Abstract
Thrombin generates fibrin and activates platelets and endothelium, causing thrombosis and inflammation. Endothelial thrombomodulin (TM) changes thrombin's substrate specificity toward cleavage of plasma protein C into activated protein C (APC), which opposes its thrombotic and inflammatory activities. Endogenous TM activity is suppressed in pathologic conditions, and antithrombotic interventions involving soluble TM are limited by rapid blood clearance. To overcome this problem, we fused TM with a single chain fragment (scFv) of an antibody targeted to red blood cells. scFv/TM catalyzes thrombin-mediated generation of activated protein C and binds to circulating RBCs without apparent damage, thereby prolonging its circulation time and bioavailability orders of magnitude compared with soluble TM. In animal models, a single dose of scFv/TM, but not soluble TM, prevents platelet activation and vascular occlusion by clots. Thus, scFv/TM serves as a prodrug and provides thromboprophylaxis at low doses (0.15 mg/kg) via multifaceted mechanisms inhibiting platelets and coagulation.
Introduction
Thrombin is a protease that converts fibrinogen into fibrin and activates platelets and endothelium by cleaving protease-activated receptors (PARs), which promotes vascular adhesion of blood cells, thrombus formation, and inflammation.1-6 These effects of thrombin are quenched by thrombomodulin (TM), a transmembrane endothelial thrombin-binding glycoprotein. TM alters thrombin's substrate specificity toward cleavage of plasma protein C into activated protein C (APC), which inactivates coagulation factors Va and VIIIa and inhibits PAR-mediated prothrombotic and proinflammatory effects of thrombin.7-9 In pathologies, including ischemia, oxidative stress, thrombosis, inflammation, sepsis, and diverse vascular diseases, endogenous TM is suppressed through inactivation by oxidants, decreased synthesis, internalization, and shedding.10-13 As a result, prothrombotic and proinflammatory mechanisms may overwhelm the effectiveness of the endogenous TM/APC system.11-17
Infusion of recombinant APC helps counteract these pathologic mechanisms, but the systemic effects of this active protease predispose to bleeding and limit its clinical utility.18-23 In contrast, TM acts only in the presence of pathologic mediators eg, thrombin. In theory, replenishment of TM might provide a safe and effective alternative to inhibit thrombosis and inflammation selectively at sites of thrombin generation. In support of this concept, gene transfection and infusion of recombinant TM alleviate thrombosis and inflammation in animal models.24 However, successful translation of this approach to thromboprophylaxis into the clinical domain is hindered by the inapplicability of gene therapy to acute settings and the unfavorable pharmacokinetics of soluble recombinant TM (sTM), which is rapidly eliminated from the circulation.25
To overcome these problems, we targeted recombinant TM to red blood cells (RBCs) as carriers, using a strategy that optimizes the bioavailability of therapeutics that act within the vascular lumen.26,27 Coupling of recombinant therapeutic proteins to RBCs markedly prolongs their circulation, enhances their potency, and reduces systemic side effects.28,29 For example, a recombinant pro-urokinase variant fused to a single chain antibody fragment30 derived from the monoclonal antibody (mAb),31 which recognizes a determinant on mouse glycophorin A (GPA), anchored safely on circulating RBCs membranes and provided prolonged thromboprophylaxis.32 In our current work, we studied the function and efficacy of a novel biotherapeutic protein consisting of an antibody fragment derived from the mAb Ter-119 (scFv Ter-119) fused to the extracellular portion of mouse TM (scFv/TM).
The advantage of the newly synthesized fusion protein compared with those reported previously32-35 is based on differences in mechanism. Plasminogen activation promotes lysis of existing clots, which may be associated with a delay in reperfusion. In contrast, scFv/TM prevents platelet and endothelial cell activation and clot formation. In theory, the fact that TM does not affect hemostatic clots directly may also enhance efficacy and safety during thromboprophylaxis. Moreover, our data show that RBC-targeted scFv/TM has a markedly longer life span in the circulation than sTM, providing protracted thromboprophylaxis unattainable with similar doses of sTM, further enhancing its safety profile.
Methods
Materials
Chemicals were from Sigma-Aldrich if not otherwise specified. Proteins were labeled with Na[125I] (PerkinElmer Life and Analytical Sciences). RBCs from fresh anticoagulated mouse blood were labeled with [51Cr]Cl2.28 All animal experiments were performed with approval from the University of Pennsylvania School of Medicine Institutional Animal Care and Use Committee.
