis. Renal AFib amyloid has a characteristic morphologic appearance, whereas the commercial antibodies usually stain only some areas of the amyloid deposits. Therefore, a diagnosis of AFib amyloidosis was reached by the combination of characteristic morphology and immunostaining.25 AL amyloidosis was diagnosed when at least 1 antibody stained the amyloid deposits (AL1, AL3, AL7, anti–λ-light chain, anti–κ-light chain), whereas all other antibodies directed against non-AL amyloid had to be immunonegative. However, with regard to ALλ amyloidosis, usually a minimum of 3 anti–λ-light chain antibodies stained the deposits, supporting proof of the fibril protein.
Stained slides were captured by a Nikon Eclipse 80i optical microscope fitted with a Nikon 20×/0.75 Plan Apo objective (Nikon). Microphotographs were acquired using a JVC digital camera (KY-F75U) and imported using the DISKUS imaging software (both provided by AVT Horn). Microphotographs were subsequently edited (sharpness, cropping, labeling) using Adobe Photoshop CS4 Extended Version 10.0.1.
Sensitivity and specificity of immunohistochemistry
Sensitivity and specificity were determined per patient (and not per biopsy) of the group of patients who were already assigned to the amyloid subtypes based on clinical, laboratory, and genetic findings (see “Results”). Sensitivity was defined as the percentage of specific classifications. Specificity was defined as percentage of correct classifications.
DNA sequence analysis
Genomic DNA was isolated from peripheral blood leukocytes using the QIAamp DNA blood mini kit (QIAGEN). Amplification of TTR exons 1 to 4, the 3′ end of FGA exon 5, APOA1 exons 3 and 4, APOA2 exon 4, and LYZ exon 2 was performed by PCR. A 25-μL reaction mixture contained approximately 200 ng DNA, 1μM of the exon-specific primers, and 12.5 μL reaction mix plus/minus enhancer (Applied Biosystems). The following thermocycling conditions were used: initial denaturation at 95°C for 10 minutes, 40 cycles of 95°C for 20 seconds, 62°C for 20 seconds, 72°C for 30 seconds, and a final elongation step at 72°C for 5 minutes. A negative control with water instead of DNA was included in each run. The size and quantity of the generated PCR products were analyzed by agarose gel electrophoresis. Fragments were purified with the ExoSAP-IT kit for PCR product clean-up (USB) and sequenced with the ABI PRISM BigDye Terminator Version 3.1 Ready Reaction Cycle Sequencing kit (Applied Biosystems). Sequences were analyzed on an ABI PRISM 3130 Genetic Analyzer.
Results
A total of 135 Congo-red-positive biopsies from 117 patients were examined. More than 1 organ was biopsied in 15 patients (range, 2-4 biopsies). Among these 15 patients, the immunohistochemical classification of the amyloid deposits was identical in all biopsies of 13 patients. In 2 patients, the amyloid deposits were unclassifiable in the first biopsy (gastric and heart biopsy) and classifiable in a second biopsy (liver and gastric biopsy).
For the analyses, we divided the 117 patients into 2 groups. The first group included 51 patients, who were already assigned to the amyloid subtypes based on clinical, laboratory, and genetic findings. It was used to independently test the sensitivity and specificity of immunohistochemistry as a diagnostic method for correct classification of systemic amyloidosis. The second group included 66 patients, whose clinical, laboratory, and genetic data were insufficient for a definite subclassification. In this group, the diagnosis of the amyloidosis subentity relied on the immunohistochemical findings.
