Abstract
Severe bacterial sepsis often leads to a systemic procoagulant and proinflammatory condition that can manifest as disseminated intravascular coagulation, septic shock, and multiple organ failure. Because activation of the contact proteases factor XII (FXII), prekallikrein, and factor XI (FXI) can trigger coagulation and inflammatory responses, the contact factors have been considered potential targets for the treatment of sepsis. However, the pathogenic role of contact activation in severe infections has not been well defined. We therefore investigated whether an anticoagulant antibody (14E11) that selectively inhibits prothrombotic FXI activation by activated FXII (FXIIa) modifies the course of bowel perforation-induced peritoneal sepsis in mice. Early anticoagulation with 14E11 suppressed systemic thrombin- antithrombin complex formation, IL-6, and TNF-α levels, and reduced platelet consumption in the circulation and deposition in the blood vessels. Treatment with 14E11 within 12 hours after bowel perforation significantly improved survival compared with vehicle treatment, and the saturating dose did not increase tail bleeding. These data suggest that severe polymicrobial abdominal infection induces prothrombotic FXI activation, to the detriment of the host. Systemic anticoagulation by inhibiting FXI activation or FXIIa procoagulant activity during sepsis may therefore limit the development of disseminated intravascular coagulation without increasing bleeding risks.
Introduction
Infection-associated intravascular blood coagulation is common in patients with severe sepsis. The resulting coagulopathy is probably driven by bacterial cell components, including peptidoglycans, teichoic acid, polyphosphates, and lipopolysaccharides (LPSs), which have been shown to activate contact proteases and tissue factor-expressing leukocytes.1-3 The host response to bacteria can also produce a systemic inflammatory response syndrome that can contribute to intravascular coagulation and defective fibrinolysis, resulting in disseminated intravascular coagulation (DIC)–associated consumption of platelets, leukocytes, and coagulation factors that cause both thrombosis and secondary hemorrhage. Activation of the contact protease factor XII (FXII) on negatively charged surfaces, including bacterial components, activates prekallikrein and factor XI (FXI) in terrestrial mammals,4 which results in thrombin generation through the intrinsic coagulation pathway, activation of the complement system, and release of the inflammatory peptide bradykinin from high-molecular-weight kininogen.5,6 Although the contact proteases appear to play a significant prothrombotic role as part of the intrinsic coagulation pathway, the importance of contact system activation in infection-related host-response remains uncertain.
Most persons with inherited contact protease deficiencies, including FXII and its substrate prekallikrein, do not have an obvious abnormal phenotype and have normal hemostasis.7-9 FXI deficiency (hemophilia C) is associated with excessive trauma-induced bleeding in a subset of affected persons,10,11 indicating that FXI can contribute to normal hemostasis. Despite its apparently modest hemostatic role, persons with high plasma FXI levels are at an increased risk for arterial and venous thrombosis,12-14 and FXI-deficient patients are protected against ischemic stroke and deep vein thrombosis.15,16 In various animal models, decreasing or eliminating FXI procoagulant activity through gene knockout, pharmacologic inhibition, or antisense oligonucleotide-mediated knockdown is also antithrombotic without significantly impairing hemostasis,17-21 suggesting that FXI is an important driver of pathologic coagulation with only a supportive function in normal hemostasis. Interestingly, FXII and prekallikrein have also been shown to contribute to the development of experimental thrombosis in mice,22,23 despite the normal to possibly prothrombotic phenotype associated with deficiency of either of these proteins (Hagemen trait and Fletcher trait, respectively) in humans.14,24-26
We previously demonstrated that FXI deficiency was associated with improved survival and reduced coagulopathy relative to wild-type mice during polymicrobial peritoneal sepsis.27 The data suggested that FXI activity may have contributed to the pathogenesis of abdominal sepsis by promoting DIC. To further investigate the mechanism by which contact activation could contribute to sepsis mortality, we generated a monoclonal antibody, 14E11, which has been shown in vitro both in plasma and in purified systems to selectively inhibit the activation of FXI by FXIIa while not inhibiting FXI activation by thrombin.28,29 The effects of 14E11 treatment on sepsis outcome in mice were compared with vehicle and activated protein C (APC) treatment.
Methods
Experimental animals
Age-matched (2- to 4-month-old) male C57BL/6 mice fed a standard diet were used in all experiments. Animals were housed individually in micro-isolation cages under a 12-hour day/night cycle and had free access to food and water. Experiments were approved by the animal care and use committee of the Oregon Health & Science University.