Expression of scFv/TM
We used a serine-rich linker peptide to fuse TM to scFv cloned from Ter-119, a rat mAb to mouse GPA.31-35 Stable Drosophila S2 cell lines expressing scFv/TM and sTM were established.26 Proteins were purified from media using an M2 (anti-flag) affinity column.33 Protein migrated on SDS-PAGE as a single approximately 85-kDa band.
APC generation by sTM and scFv/TM in vitro
Free scFv/TM or sTM (1-16nM) were incubated with 5nM bovine thrombin and 300nM human protein C for 20 minutes at room temperature. Thrombin was quenched with hirudin (50 U/mL), and APC was measured using Spectrozyme (OD405 nm).26
Binding of scFv/TM to mouse RBCs
Binding of 125I-scFv/TM to mouse versus human RBCs was measured.29,32 To assess dissociation kinetics, mouse RBCs loaded with 125I-scFv/TM and washed to remove unbound material were resuspended in PBS/BSA for various times, and radioactivity in the supernatant and pellet was measured. To test RBC agglutination, suspensions of mouse RBCs in a 96-well V-shaped plate wells were incubated for 1 hour with increasing concentrations of scFv/TM or parental scFv antibody (eBioscience).36
Thrombin-mediated protein C activation by RBCs loaded with scFv/TM
Mouse RBCs coated with approximately 2.5 × 104 molecules of scFv/TM per RBC were resuspended at 2% hematocrit (final concentration ∼ 20nM of RBC-bound scFv/TM) and incubated with 20nM bovine thrombin and 300nM protein C for 1 hour at room temperature. RBCs were removed by centrifugation, thrombin was quenched with hirudin, and APC amidolytic activity in the supernatants was measured.26
Tissue distribution and blood clearance of RBC-scFv/TM in mice
51Cr-labeled mouse RBCs (51Cr-RBC) or 51Cr-RBC loaded with 125I-anti–GPA scFv/TM (∼ 4 × 104 copies per RBC) were injected into anesthetized mice via the jugular vein (intravenously). At designated times, blood was withdrawn in heparin, centrifuged at 1200g, and radioactivity in plasma, RBC pellets, and organs was measured.29 In other cohorts, distribution of 125I-scFv/TM and 125I-sTM was measured in the same way.
APC generation in vivo
scFv/TM in a mouse model of jugular vein thrombosis
We used a mouse model of acute thrombotic occlusion.32 Equimolar amounts of scFv/TM (0.15 mg/kg), sTM (0.1 mg/kg), or PBS was injected via the jugular vein. One hour later, thrombosis was induced in exposed contralateral jugular veins with 15% FeCl3 applied to the adventitia for 2 minutes. Time to vascular occlusion and total blood flow over the ensuing 30 minutes were measured using a 0.5-VB flow Doppler probe (Transonic Systems).
scFv/TM and platelet activation by thrombin
Platelet activation was detected using fluorescently labeled P-selectin antibody. Isolated mouse platelets11,39,40 were suspended (1 × 108/mL) in Tyrode buffer containing 3 mg/mL albumin11 and incubated with RBCs loaded with scFv/TM (∼ 2.5 × 104 molecules/RBC) or control RBCs. Thrombin (0.2 U/mL) or AYP-PAR4 peptide (500 μg/mL) was added for 30 minutes at 37°C followed by addition of FITC-labeled P-selectin antibody for 30 minutes at room temperature. The reaction was stopped by dilution with 1 mL Tyrode buffer. P-selectin was measured by FACS analysis. We also injected mice intravenously with soluble scFv/TM or sTM (4 mg/kg). Blood was collected 1 hour later, diluted 10 times with Tyrode buffer, stimulated with thrombin (0.5 U/mL) or vehicle for 30 minutes, and P-selectin expression was measured.
scFv/TM in a cremaster vessel laser injury model
Intravital video microscopy was used to monitor clot formation in the cremaster muscle vasculature. Microvessels were studied using an Olympus BX61WI microscope with a 40 × /0.8 numeric aperture water-immersion objective lens. Animals were given scFv/TM (4 mg/kg) or equimolar sTM via the jugular vein 1 hour before injury. Labeled antibodies to mouse fibrin (kindly provided by Dr Hartmut Weiler, University of Wisconsin), and platelets were injected 5 minutes before injury.41 Injury to vessels 20 to 40 μm in diameter was induced using an SRS NL100 Nitrogen Laser system at 65% energy level at the edge of the vessel wall. Colocalization was studied using 2 fluorescent channels, 490 and 647 nm, plus bright field. Data were collected over 2.5 minutes at 5 frames per second (f/s; 750 frames per study). A maximum of 5 arterioles and 5 venules were injured per mouse.