In detail, the first group (n = 51; Table 2) included 39 patients with typical clinical signs, such as macroglossia or periorbital bleeding, which, in combination with a monoclonal gammopathy and in the absence of an amyloidogenic germline mutation, pointed to AL amyloidosis. Another 11 patients tested positive for a hereditary amyloidosis, and 1 patient had a history of chronic inflammation (Bechterew disease) suggestive of AA amyloidosis. These latter 12 patients showed no serologic evidence of a monoclonal gammopathy. Immunohistochemistry was successful in determining the subentity in 49 cases, and in each of these 49 cases the immunohistochemical result was in concordance with the final diagnosis. This corresponds to an overall sensitivity of 49 of 51 (96%) and a specificity of 49 of 49 (100%). In a mere 2 patients, immunohistochemistry did not permit further specification, both of them belonging to the hereditary amyloidosis group (ATTR and AFib).
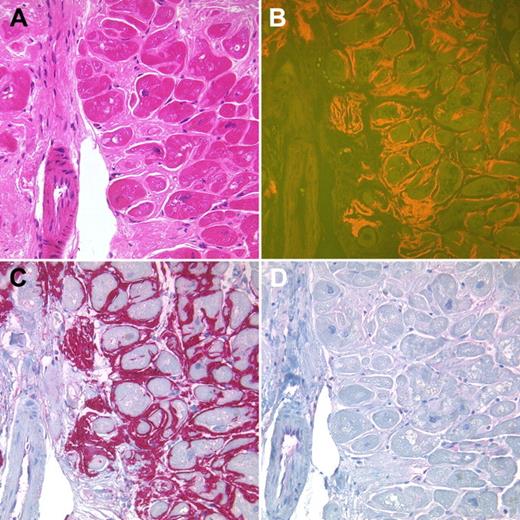
The second group (n = 66; Table 3) consisted of 56 patients with a monoclonal gammopathy suggestive of AL amyloidosis who, however, displayed no typical clinical signs and are referred to as “AL suspected.” Three patients were categorized as “suspected ATTR,” in 2 of them because of concomitant TTR mutation and monoclonal gammopathy (Figure 1). In the third patient with cardiac amyloidosis and a positive family history, the detected TTR mutation had not been previously described as amyloidogenic (p.Ala19Asp-/A19D substitution, Table 4). Seven cases with cardiac amyloidosis were suggestive of senile transthyretin-derived ATTR amyloidosis (median age, 76 years; 5 males, diagnosed in 6 patients by cardiac and in 1 by bladder biopsy) because they had a negative family history, no monoclonal gammopathy, no amyloidosis-related gene mutation, and no evidence of an underlying inflammatory disorder. Immunohistochemistry confirmed the suspected subtype in 52 of 56 of AL, in 3 of 3 of ATTR, and 6 of 7 cases of senile ATTR amyloidosis. Thus, immunohistochemistry was diagnostic in 61 of 66 patients (92%), and there was no immunohistochemical misclassification in the second group.
Endomyocardial biopsy of a 69-year-old female patient with a monoclonal gammopathy type IgG lambda. Interstitial amyloid deposits showed a homogeneous eosinophilic staining in the H&E-stained tissue section (A) and yellow-orange fluorescence in Congo red-stained sections (B). The amyloid deposits strongly immunoreacted with an antibody directed against TTR (C). No immunoreactions were found with antibodies directed against λ-light chain (D). A TTR mutation was detected (V30M), making the diagnosis of a hereditary ATTR amyloidosis. Original magnification ×200.
Endomyocardial biopsy of a 69-year-old female patient with a monoclonal gammopathy type IgG lambda. Interstitial amyloid deposits showed a homogeneous eosinophilic staining in the H&E-stained tissue section (A) and yellow-orange fluorescence in Congo red-stained sections (B). The amyloid deposits strongly immunoreacted with an antibody directed against TTR (C). No immunoreactions were found with antibodies directed against λ-light chain (D). A TTR mutation was detected (V30M), making the diagnosis of a hereditary ATTR amyloidosis. Original magnification ×200.