Anticoagulants
Derivation and activity of the murine anti–mouse FXI monoclonal antibody 14E11 have been described in detail elsewhere.28,29 In brief, the antibody was generated by immunizing FXI-deficient mice with recombinant mouse FXI. The inhibitory antibody 14E11, which binds to a highly conserved region of the apple 2 (A2) domain of FXI, has been shown to inhibit the activation of FXI by FXIIa in vitro while not significantly inhibiting FXI feedback activation by thrombin.29 The antibody is anticoagulant in mammalian plasma, and antithrombotic both in mouse and primate disease models.28 Human plasma–derived APC was a gift from the American Red Cross. This vitamin K–dependent protease acts as an anticoagulant by proteolytic inactivation of activated FV and FVIII, and as a cytoprotective enzyme by proteolytic activation of PAR1.30 APC has been shown to be antithrombotic in both mouse and primate disease models,31,32 to reduce the mortality of experimental Escherichia coli sepsis in baboons,33 and to reduce the mortality of severe sepsis in human patients,34 although recent clinical data have called into question the efficacy of APC in sepsis.35
To confirm anticoagulant activity of both APC and 14E11, activated partial thromboplastic time (aPTT) assays were performed on pooled citrated (one-tenth volume 3.2% sodium citrate) normal mouse plasma. Plasma (50 μL) was anticoagulated with increasing concentrations of 14E11 or APC, and then incubated at 37°C for 3 minutes with 50 μL of aPTT reagent (aPTT-ES; Helena Laboratories) followed by the addition of 50 μL of CaCl2 (25mM) and time to clot formation determined on an Amelung KC4 Analyzer (Sigma-Aldrich). The in vivo anticoagulant effect of APC (6 mg/kg) and 14E11 (4 mg/kg) was measured and, in the case of 14E11, verified for up to 72 hours after treatment. The dose of 14E11 that was chosen achieved lasting and saturating systemic anticoagulation that was comparable with the endogenous anticoagulation of FXI-deficient mice. The dose of APC was chosen to achieve, at least temporarily, some level of systemic anticoagulation. Single subcutaneous injections were administered to mice that were then killed (3-12 mice per time point) and blood drawn via cardiac puncture into one-tenth volume 3.2% sodium citrate. Blood was immediately centrifuged for platelet-poor plasma and aPTT assays were performed immediately to limit the extent of APC inactivation ex vivo.
Mouse model of polymicrobial peritonitis
To determine whether treatment with 14E11 could improve sepsis survival, we tested the antibody using the cecal ligation and puncture (CLP) model that induces primary peritoneal infection.36 Mice were anesthetized in an inhalant anesthetic chamber using a veterinary anesthetic vaporizer (LEI Medical) with 5% isofluorane. Anesthesia was maintained by flow mask with the isofluorane concentration reduced to 2% for the duration of the procedure, which lasted approximately 10 minutes. Abdominal skin was prepared by shaving a 2-cm area with electric clippers and swabbing the skin with 70% isopropanol followed by 10% povidone-iodine. A 1-cm vertical midline incision was made and the cecum exposed and isolated using dressing forceps. The cecum was ligated approximately 1 cm from its distal end using a 6.0-braided silk suture (Teleflex Medical). The ligated section was punctured through and through twice using a 22-gauge hypodermic needle. The cecum was replaced in the abdominal cavity, and the abdominal wall closed in layers using a 6-0 Vicryl suture (Ethicon). Surgical instruments were cleaned and sterilized between each animal using 70% isopropanol and a Germinator 500 (Cell Point Scientific). Sham mice were subjected to the initial surgical incisions without subsequent cecal ligation or puncture. We have shown previously that the CLP procedure creates a mixed bacterial infection within the peritoneal cavity that is abundant in both Gram-negative and Gram-positive bacteria.27 For survival studies, mice were given single subcutaneous injections of vehicle (PBS, pH 7.2), 14E11 (4 mg/kg), human plasma–derived APC (American Red Cross; 6 mg/kg), or a non–cross-reacting control IgG (4 mg/kg) at designated time points after CLP surgery. Survival was recorded over the next 7 days, after which all surviving mice were killed. In a separate experiment, mice were given subcutaneous injections immediately after CLP and then killed at 12, 24, or 36 hours after the surgery, with samples processed as described in the next section.