Data analysis
Data are presented as the mean ± SEM of at least 3 separate experiments. Differences between groups were tested for statistical significance using Student t test or ANOVA. Statistical significance was set at P less than .05.
See supplemental Methods for more details (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Results
Binding of scFv/TM fusion protein to target RBCs
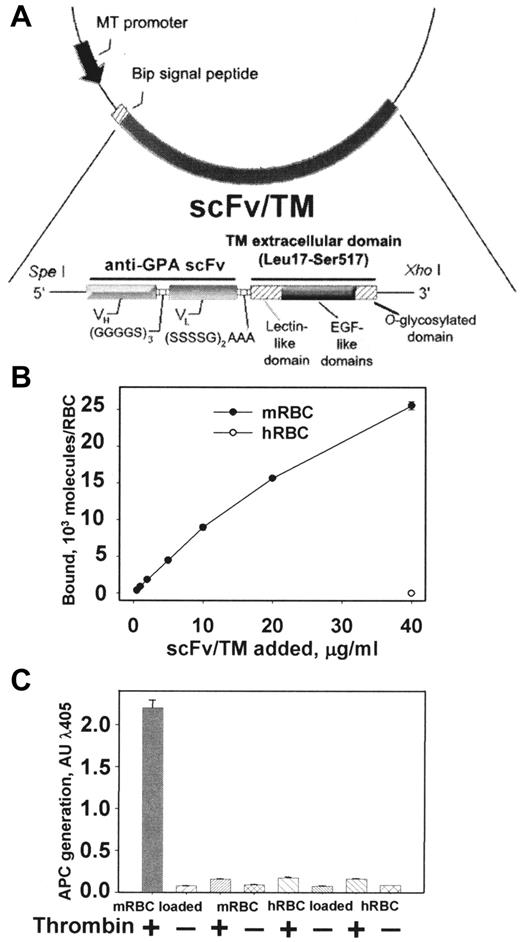
Figure 1A shows the design of the cDNA encoding scFv/TM targeted to mouse GPA. Transfected S2 cells secrete a protein that migrates as a single band (molecular weight ∼ 85 kDa) on SDS-PAGE and Western blotting (not shown). 125I-labeled scFv/TM bound to mouse RBCs in a dose-dependent manner, reaching a level of 2.5 × 104 versus less than 100 molecules per human RBCs at the maximal concentration used (Figure 1B). Binding to mouse RBCs was rapid, reaching 50% of Bmax within 10 minutes (supplemental Figure 1A). As expected, the monovalent scFv/TM did not agglutinate mouse RBCs and highlights the expected monovalent nature of the fusion protein. The parental mAb Ter-119, on the other hand, caused substantial RBCs agglutination consistent with the bivalent nature of G-type immunoglobulins, even at concentrations as low as 4 μg/mL (supplemental Figure 1A inset). Binding of scFv/TM to mouse RBCs was stable (ie, no more than 10% of bound fusion protein detached from RBCs over 3 hours; supplemental Figure 1B).
Molecular design, binding, and APC-generating activities of scFv/TM. (A) Schematic diagram describing the cloning strategy for the fusion construct scFv/TM. The final construct contains a triple FLAG tag at the C-terminus introduced for purification (not shown). (B) Binding of 125I-scFv/TM to mouse RBCs after 1-hour incubation with a 1% suspension of washed RBCs and removal of free protein (precoating). Open circle in bottom right corner represents binding to human RBCs to control for specificity. (C) In vitro generation of APC by thrombin is stimulated by mouse RBCs preloaded with scFv/TM. Human RBCs preloaded with scFv/TM and unloaded mouse RBCs serve as negative controls. Unless indicated otherwise, data in this and figures that follow are shown as mean ± SEM (n = 3). Please note that deviation bars are often too small to be evident.
Molecular design, binding, and APC-generating activities of scFv/TM. (A) Schematic diagram describing the cloning strategy for the fusion construct scFv/TM. The final construct contains a triple FLAG tag at the C-terminus introduced for purification (not shown). (B) Binding of 125I-scFv/TM to mouse RBCs after 1-hour incubation with a 1% suspension of washed RBCs and removal of free protein (precoating). Open circle in bottom right corner represents binding to human RBCs to control for specificity. (C) In vitro generation of APC by thrombin is stimulated by mouse RBCs preloaded with scFv/TM. Human RBCs preloaded with scFv/TM and unloaded mouse RBCs serve as negative controls. Unless indicated otherwise, data in this and figures that follow are shown as mean ± SEM (n = 3). Please note that deviation bars are often too small to be evident.