Finally, we analyzed in detail all 7 cases of our study, which could not be classified by immunohistochemistry. Of note, there was only 1 biopsy per patient taken from kidney (2 patients), heart (2 patients), and liver (1 patient), nerve (1 patient), and gut (1 patient). Two patients had hereditary forms (1 patient with a TTR and 1 patient with an FGA mutation). The final diagnosis was made because of the following criteria: missing monoclonal gammopathy, no periorbital bleeding and macroglossia, and presence of an amyloidogenic mutation. Of the 5 remaining patients, 4 had AL amyloidosis. Final diagnosis was made because of the following criteria: presence of a monoclonal gammopathy, absence of an amyloidogenic mutation, and typical organ involvement pattern. One patient had the final diagnosis of senile ATTR amyloidosis based on the following criteria: missing monoclonal gammopathy, no periorbital bleeding and macroglossia, absence of an amyloidogenic mutation, male gender, an age of 76 years, and a TTR-positive test result in the immunoelectron microscopy (performed in Pavia, Italy).
Discussion
Previous studies have shown the feasibility and diagnostic value of immunohistochemistry as a tool for amyloid subclassifica-tion8,14–17,24,26–28 ; an overview is given in Table 6. However, they largely represent histologic case series collected for the sake of methodologic feasibility and epidemiology. Clinical data to corroborate the respective immunohistochemical diagnosis were mostly fragmentary, so that the validation was generally done by a thorough restaining and reanalysis of the samples.
In this study, we aimed to reevaluate the role of immunohistochemistry for the classification of systemic amyloidosis in synopsis with clinical and laboratory findings. Typical signs for AL amyloidosis, laboratory gammopathy screening, genetic analysis, and the family history were used to test the accuracy of the immunhistochemical results.
In our hands, immunohistochemistry proved to be a very valuable tool for amyloid subtyping, permitting a definite classification in 110 of 117 (94%) specimens tested. In 49 of 51 cases, where the amyloid subentity was already diagnosed, the immunohistochemical findings were in accordance with the clinical and genetic data. We therefore could validate immunohistochemistry as a highly specific and sensitive method. However, a limitation of our study is the rather low number of non-AL patients.
As for the distribution of subentities in our Western European patient population, AL amyloidosis was by far the prevailing entity with 95 of 117 patients (81%), but there was also a substantial number of hereditary (14 of 117 patients, 12%) and of senile transthyretin cases (7 of 117 patients, 6%). Only a single patient was affected by AA amyloidosis. In our opinion, 3 factors account for this low incidence in our series compared with previous studies. First, the current Western European medical standard in treating chronic inflammation appears to translate into a low incidence of AA amyloidosis. Second, our study population contains a cross section of all organ biopsy sites. Because AA is known to predominantly affect the kidneys, this form is logically over-represented in most previous studies, which focused on kidney biopsies.14,16,28 Finally, the diagnosis of AA amyloidosis was within the scope of some primary renal pathologists, so these cases were not included for reference pathology in our study, where renal specimens (numbering a mere 14) were accordingly under-represented. Correspondingly, we have observed a high number of patients with typical symptoms of AL amyloidosis (macroglossia and periorbital bleeding in 41% of AL amyloidosis patients), which probably reflects selection of patients having their biopsies primarily not in the kidney and far advanced disease.