Sample collection and analysis
Blood samples were drawn by cardiac puncture into a one-tenth volume of 3.2% sodium citrate at 12, 24, and 36 hours after surgery. Red blood cell, leukocyte, and platelet counts were measured using a Hemavet 950 FS (Drew Scientific) with discriminators set for mice. Blood was immediately centrifuged for platelet poor plasma and frozen at −80°C. Thrombin/antithrombin complexes (TAT), IL-6, and TNF-α levels in the collected plasma were measured using commercial ELISA test kits (TAT-micro, Enzygnost, Siemens; TNF and IL-6, BD OptEIA). Leukocyte infiltration into the peritoneum was measured by lavage with 3 mL of sterile saline, and cells were counted using the automated cell counter. To assess bacteria numbers in the peritoneum at the 24-hour time point, the lavage samples were diluted 1:1000 in sterile saline and 500 μL was plated on trypticase soy agar plates with 5% sheep blood (HealthLink) and then incubated at 37°C for 24 hours. The colony-forming units were hand counted.
Histopathology
Thirty-six hours after CLP or sham surgery, mice were killed and immediately perfused with 50 mL saline. Livers were excised and fixed in 10% zinc-buffered formalin (Protocol; Fisher Healthcare) for 24 hours and then transferred to 70% ethanol before being embedded in paraffin and sectioned. Sections were stained with H&E to assess the prevalence of microthrombi in the liver. An investigator who was blinded to the treatment of the animals performed the histologic analysis. Randomly selected sections were photographed and similarly sized vessels evaluated to determine the prevalence of microthrombi. Bile ducts and transverse vessel sections were not included in the analysis. Within the vessel lumen, microthrombi were defined as dense formations of aggregated platelets greater than 20 μm. For each mouse, the number of vessels containing microthrombi were enumerated and then divided by the total number of vessels evaluated. These numbers were averaged to yield the percentage of vessels with microthrombi for each mouse. Mice having microthrombi present in greater than 25% of the vessels were chosen to be positive for pathologic coagulation. For each mouse, 13 to 25 vessels were analyzed with 3 to 5 mice evaluated per group.
Hemostasis assessment
The effects of 14E11 or APC treatment on bleeding times were assessed using the tail-transection method.37 Briefly, naive mice were anesthetized in an inhalant anesthetic chamber using a veterinary anesthetic vaporizer (LEI Medical) with 5% isofluorane. They were then given a single subcutaneous injection of vehicle (PBS, pH 7.2), 14E11 (4 mg/kg), or human plasma-derived APC (American Red Cross; 6 mg/kg) and transferred to a restraining device that allowed free access to the tail. Early absorption and systemic distribution of the antibody resulted maximum plasma anticoagulation within 30 minutes. The tail was transected 30 minutes after injection at a diameter of 1.5 mm and quickly placed into a 1-mL microcentrifuge tube containing 500 μL of room temperature water. Bleeding time and total blood volume lost were recorded for up to 30 minutes.
Data analysis
Data are mean ± SEM. Survival data were analyzed using Kaplan-Meier analysis, and comparisons between treatment groups were calculated using the log-rank test using Med-Calc. Bleeding time data were also analyzed using the nonparametric log-rank test for right censored data. The 2-tailed Student t test was used for single pair comparisons. P less than or equal to .05 was considered significant.
Results
Anticoagulant effects of 14E11 and APC
Both 14E11 and APC prolonged the aPTT of mouse plasma (Figure 1A-B), with 14E11 approaching maximum inhibition at 2 μg/mL, which is similar to the plasma FXI concentration in humans (3-7 μg/mL) and likely in mice.38 The prolongation of the clotting time was similar to that of FXI-deficient mice. Human plasma derived APC also dose-dependently prolonged the aPTT of mouse plasma, doubling the clotting time at 2 to 4 μg/mL. Mice given single subcutaneous injections of both APC (6 mg/kg) and 14E11 (4 mg/kg) showed rapidly developing anticoagulant responses after injections. At peak, the aPTT was elevated 124% by 14E11 (Figure 1C) and 43% by APC (Figure 1D) administration compared with vehicle-treated animals (66.0 ± 4.6 seconds, 42.2 ± 3.4 seconds, and 29.5 ± 1.7 seconds, respectively, n = 12 for each; P < .01 for both 14E11 and APC compared with vehicle) 30 minutes after injections. The anticoagulant effects of 14E11 persisted for at least 72 hours, whereas the aPTT of APC treated mice returned to the control average within 2 hours.