RBC-loaded scFv/TM facilitates APC generation by thrombin
Purified scFv/TM and control nontargeted soluble TM (sTM) stimulated thrombin-mediated conversion of protein C into APC in a dose-dependent manner with nearly identical activity (supplemental Figure 1C). This finding suggests that the N-terminal extension (scFv) does not have a negative impact on the bioactivity of TM being the effector domain of the fusion protein. APC activity was only generated by mouse RBCs preloaded with scFv/TM and only in the presence of thrombin, whereas minimal APC activity was generated under all other experimental conditions (Figure 1C). These results indicate that the membrane-targeted fusion protein is biologically fully active.
Binding to the RBCs extends the circulatory half-life of scFv/TM without causing detrimental effects on the carrier cells
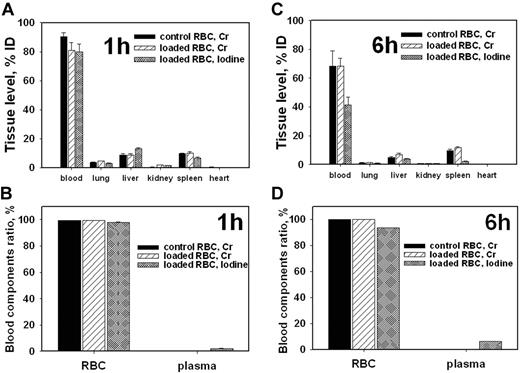
Next, we tested the effect of scFv/TM on RBCs survival. 51Cr-labeled mouse RBCs were precoated with 125I-scFv/TM (∼ 2.5 × 104 copies bound per RBC) and injected into mice. The level of 51Cr in the blood and major organs remained stable for at least 6 hours after injection of scFv/TM-loaded 51Cr-RBC vs control 51Cr-RBCs (Figure 2; supplemental Figure 2). Almost 100% of the radioactivity in blood was found in the RBCs pellet with only trivial amounts present in the plasma (Figure 2B,D; supplemental Figure 2B). Therefore, scFv/TM was not trapped in capillaries, nor did it accelerate the elimination of carrier RBCs.
Preloading of scFv/TM does not affect RBCcirculation and tissue distribution. Blood and tissue radioactivity 1 hour (A-B) or 6 hours (C-D) after intravenous injection of 51Cr-labeled mouse RBCs preloaded with 125I-scFv/TM (n = 5). (B,D) Radioactivity in RBCs pellet and in the plasma, respectively.
Preloading of scFv/TM does not affect RBCcirculation and tissue distribution. Blood and tissue radioactivity 1 hour (A-B) or 6 hours (C-D) after intravenous injection of 51Cr-labeled mouse RBCs preloaded with 125I-scFv/TM (n = 5). (B,D) Radioactivity in RBCs pellet and in the plasma, respectively.
The distribution of 125I-labeled scFv/TM and 51Cr-labeled RBCs was nearly identical 1 hour after injection (Figure 2A-B), indicating stable binding to the RBCs. Over time, the ratio between 125iodine and 51Cr in blood progressively declined from approximately 1 to approximately 0.75 and approximately 0.55 at 1, 3, and 6 hours after injection, respectively (Figure 2A,C; supplemental Figure 2A). As a result, the blood 125I-labeled scFv/TM declined slowly after injection of RBC-scFv/TM, from 85% of the injected dose (ID) at 1 hour to approximately 50% at 3 hours and approximately 40 at 6 hours (Figure 2; supplemental Figure 2). Thus, a substantial fraction of scFv/TM remained associated with RBCs for many hours after injection of preloaded RBC-scFv/TM in vivo.