Our study also shows problems inherent to immunohistochemical amyloid subtyping. In 4 cases with a final diagnosis of AL amyloidosis, the diagnosis could not be made by immunohistochemistry. This lack of diagnostic accuracy has been previously described.6,8,17,27,28 In a large study by Lachmann et al, AL fibrils were identified by immunohistochemical staining only in 121 of 316 patients (38%) with confirmed AL disease.9 This weakness of immunohistochemistry with respect to AL amyloid has been attributed to intrinsic difficulties of light chain detection-like conformational differences between native versus tissue-fixed light chains, antigen masking, the heterogeneity of light chains because of their prominent variable domains, and light chain fragmentation during amyloid fibril formation rather than to technical issues, although the quality of some commercially available antibodies may be a contributing factor.29–31 We circumvented the intrinsic problem of light chain variability by the routine application of 4 different antibodies directed against λ light chain.28 This improved the diagnostic accuracy of our immunotyping, which is probably not possible in routine pathology laboratories. In addition, in 2 patients, the investigation of a second amyloid-containing tissue sample finally allowed the classification of a primarily unclassifiable type. This indicates that tissue processing may prevent immunotyping in some cases. The vast majority of our biopsy specimens were obtained from a variety of different departments of pathology, and we cannot exclude the possibility that variability in the tissue processing (ie, the type of formalin, the duration and temperature of fixation) may have compromised our standardized immunostaining procedure. However, the rate of 91 of 95 (96%) cases with unequivocal detection of the light chain involved, which in all cases was congruent with the respective light chain of the monoclonal gammopathy, compares very favorably with previous studies9,17 and is well within the same range of diagnostic sensitivity as observed for other types of amyloidosis (eg, AA, ATTR), which are known for a strong and conclusive antibody staining.
Our study also highlights other pitfalls of amyloid subclassification. Most critically, the diagnosis of hereditary amyloidosis can be easily missed, mainly because of 2 factors. First, despite its autosomal dominant mode of inheritance, 9 of our 14 patients with a hereditary amyloidosis had an unremarkable family history, a known finding that has largely been attributed to a variable penetrance and to the late onset of symptoms in many patients.9,15,31–33 Second, 2 patients with a hereditary form coincidentally also had a monoclonal gammopathy, which would have suggested the wrong diagnosis of AL amyloidosis if not validated by immunohistochemistry (Figure 1). This phenomenon has also been observed in previous studies, which reported a frequency of monoclonal gammopathy ranging from 3% to 10% in patients with hereditary amyloidoses.9,32 Our study therefore supports the widely accepted doctrine that the amyloid subentity should be sought in tissue specimens and that a mere reliance on clinical and laboratory findings carries the risk of misdiagnosis.31 Obviously, a verified diagnosis of senile ATTR amyloidosis is only possible if the amyloid in the biopsy is analyzed.
In conclusion, our study shows that immunohistochemistry by a highly specialized surgical pathologist in combination with clinical and laboratory tests is accurate in reaching definite amyloid subtyping. Clinical patterns as well as laboratory and genetic testing alone cannot substitute for the identification of amyloid precursor proteins within the deposits. The described methods should be used complimentarily to obtain an unequivocal subclassification and represent the standard in our center. Recently, amyloid subtyping based on proteomic techniques and mass spectroscopy has been implemented in a few amyloidosis centers,11–13,34–36 which opens up new perspectives for unusually difficult cases or those with hitherto unknown amyloid proteins.
The project was presented in part at the EURAMY meeting in Porto, Portugal on February 15-16, 2008.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all pathologists who agreed to send the biopsy material for immunohistochemistry to C.R.; Laura Verga (Amyloidosis Center, Pavia, Italy) for providing electron microscopy immunohistochemistry in 1 sample of this study; and Ahmet Dogan (Mayo Clinic, Rochester, MN), for providing high-performance liquid chromatography and tandem mass spectrometry in 1 biospsy.
This work was supported in part by the European Union (grant EU FP6 EURAMY).
Authorship
Contribution: S.O.S., C.R., U.H., and P.L. conceived and designed the study; U.H. and S.O.S. provided study materials and patients; U.H., A.M., M.H., T.B., and S.O.S. collected and assembled the data; T.B., U.H., C.R., P.L., and S.O.S. analyzed and interpreted the data; U.H., T.B., C.R., P.L., and S.O.S. wrote the manuscript; and all authors gave final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stefan O. Schönland, Amyloidosis Center, University Hospital, Im Neuenheimer Feld 410, D-69120 Heidelberg, Germany; e-mail: stefan.schoenland@med.uni-heidelberg.de.
References
Author notes
S.O.S. and U.H. contributed equally to this study.