Activities of 14E11. Effect of 14E11 (A) or APC (B) on the aPTT of mouse plasma. Pooled mouse plasma was supplemented with APC or 14E11 and tested in the aPTT assay as described in “Anticoagulants.” 14E11 was tested in 3 separate clotting experiments using different plasma pools from 3 or 4 mice each, and data shown are averages of clotting times ± SEM. Effect of 14E11 (C) or APC (D) in vivo. A single subcutaneous injection of 14E11 (4 mg/kg) or APC (6 mg/kg) was given to mice (n = 12 each for the 0-hour and 0.5-hour groups, n = 3 each for 2- to 72-hour groups), and blood was drawn via cardiac puncture into one-tenth volume/volume 3.2% citrate at various time points. Blood was centrifuged for platelet-poor plasma, and the aPTT was measured immediately using an Amelung KC4 coagulometer. Data are mean ± SEM.
Activities of 14E11. Effect of 14E11 (A) or APC (B) on the aPTT of mouse plasma. Pooled mouse plasma was supplemented with APC or 14E11 and tested in the aPTT assay as described in “Anticoagulants.” 14E11 was tested in 3 separate clotting experiments using different plasma pools from 3 or 4 mice each, and data shown are averages of clotting times ± SEM. Effect of 14E11 (C) or APC (D) in vivo. A single subcutaneous injection of 14E11 (4 mg/kg) or APC (6 mg/kg) was given to mice (n = 12 each for the 0-hour and 0.5-hour groups, n = 3 each for 2- to 72-hour groups), and blood was drawn via cardiac puncture into one-tenth volume/volume 3.2% citrate at various time points. Blood was centrifuged for platelet-poor plasma, and the aPTT was measured immediately using an Amelung KC4 coagulometer. Data are mean ± SEM.
14E11 treatment improves CLP-induced sepsis survival
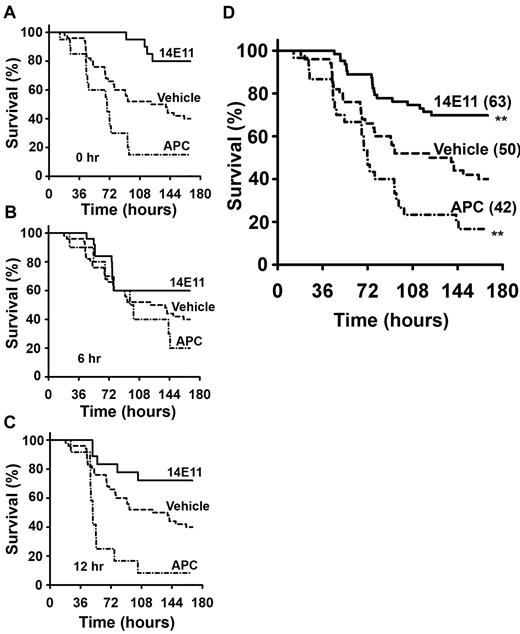
The surgical procedure of bowel perforation (CLP) lasted approximately 10 minutes, no excessive surgical bleeding was noted, and all animals recovered well from anesthesia. Signs of generalized illness (lethargy, apparent dyspnea, and piloerection) appeared in most animals within 6 to 12 hours after CLP. During the one-week observation period, mice that were treated immediately after CLP (0 hours) with 14E11 had an 80% survival (16 of 20), compared with a 45% survival (9 of 20) for vehicle-treated mice, 46% (6 of 13) for control IgG, and 15% (3 of 20) for APC treated mice (Figure 2A; P = .001 14E11 vs vehicle and P = .02 14E11 vs IgG control). In mice given 14E11 6 hours after CLP (Figure 2B), 60% survived (15 of 25), whereas only 37% (11 of 30) of vehicle-treated and 20% (2 of 10) of APC treated survived. Mice administered 14E11 at 12 hours after CLP (Figure 2C) had a survival of 72% (13 of 18) compared with 8% (1 of 12) for those administered APC. Combined data showed a 30% improvement in overall survival for the 14E11-treated group over vehicle (Figure 2D; P = .001) with an odds ratio of death of 3.5 (95% confidence interval, 1.6-7.6) for vehicle compared with 14E11-treated mice, and an overall survival of 70% for 14E11 treatment (n = 63), 14% for APC treatment (n = 42), and a 40% survival for vehicle-treated animals (n = 50).
Survival advantage of mice treated with 14E11 in peritoneal sepsis. Mice treated with the anti-FXI antibody 14E11 have a survival advantage after large bowel perforation. Kaplan-Meier survival curves showing survival of 2- to 4-month-old age-matched male C57Bl/6 mice treated with 14E11 (4 mg/kg), APC (6 mg/kg), or vehicle (PBS) at (A) 0 hours, (B) 6 hours, and (C) 12 hours after cecal ligation and puncture (n = 10-30 animals per time point). Vehicle data are combined (n = 50), as there was no significant difference in survival when administering vehicle at different time points (P = .93). Combined data from all experiments are shown in panel D, with total number of mice shown in parentheses. Combined survival curves were compared by log-rank test and showed a significant survival advantage for 14E11-treated mice (**P < .01) and a significant increase in death after APC treatment.