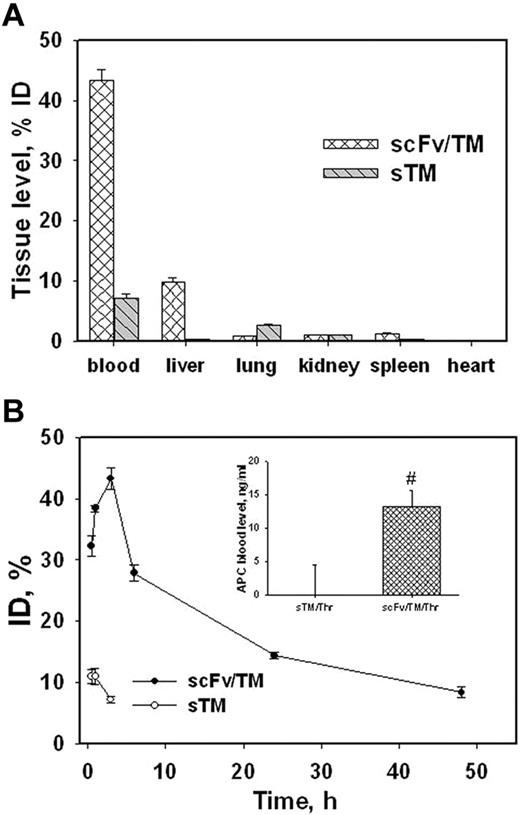
We next examined the tissue distribution and pharmacokinetics of 125I-scFv/TM versus nontargeted sTM injected directly into the bloodstream in mice. Three hours after intravenous injection, only a minor fraction of sTM was found in the blood and organs, whereas most scFv/TM remained in the blood with a minor fraction in the liver (Figure 3A). At earlier time points (0.5 and 1 hours), a higher proportion of injected scFv/TM was transiently found in the liver (supplemental Figure 3). However, anchorage of injected scFv/TM to circulating RBCs remained stable ie, 95% of the fusion protein in the blood was associated with the RBCs throughout (supplemental Figure 3 inset).
Tissue distribution and circulation of radiolabeled scFv/TM and sTM in mice. (A) Organ distribution of 125I-scFv/TM and sTM 3 hours after intravenous injection. (B) Kinetics of blood clearance of scFv/TM and sTM (n = 3). Inset: APC levels in plasma 10 minutes after injection of thrombin 1 hour after injection of scFv/TM or sTM (n = 5).
Tissue distribution and circulation of radiolabeled scFv/TM and sTM in mice. (A) Organ distribution of 125I-scFv/TM and sTM 3 hours after intravenous injection. (B) Kinetics of blood clearance of scFv/TM and sTM (n = 3). Inset: APC levels in plasma 10 minutes after injection of thrombin 1 hour after injection of scFv/TM or sTM (n = 5).
As a result of anchoring to circulating RBCs, approximately 40% of injected 125I-labeled scFv/TM was found in the blood 3 hours after injection, after which the level decreased slowly over time. More than 10% of the injected dose remained in the blood 2 days after injection (Figure 3B; supplemental Figure 3). In contrast, only approximately 10% of nontargeted sTM was detected in blood 1 hour after intravenous injection (Figure 3B open symbols in bottom left corner) because of rapid hepatic elimination. Further, scFv/TM retained its biologic activity in circulation. Thrombin was injected intravenously 1 hour after giving an equimolar amount of scFv/TM or soluble TM and APC activity in plasma was measured. Thrombin generated significantly greater APC activity in mice pretreated with scFv/TM versus sTM (Figure 3B inset, basal level of APC generated by thrombin alone is subtracted). This outcome mirrors the pharmacokinetics of scFv/TM bound to RBCs and shows that RBC carriage enhances TM bioavailability.
RBC-targeted scFv/TM prevents thrombosis
We next injected equimolar amounts of scFv/TM or sTM in mice 1 hour before inducing occlusive thrombi in the jugular vein. Doppler analysis of blood perfusion showed that prophylactic injection of sTM had no effect on the rate or duration of thrombotic occlusion compared with PBS-treated controls (Figure 4; supplemental Figure 4A-B). In contrast, mice preinjected with 0.15 mg/kg scFv/TM were protected from forming occlusive venous clots (Figure 4; supplemental Figure 4C-D). We observed temporary reduction of perfusion in only 2 of 8 scFv/TM-treated mice, but perfusion was rapidly restored to nearly preinjection levels (supplemental Figure 4C). Blood flow measured by the area under the curve of the Doppler perfusion values revealed that animals given scFv/TM retained 82% ± 3% (SEM) of normal blood flow compared with 28% ± 2% (SEM) in sTM-treated animals and 21% ± 2% (SEM) in the PBS-treated group (P = 3 × 10−10 comparing sTM and scFv/TM, and P = 2 × 10−10 comparing scFv/TM and PBS).