Survival advantage of mice treated with 14E11 in peritoneal sepsis. Mice treated with the anti-FXI antibody 14E11 have a survival advantage after large bowel perforation. Kaplan-Meier survival curves showing survival of 2- to 4-month-old age-matched male C57Bl/6 mice treated with 14E11 (4 mg/kg), APC (6 mg/kg), or vehicle (PBS) at (A) 0 hours, (B) 6 hours, and (C) 12 hours after cecal ligation and puncture (n = 10-30 animals per time point). Vehicle data are combined (n = 50), as there was no significant difference in survival when administering vehicle at different time points (P = .93). Combined data from all experiments are shown in panel D, with total number of mice shown in parentheses. Combined survival curves were compared by log-rank test and showed a significant survival advantage for 14E11-treated mice (**P < .01) and a significant increase in death after APC treatment.
Attenuation of coagulopathy and inflammation by 14E11
Previously, we described an increased survival and more limited coagulopathy in septic FXI knockout mice compared with wild-type.27 We therefore hypothesized that inhibiting FXI activity after sepsis onset could also provide protection from lethal sepsis by limiting consumptive coagulopathy and possibly by reducing the systemic inflammatory response. Mice that were anticoagulated with 14E11 immediately after CLP (0 hours) had a 31% higher average platelet count 36 hours after surgery compared with vehicle-treated controls (935 ± 85 × 103 platelets/μL vs 646 ± 76 × 103 platelets/μL, respectively, n = 8 each, P < .05; Figure 3A), suggesting reduced platelet consumption. Circulating leukocytes decreased similarly in all CLP groups (Figure 3B), with an increase in leukocytes in the peritoneum within 12 hours after CLP surgery, consistent with leukocyte migration to the site of infection (Figure 3C). Bacterial counts within the peritoneal cavity were not significantly different among the CLP groups 24 hours after surgery (785 ± 362/μL of peritoneal lavage fluid [plf], 953 ± 347/μL, and 575 ± 380/μL, for vehicle, 14E11, and APC treatments, respectively, n = 8 each), with no bacteria found in normal mice. On histologic examination of liver sections from animals that were killed 36 hours after surgery, 75% (3 of 4) of vehicle-treated mice had microthrombi within blood vessels compared with 40% of mice for both 14E11 and APC treatment (2 of 5 mice each; Figure 3D). We found no occluded vessels in normal mouse livers (0 of 4 mice), and occlusions in 1 of 3 sham-operated mice. TAT levels initially increased similarly in all groups 12 hours after CLP but remained significantly elevated in vehicle-treated animals 24 hours after CLP compared with sham-operated mice (229% ± 54% vs 92% ± 6% of normal mouse TAT levels, respectively, n = 8 each; P < .05), whereas TAT levels returned to normal by 24 hours in 14E11- and APC-treated animals (100% ± 7% and118% ± 7%, respectively, n = 7 and 8; Figure 3E). The TAT data combined with the platelet counts and liver histology suggested that the CLP resulted in a greater procoagulant response in vehicle-treated mice than in anticoagulated animals.
Effects of 14E11 and APC treatment on coagulation and inflammation. Mice treated with 14E11 have less platelet consumption and decreased inflammation after CLP sepsis. (A) Platelet counts were measured in cohorts killed at designated time points after CLP (n = 7 or 8 for each group per time point). Vehicle-treated mice show a progressive drop in platelet count after bowel perforation (36 hours after infection). *P < .05 (vehicle vs sham). (B) All CLP groups show a similar decrease in circulating leukocytes (WBCs) and (C) similar migration of leukocytes into the peritoneal cavity. (D) Microscopic images of representative liver sections from normal, sham-operated, and vehicle-, 14E11-, and APC-treated septic mice (hematoxylin and eosin stain). For histologic evaluation, all animals received vehicle, APC, and 14E11 immediately after CLP (0 hours). Mice were killed 36 hours after CLP, saline perfused, and the organs were fixed in 10% zinc-buffered formalin. Vehicle-treated mice show multifocal thrombosis in the liver, whereas 14E11- and APC-treated mice had a lower density of occluded vessels per field. Histologic sections were visualized with a Nikon Optiphot-2 upright microscope using a 10× objective. Bright-field images were captured with a Hitachi HV-C20 CCD camera and processed using SPOT Basic Version 3.5.0 software. Scale bar represents 150 μm. (E) TAT levels in the plasma are elevated for all groups at 12 hours after surgery, and TAT remains elevated in the vehicle-treated group at 24 hours, suggesting sustained thrombin generation. *P < .05 (vehicle vs sham). (F) TNF-α and (G) IL-6 levels are increased in vehicle-treated mice compared with 14E11-treated animals 12 hours after CLP (n = 7 or 8 for each group per time point), suggesting an anti-inflammatory mechanism for 14E11 during sepsis. *P < .05. Data are mean ± SEM.
Effects of 14E11 and APC treatment on coagulation and inflammation. Mice treated with 14E11 have less platelet consumption and decreased inflammation after CLP sepsis. (A) Platelet counts were measured in cohorts killed at designated time points after CLP (n = 7 or 8 for each group per time point). Vehicle-treated mice show a progressive drop in platelet count after bowel perforation (36 hours after infection). *P < .05 (vehicle vs sham). (B) All CLP groups show a similar decrease in circulating leukocytes (WBCs) and (C) similar migration of leukocytes into the peritoneal cavity. (D) Microscopic images of representative liver sections from normal, sham-operated, and vehicle-, 14E11-, and APC-treated septic mice (hematoxylin and eosin stain). For histologic evaluation, all animals received vehicle, APC, and 14E11 immediately after CLP (0 hours). Mice were killed 36 hours after CLP, saline perfused, and the organs were fixed in 10% zinc-buffered formalin. Vehicle-treated mice show multifocal thrombosis in the liver, whereas 14E11- and APC-treated mice had a lower density of occluded vessels per field. Histologic sections were visualized with a Nikon Optiphot-2 upright microscope using a 10× objective. Bright-field images were captured with a Hitachi HV-C20 CCD camera and processed using SPOT Basic Version 3.5.0 software. Scale bar represents 150 μm. (E) TAT levels in the plasma are elevated for all groups at 12 hours after surgery, and TAT remains elevated in the vehicle-treated group at 24 hours, suggesting sustained thrombin generation. *P < .05 (vehicle vs sham). (F) TNF-α and (G) IL-6 levels are increased in vehicle-treated mice compared with 14E11-treated animals 12 hours after CLP (n = 7 or 8 for each group per time point), suggesting an anti-inflammatory mechanism for 14E11 during sepsis. *P < .05. Data are mean ± SEM.
To assess the systemic inflammatory response to CLP surgery, we measured circulating levels of the inflammatory cytokines TNF-α and IL-6. TNF-α levels increased significantly in all CLP groups 12 hours after surgery; however, 14E11 treatment attenuated the increase by more than 50% compared with vehicle-treated mice (57.3 ± 11.5 pg/mL vs 120.2 ± 19.8 pg/mL, P < .05 n = 7 and 8, respectively; Figure 3F). IL-6 was significantly increased in all CLP groups 12 hours after surgery, again with the 14E11-treated group having significantly lower levels compared with vehicle (21.5 ± 6.5 ng/mL vs 41.6 ± 1.2 ng/mL, respectively, n = 8 each, P < .05; Figure 3G). APC treatment did not significantly affect levels of the systemic inflammatory markers. Both TNF-α and IL-6 decreased by 24 hours after CLP, with no significant differences between the treatment groups.
Absence of detectable hemostasis impairment after 14E11 treatment
The tail-clip bleeding time is typically prolonged by both antiplatelet agents and anticoagulants in mice,37,39 yet 14E11 had no effect on bleeding time or volume compared with vehicle-treated controls (12.1 ± 1.8 minutes vs 12.8 ± 1.0 minutes, and 100 ± 22 μL vs 134 ± 40 μL respectively, n = 12; Figure 4) 30 minutes after treatment. Anticoagulation with APC increased the bleeding time and volume to 17.9 ± 1.8 minutes and 245 ± 61 μL (n = 12, P = .01 for APC bleeding time vs vehicle, and P < .05 for APC bleeding volume vs 14E11). No rebleeding from the tail-clip wounds or other adverse bleeding events were noted in any of the animals. Taken together, these results show that early anticoagulation with 14E11 improved the survival of large bowel perforation without significant impairment of hemostasis, compared with early anticoagulation with APC, which both decreased survival and increased bleeding in the mice.