Thromboprophylaxis by scFv/TM in venous thrombosis model in mice. Occlusion of the jugular vein was induced with FeCl3 1 hour after injection of PBS or equimolar doses of scFV/TM versus sTM, and blood flow was monitored using Doppler (supplemental Figure 4). The plot shows the data from each group presented as percentage of the preinjury blood flow in the jugular vein during the first 30 minutes after induction of thrombosis; N = 8 per group. *P = 3 × 10−10, sTM versus scFv/TM. #P = 2 × 10−10, scFv/TM versus PBS.
Thromboprophylaxis by scFv/TM in venous thrombosis model in mice. Occlusion of the jugular vein was induced with FeCl3 1 hour after injection of PBS or equimolar doses of scFV/TM versus sTM, and blood flow was monitored using Doppler (supplemental Figure 4). The plot shows the data from each group presented as percentage of the preinjury blood flow in the jugular vein during the first 30 minutes after induction of thrombosis; N = 8 per group. *P = 3 × 10−10, sTM versus scFv/TM. #P = 2 × 10−10, scFv/TM versus PBS.
RBC-loaded scFv/TM inhibits platelet activation by thrombin
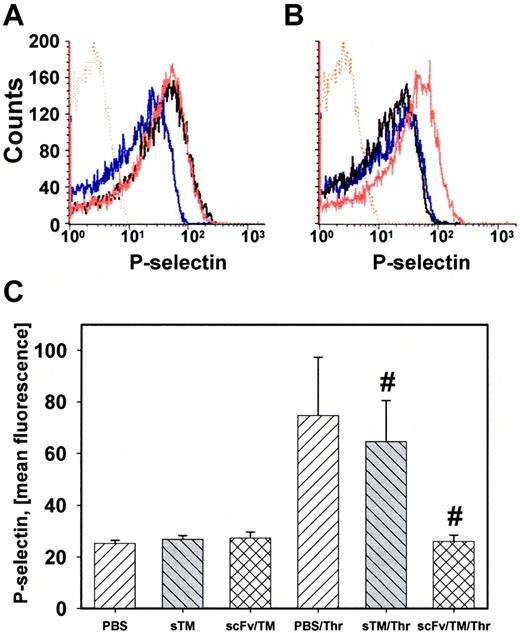
The antithrombotic effect of scFv/TM can be mediated by the anticoagulant properties of APC (Figure 3B insert) or by inhibiting cellular signaling of thrombin. We tested whether RBC-loaded scFv/TM directly inhibits thrombin-mediated activation of platelets. RBCs loaded with scFv/TM inhibited platelet activation by thrombin (detected by P-selectin exposure), but not by AYP-PAR4 peptide (Figure 5A-B). To assess whether this occurs in vivo, blood was collected 1 hour after injection of equimolar amounts of scFv/TM or sTM, diluted to a 5% hematocrit and thrombin was added to initiate platelet activation. FACS analysis showed that thrombin caused P-selectin expression on platelets from mice injected with PBS or sTM, but not from mice given scFv/TM (Figure 5C).
RBC-loaded scFv/TM inhibits platelet activation by thrombin. (A) P-selectin expression on platelets mixed with RBCs (5% hematocrit). Resting platelets (blue line) and platelets stimulated with thrombin (black line) or AYP-PAR4 peptide (red line). (B) Same as in panel A, but RBCs were preincubated with scFv/TM. scFv/TM-loaded RBCs inhibited platelet activation by thrombin (black line) but not by AYP (red line). (C) Mice (n = 3 for each arm) were injected with PBS, sTM, or scFv/TM 1 hour before collecting blood. P-selectin expression was measured as in panel A. Injection of scFv/TM prevents platelet activation by thrombin compared with sTM (P = .0357).
RBC-loaded scFv/TM inhibits platelet activation by thrombin. (A) P-selectin expression on platelets mixed with RBCs (5% hematocrit). Resting platelets (blue line) and platelets stimulated with thrombin (black line) or AYP-PAR4 peptide (red line). (B) Same as in panel A, but RBCs were preincubated with scFv/TM. scFv/TM-loaded RBCs inhibited platelet activation by thrombin (black line) but not by AYP (red line). (C) Mice (n = 3 for each arm) were injected with PBS, sTM, or scFv/TM 1 hour before collecting blood. P-selectin expression was measured as in panel A. Injection of scFv/TM prevents platelet activation by thrombin compared with sTM (P = .0357).