Bleeding time and volume after 14E11 administration. Mice treated with the anti-FXI antibody 14E11 (4 mg/kg subcutaneously) 30 minutes before tail cut show normal tail bleeding time (A) and volume (B), whereas APC (6 mg/kg subcutaneously) treatment significantly increases bleeding time compared with PBS vehicle-treated controls; n = 12 each. **P = .01 (log-rank test for right censored data). During experiments, mice were sedated with 5% isoflurane and immobilized for tail transection at 1.5-mm tail diameter. After transection, the tail was placed into a 1-mL microcentrifuge tube of room temperature water and time until bleeding cessation was recorded. The experiment was ended, and data were censored if bleeding continued beyond 30 minutes.
Bleeding time and volume after 14E11 administration. Mice treated with the anti-FXI antibody 14E11 (4 mg/kg subcutaneously) 30 minutes before tail cut show normal tail bleeding time (A) and volume (B), whereas APC (6 mg/kg subcutaneously) treatment significantly increases bleeding time compared with PBS vehicle-treated controls; n = 12 each. **P = .01 (log-rank test for right censored data). During experiments, mice were sedated with 5% isoflurane and immobilized for tail transection at 1.5-mm tail diameter. After transection, the tail was placed into a 1-mL microcentrifuge tube of room temperature water and time until bleeding cessation was recorded. The experiment was ended, and data were censored if bleeding continued beyond 30 minutes.
Discussion
Infection-associated activation and consumption of contact factors, including FXI and FXII, have been well documented in both animal models and patients.40-42 Baboon studies of lethal experimental E coli bacteremia showed that partial inhibition of FXII reduced hypotension and extended life.43 Furthermore, work with FXI-deficient mice has shown that mixed bacterial sepsis and the associated coagulopathy that develops after CLP are less severe in FXI-deficient animals than in their wild-type counterparts.27 Because activation of FXI by either thrombin or FXIIa can activate the coagulation cascade via FIX,44 causing intravascular coagulation and perhaps contributing either directly or indirectly to acute inflammation, we explored whether activation of FXI through the contact pathway contributes to the pathogenesis of severe polymicrobial abdominal sepsis in mice.
Our present data using a mouse model of CLP-induced mixed bacterial sepsis show that anticoagulation with 14E11 significantly improves survival when administered up to 12 hours after initiating infection. The survival benefit appears to be associated with a decrease in platelet consumption, thrombin generation, and thus a reduced systemic coagulopathy that is often characteristic of DIC. The coagulation-related pathogenesis of abdominal sepsis was directly supported by histologic evidence showing more multifocal thrombi within the liver and a higher systemic TAT level in vehicle-treated animals compared with 14E11-treated mice. These findings were consistent with our previous study in FXI-deficient mice27 and are also supported by other studies that show pathologic activation of FXI can occur during infections of various origins.41,42
The observed increase in mortality in the APC-treated mice was surprising. Anticoagulation with APC was chosen for these studies as a positive control for 14E11 since the FDA approved the use APC for severe sepsis based on results from the PROWESS trial that showed a survival benefit of low-dose APC.34 However, the subsequent ADDRESS trial failed to demonstrate the efficacy of APC (APC mortality of 18.5% vs 17% with placebo, P = .34).45 The recent PROWESS-SHOCK trial also showed that the 28-day all-cause mortality of low-dose APC-treated patients was slightly higher (26.4%) compared with placebo treatment (24.2%), the results of which have led to the withdrawal of APC from the market.35 These studies suggest that APC administration may not produce a significant benefit in septic patients, at least when combined with more recent advances in supportive care; therefore, APC was not a useful positive control for our studies.
The significant increase in mortality of the APC-treated animals in the current study cannot at this point be completely explained because this study was not designed to quantify extraperitoneal bleeding or bacterial burden to characterize the observed effects of high-dose human APC in septic mice. The increased mortality may have resulted from the anticoagulant effects of high dose APC, which increased bleeding times and perhaps led to a critical lowering of early protective fibrin, although no overt internal bleeding was evident during necropsy. Indeed, anticoagulants that target the common pathway of blood coagulation have been shown to exacerbate bacterial sepsis in mice.46 However, FXI deficiency does not appear to critically lower protective fibrin formation,47 suggesting that specific coagulation-related molecular mechanisms affect microbial dissemination and pathogenicity. These data support the concept that, although coagulation can be vitally important to the innate immune response against invading microbes, some infectious pathogens may have evolved to use the contact system of the mammalian host to promote virulence.
In addition to its role in hemostasis and thrombosis, FXI activity may be involved in pathways that affect platelet and leukocyte functions and trafficking,48 and possibly the generation of the potent vasoregulatory peptide, bradykinin.49 Although bradykinin generation was not evaluated in our current study, 14E11-mediated inhibition of FXI activation during sepsis was associated with a reduction in the inflammatory plasma markers TNF-α and IL-6. At present, there is no known direct mechanistic link between FXI activation and these cytokines, although there is evidence that FXIa has direct cellular signaling functions through the receptor apoER2 and possibly others.48,50 We speculate that the limited inflammatory response associated with 14E11 treatment may be linked with the suppression of pathologic intravascular thrombin generation, as evidenced by the attenuated TAT levels, platelet consumption, and microvascular thrombosis. Disseminated thrombosis causes a mechanical disruption of blood flow and organ perfusion deficits, leading to moderate to severe tissue ischemia that produces an acute inflammatory response. Thrombin is also capable of cleaving PARs on numerous cells, and is directly inflammatory. Regardless of the exact mechanism associated with the observed decrease in inflammation, early treatment with 14E11 attenuated the elevation of plasma TNF-α and IL-6 levels 12 hours after initiating infection, which may have also played a role in improving sepsis survival.
In our previous studies, FXI deficiency did not confer a survival benefit after lethal endotoxin injection.27 Endotoxin can produce profound and generalized inflammation, including the promotion of tissue factor exposure on cellular surfaces,3 which could readily bypass the contact activation pathway. However, FXI deficiency or inhibition reduces the lethality of tissue factor infusions,28 indicating that tissue factor expression may not be the critical mechanism of death. Endotoxin injection is an experimental challenge that, in our experience, seems to be substantially different from that of the mixed infection caused by peritoneal contamination after cecal perforation in the mouse. The presence of a bacterial “surface” to induce contact activation after CLP may have led to the different outcomes between the 2 distinct challenges.1 Future studies could identify specific microbes that use contact activation to invade, infect, and/or proliferate within the mammalian host, as these infections could be more likely to respond to FXI or FXII inhibitor treatment.
In conclusion, we have shown that mice anticoagulated with a unique inhibitor of FXI activation had reduced inflammatory and coagulopathic responses and reduced mortality in primary abdominal sepsis. The apparent disconnect between thrombosis and hemostasis at the FXII/FXI axis indeed opens up the possibility for a more specific targeting of pathologic coagulation without a critical impairment of vital hemostasis, which is particularly important in septic coagulopathy. Selectively targeting the contact factors rather than downstream hemostatic enzymes may also allow sufficient procoagulant capacity to serve both hemostasis and the thrombin-dependent innate immune response while limiting the potential for what appears to be contact-initiated enhanced microbial virulence. Whereas our former studies in FXI-deficient mice indicated a role for FXI in abdominal sepsis, the current data suggest that FXI activation by the contact factor FXIIa is a molecular event that is responsible, at least in part, for the detrimental effects of abdominal sepsis after bowel perforation. Our data thus support the early hypothesis of Pixley et al that selectively inhibiting the procoagulant activity of FXIIa may be an attractive target for safe pharmacologic anticoagulation in sepsis.43
Presented in part at the 52nd annual meeting of the American Society of Hematology, Orlando, FL, December 6, 2010, and the XXIII Congress of the International Society on Thrombosis & Haemostasis, Kyoto, Japan, July 25, 2011.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Daniel Cawley (Oregon Health & Science University) and Melani Helm (Aronora LLC) for their technical assistance.
This work was supported in part by the National Institutes of Health (grant AI088937, E.I.T. and A.G.; grant HL106919, E.I.T.; grant HL101972, O.J.T.M.; and grant HL081326, D.G.), and Bayer Healthcare (unrestricted grant, E.I.T. and A.G.).
National Institutes of Health
Authorship
Contribution: E.I.T. designed and managed the study, wrote the final paper, and prepared the figures; A.G. designed and managed the study and wrote the final paper; N.G.V., S.H., E.I.T., and P.Y.L. performed experiments and collected and analyzed the data; and D.G. and O.J.T.M. discussed the results, assisted in design of the studies, and contributed to the drafts.
Conflict-of-interest disclosure: A.G., E.I.T., N.G.V., P.Y.L., and Oregon Health & Science University have a significant financial interest in Aronora LLC, a company that may have a commercial interest in the result of this research. This potential conflict of interest has been reviewed and managed by the Oregon Health & Science University Conflict of Interest in Research Committee. The remaining authors declare no competing financial interests.
Correspondence: Erik I. Tucker, Department of Biomedical Engineering, Oregon Health & Science University School of Medicine, 3303 SW Bond Ave, CH13B, Portland, OR 97239; e-mail: tuckeeri@ohsu.edu.