Combined inhibition of platelet aggregation and fibrin deposition in the microvasculature by scFv/TM
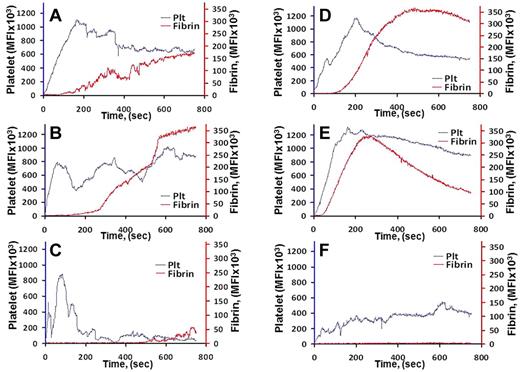
Lastly, we examined the effect of scFv/TM in a mouse model of thrombus formation caused by laser vascular injury using real-time in situ fluorescence microscopy. Fibrin and activated platelets forming the clot were identified using fluorescent labels (“scFv/TM in a cremaster vessel loser injury model”). Supplemental videos reveal rapid deposition of platelets followed by fibrin at sites of injury in small arteries and veins of untreated mice. Mice injected with PBS or sTM 1 hour before injury showed comparable platelet and fibrin deposition damage (Figure 6A-B,D-E; supplemental Videos 1-4), whereas scFv/TM markedly inhibited deposition of platelets and fibrin at all sites of vascular injury (Figure 6C,F; supplemental Videos 5-6).
Effect of scFv/TM on formation of blood clots in arterioles and venules detected using real-time in situ fluorescence microscopy. Saline (A,D), sTM (3 mg/kg; B,E), or an equimolar dose of scFv/TM (C,F) was injected in the jugular vein 1 hour before injury; N = 3 per group. Lanes indicate time-dependent platelet and fibrin accumulation at sites of arteriolar (A-C) and venular (D-F) injury. P = .0318, sTM versus scFv/TM.
Effect of scFv/TM on formation of blood clots in arterioles and venules detected using real-time in situ fluorescence microscopy. Saline (A,D), sTM (3 mg/kg; B,E), or an equimolar dose of scFv/TM (C,F) was injected in the jugular vein 1 hour before injury; N = 3 per group. Lanes indicate time-dependent platelet and fibrin accumulation at sites of arteriolar (A-C) and venular (D-F) injury. P = .0318, sTM versus scFv/TM.
Discussion
Thrombosis and hemorrhage are among the most common causes of death and disability in the United States.1,2 There is a need for more effective prophylaxis in patients at acute risk of thrombosis, such as in the early postoperative period, in unstable angina, transient ischemic attack, and non-ST–elevated acute myocardial infarction when the risk of bleeding is substantial because of the underlying disease or concurrent management. Currently available antithrombotic drugs, including anticoagulants, such as coumadin, factor Xa, or thrombin inhibitors,3-5 and inhibitors of platelets, including aspirin and purinergic receptor antagonists, reduce the risk of subsequent cardiovascular events by approximately 25% to 50%.6-15 Thus, no agent prevents thrombosis in the majority of patients, and all increase the risk of bleeding because of systemic activity. A more ideal thromboprophylactic agent would have the following properties: (1) circulate as a prodrug for a sufficient time to cover the period of greatest risk, (2) target incipient occlusive thrombi while sparing existing mural hemostatic clots, and (3) inhibit multiple interactive prothrombotic mechanisms.
In theory, replenishment of the TM axis of the natural antithrombotic pathway may exemplify these attributes. Recombinant TM is an especially attractive agent for rapid-onset thromboprophylaxis in acute and subacute settings, but its translational potential is limited by rapid elimination. TM fused to a tissue factor (TF) antibody showed antithrombotic activity in an animal model,42 but TF rapidly disappears once the provocation for its expression has past, whereas free fusion proteins circulate for a limited time. Therefore, insufficient lifetime in the bloodstream limits the potential of existing molecular iterations of recombinant TM as an approach to thromboprophylaxis.
Results shown in this paper indicate that a new recombinant fusion protein combining the extracellular domain of TM with a scFv fragment binding to RBCs resolves the issue by markedly prolonging its intravascular pharmacokinetics and bioavailability without damaging the carrier RBCs. The major fraction of scFv/TM binds rapidly to circulating RBCs and dissociates slowly, endowing the drug with an intravascular half-life of many hours. The detachment kinetics in vivo is faster than in vitro (Figure 3B vs supplemental Figure 1), which is probably the result of the effect of mechanical factors (eg, repetitive cycles of high shear stress), which merit further investigation.
Studies in 2 distinct models of thrombosis demonstrate that the scFv/TM confers more durable and potent thromboprophylaxis than soluble TM both on the venous and arterial sides of the vasculature and in both large and small vessels that differ in expression of intrinsic components of the TM/APC system. Of note, a single low dose of scFv/TM (0.15 mg/kg) conferred thromboprophylaxis, whereas antithrombotic proteins, including APC and plasminogen activators, require orders of magnitude higher therapeutic doses and have lack prophylactic utility.
Studies in vitro and in vivo suggest that scFv/TM inhibits thrombosis via both APC-dependent and independent pathways. Of note, there was no protein C in the experiment in which scFv/TM inhibited platelet activation (Figure 5A-B). Studies showing that scFv/TM inhibits platelet activation by thrombin may indicate that RBC/scFv/TM sequesters thrombin and/or that thrombin captured by RBC/scFv/TM is less able to cleave PAR4 and generate receptor activating peptide. This is consistent with the finding that the complex does not protect against platelet activation when the AYP-PAR4 peptide is added directly.
These studies were intended to test the efficacy of scFv/TM as a short-term rapid-onset mode of thromboprophylaxis in patients deemed to be an imminent risk of thrombosis or rethrombosis, such as postsurgical patients, rather than as a means to ameliorate the impact of preexisting clots. However, scFv/TM, alone or given in conjunction with other thromboprophylaxis agents may facilitate clot turnover, which depends on persistent generation of thrombin and recruitment of fibrin to compensate for fibrinolysis. Additional studies will be needed to fully delineate the therapeutic window of scFv/TM in the prevention and treatment of thrombosis.
The mAb Ter-119 was initially thought to recognize a GPA-associated protein on mouse RBCs31 but later proven to interact directly with this sialoglycoprotein, which has surface density approximately 106 molecules per cell.43-45 The same authors have also established that GPA and band3, an anion exchanger as abundant on the RBC membrane as GPA itself, interact with each other in the RBC membrane.43 Further, ligation of GPA with bivalent (mAb) and also monovalent antibody preparations, (Fab), could lead to a rigidification of the RBC membrane, suggesting enhanced sensitivity to degradation because of physical stress, such as passage through small capillary vessels in vivo.46 However, in a more recent study, mouse RBCs coated with a bivalent Ter-119–derived F(ab′)2 fragment survived much longer in circulation than those coated with the Ter-119 mAb after infusion into C57BL/6 mice.47 Therefore, we do not expect detrimental structural consequences on RBC homeostasis because of the monovalent nature of scFv/TM; and, from a sheer quantitative perspective, less than 4% of the available binding sites are required to achieve effective protection against clot formation. The data presented in our current study and several previous reports using other targeted therapeutics directed to this RBC30,32,35,48 antigen support the notion that harmful alterations in RBC function and survival are not likely to occur using such an approach. From a translational viewpoint, we are currently in the process of capitalizing on unique phage display libraries for the generation of scFvs that bind to human RBCs. Of course, multifaceted aspects of safety of this approach, including potential adverse effects on RBC homeostasis, will be meticulously studied before entering the clinical domain.
In conclusion, this study shows that targeting of scFv/TM to RBCs forms stable, circulating, and highly effective antithrombotic complexes that inhibit APC generation and thrombin-mediated platelet inhibition, and thereby prevents thrombosis. TM may also act by inhibiting inflammation, which commonly precedes thrombosis.2,49,50 Additional studies involving humanizing the fusion partners, extending the complexity and relevance of the thrombosis models, and deeper inquiry into safety are needed to help determine whether the strategy of immediate thromboprophylaxis provided by RBC carriage of scFv/TM or subsequent iterations is poised for translation into the clinical domain.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) RO1 HL090697 and HL091950 (V.R.M.) and NIH RO1 AI 037618 and R01 AI041592 (J.P.A.).
National Institutes of Health
Authorship
Contribution: S.Z., V.R.M., D.B.C., and M.P. designed the study; D.S., C.T.E., and J.P.A. contributed vital new reagents; S.Z., B.-S.D., S.T., M.A.K., M.N., R.C., and A.S. performed the experiments; S.Z., D.B.C., D.S., J.P.A., M.A.K., C.T.E., S.T., V.R.M., and M.P. analyzed the data; and S.Z., D.B.C., M.P., C.T.E., and V.R.M. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Vladimir R. Muzykantov, Perelman School of Medicine, University of Pennsylvania, Institute of Translational Medicine and Therapeutics, Translational Research Center, 3400 Civic Center Blvd, Bldg 421, TRC 10-125, Philadelphia, PA 19104-5158; e-mail: muzykant@mail.med.upenn.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal