Abstract
Inappropriately low expression of the key iron regulator hepcidin (HAMP) causes iron overload in untransfused patients affected by β-thalassemia intermedia and Hamp modulation provides improvement of the thalassemic phenotype of the Hbbth3/+ mouse. HAMP expression is activated by iron through the bone morphogenetic protein (BMP)–son of mothers against decapentaplegic signaling pathway and inhibited by ineffective erythropoiesis through an unknown “erythroid regulator.” The BMP pathway is inactivated by the serine protease TMPRSS6 that cleaves the BMP coreceptor hemojuvelin. Here, we show that homozygous loss of Tmprss6 in Hbbth3/+ mice improves anemia and reduces ineffective erythropoiesis, splenomegaly, and iron loading. All these effects are mediated by Hamp up-regulation, which inhibits iron absorption and recycling. Because Hbbth3/+ mice lacking Tmprss6 show residual ineffective erythropoiesis, our results indicate that Tmprss6 is essential for Hamp inhibition by the erythroid regulator. We also obtained partial correction of the phenotype in Tmprss6 haploinsufficient Hbbth3/+ male but not female mice and showed that the observed sex difference reflects an unequal balance between iron and erythropoiesis-mediated Hamp regulation. Our study indicates that preventing iron overload improves β-thalassemia and strengthens the essential role of Tmprss6 for Hamp suppression, providing a proof of concept that Tmprss6 manipulation can offer a novel therapeutic option in this condition.
Introduction
The liver antimicrobial peptide hepcidin (HAMP) is the central regulator of systemic iron homeostasis. HAMP controls the surface expression of the iron exporter ferroportin on duodenal enterocytes and macrophages, modulating iron absorption and recycling. HAMP is activated by the bone morphogenetic proteins (BMP)–son of mothers against decapentaplegic (SMAD) signaling pathway, in response to increased body iron and by the IL-6–signal transducer and activator of transcription (STAT)3 pathway in inflammation.1 The glycosylphosphatidylinositol (GPI)–anchored protein hemojuvelin (HJV) is a BMP coreceptor and homozygous mutations of HAMP or HJV cause juvenile hemochromatosis in humans2,3 and severe iron overload in mice.4,5
The BMP pathway is inhibited by matriptase-2 (MT-2), a type II transmembrane serine protease encoded by the transmembrane proteaSe serine 6 (TMPRSS6) gene and mainly expressed in the liver.6,7 MT-2, by cleaving HJV from the hepatocyte surface, attenuates the BMP-SMAD signaling and down-regulates HAMP expression.8 Mice deficient for both Tmprss6 and Hjv show markedly decreased Hamp mRNA levels and systemic iron overload, as do Hjv deficient mice,9 in agreement with Hjv being the serine protease substrate.
TMPRSS6 plays an essential role for erythropoiesis: homozygous inactivation of the Tmprss6 gene leads to excessive Hamp production, impaired dietary iron absorption and microcytic anemia in mice,10,11 and iron-refractory iron deficiency anemia (IRIDA) in humans.12-17
The important role of TMPRSS6 in erythropoiesis is highlighted also by genome-wide association studies. Common TMPRSS6 genetic variants, such as rs855791, associate with serum iron and transferrin saturation, hemoglobin (Hb), and erythrocyte (MCV and MCH) traits in different populations.18-24 We and others have shown that Tmprss6 haploinsufficient mice have an increased susceptibility to iron deficiency.9,25 Altogether these results suggest that TMPRSS6 gene dosage may modify erythropoiesis and influence HAMP expression.
β-thalassemias are severe recessive disorders because of mutations of β-globin genes that lead to defective globin chain synthesis, transfusion-dependent microcytic anemia, ineffective erythropoiesis, shortened red cell survival, and secondary iron overload. β-thalassemia intermedia results from globin gene mutations with less profound effects.26 The degree of anemia is compatible with survival even without transfusions, but iron overload develops in the liver, heart, pancreas, and other organs. Excess iron leads to oxidative damage as a result of the generation of reactive oxygen species and cardiac iron toxicity is the primary cause of death in patients with β-thalassemia syndromes.27
The Hbbth3/+ mouse exhibits features similar to β-thalassemia intermedia in humans, including Hb levels between 7 and 9 g/dL, aberrant erythrocyte morphology, increased reticulocyte count, ineffective and extramedullary erythropoiesis, hepato-splenomegaly, and liver and spleen iron overload,28 a complex phenotype which worsens with aging.29,30
HAMP is deficient in humans with thalassemia intermedia.31,32 Liver Hamp mRNA is decreased in young Hbbth3/+ mice,29,33,34 but increases with age, eventually reaching the same level of WT mice.30 However, the up-regulation of Hamp is not proportional to the increased iron accumulation observed in the Hbbth3/+ animals,30 suggesting that the inappropriately low Hamp plays a role in iron overload.
Iron manipulation may improve anemia in thalassemic mice.35 Infusions of transferrin were first used to ameliorate anemia in a model (th1/th1) of mild thalassemia intermedia.36 Limited dietary iron restriction might be beneficial in the short-term in Hbbth3/+ mice, reducing iron overload and improving anemia and splenomegaly.37 In Hamp transgenic animals transplanted with a thalassemic bone marrow, a partial correction of the phenotype was observed, but the positive effect was strictly dependent on the Hamp gene copy number.37 Whether the effect is dependent solely on iron reduction, solely on an increase in Hamp, or a combination of both remains unclear.
These studies suggest that therapeutic strategies aimed at increasing HAMP levels or the use of HAMP agonists might decrease abnormal iron absorption and improve the anemia in humans with β-thalassemia.35
Here we asked whether inactivation of the Hamp inhibitor Tmprss6 would up-regulate Hamp and rescue the phenotype of the Hbbth3/+ mouse model.
Methods
Mouse models
C57BL6/Hbbth3 (th3/+) mice (The Jackson Laboratory) were maintained in heterozygosity by breeding with C57BL/6N mice (Charles River) and genetic screening.
Tmprss6−/− mouse on a mixed C57BL/6-Sv129 background was kindly provided by Prof C. Lopez-Otin (University of Oviedo, Spain). The animals were maintained in the animal facility of San Raffaele Scientific Institute (Milan, Italy) in accordance with the European Union guidelines. The study was approved by the Institutional Animal Care and Use Committee of the San Raffaele Scientific Institute.
We bred Hbbth3/+ to Tmprss6+/− mice and then intercrossed the Tmprss6+/−Hbbth3/+ and the Tmprss6+/− progeny to generate various genotype combinations. Mice were given a standard diet and males and females were analyzed separately. For hematologic analyses, blood was collected by tail vein puncture into tubes containing 40 mg/mL EDTA (ethylenediaminetetraacetic acid) in 1-, 2-, 4-, and 6-month-old animals. Only for 6-month-old animals, blood was collected for erythropoietin (Epo) quantification before sacrifice. After sacrifice, livers and spleens were weighed, dissected, and snap-frozen immediately for RNA analysis or dried for tissue iron quantification or processed for fluorescence-activated cell sorter (FACS) analysis.
Hematologic analysis
Hemoglobin (Hb) concentration, red blood cell (RBC) counts, and erythrocyte indexes (MCV, MCH) were measured on the Sysmex KX-21 automated blood cell analyser (Sysmex America). Blood smears were stained with May-Grunwald-Giemsa and photomicrographs were obtained using a Nikon Eclipse E6000 Microscope with a Nikon DXM1200 Digital Camera and analyzed with the Nikon Act-1 Version 2.20 software (Nikon).
Serum Epo was measured using mouse Epo quantikine set (R&D Systems), according to the manufacturer's instructions.
Tissue iron content
To measure iron concentration, tissue samples were dried at 110°C overnight, weighed, and digested in 1 mL of acid solution (3M HCl, 0.6M trichloroacetic acid) for 20 hours at 65°C. The clear acid extract was added to 1 mL of working chromogen reagent (1 volume of 0.1% bathophenanthroline sulfate and 1% thioglycolic acid solution, 5 volumes of water, and 5 volumes of saturated sodium acetate). The solutions were then incubated for 30 minutes at room temperature until color development and the absorbance measured at 535 nm. A standard curve was plotted using an acid solution containing increasing amounts of iron diluted from a stock solution of Titrisol iron standard (Merck).
Quantitative RT-PCR
Total RNA was extracted from murine liver and spleen using the guanidinium thiocyanate-phenol-chloroform method (Trizol Reagent), following the manufacturer's (Invitrogen) recommendations. RNA (2 μg) was used for quantitative polymerase chain reaction (qPCR) analysis for first-strand synthesis of cDNA with the High Capacity cDNA reverse transcription kit (Applied Biosystems), according to the manufacturer's instructions. For real-time PCR analysis, specific murine assays-on-demand products (20×) and TaqMan master mix (2×) from Applied Biosystems were used, according to the manufacturer's instructions, and the reactions were run on 7900HT Fast real-time PCR System (Applied Biosystems) in a final volume of 20 μL. Each cDNA sample was amplified in triplicate and the RNA level was normalized to the corresponding level of Hprt1 mRNA. Primers used for qRT-PCR are shown in supplemental Table 1 (available on the Blood Web site, see the Supplemental Materials link at the top of the online article).
Flow cytometry
Splenic cells were incubated with purified rat anti–mouse CD16/CD32 antibody (Mouse BD FcBlock, 2.4G2; BD Pharmingen) and 1% FBS to block unspecific binding, stained with a phycoerythrin (PE)–conjugated rat anti–mouse CD71 antibody (C2; PharMingen) and a fluorescein isothiocyanate (FITC)–conjugated rat anti–mouse TER-119 antibody (TER-119; BD PharMingen). Analyses by FACS were performed using FACSCanto flow cytometer (Becton Dickinson).
Percentages of reticulocytes were determined by flow cytometry after staining with thiazole orange dye (BD Biosciences).
Statistics
Data are presented as mean ± SD. Unpaired 2-tailed Student t test was performed using Prism 4.0 (GraphPad). P < .05 was considered statistically significant.
Results
Homozygous loss of Tmprss6 ameliorates anemia and improves ineffective erythropoiesis of Hbbth3/+ mice
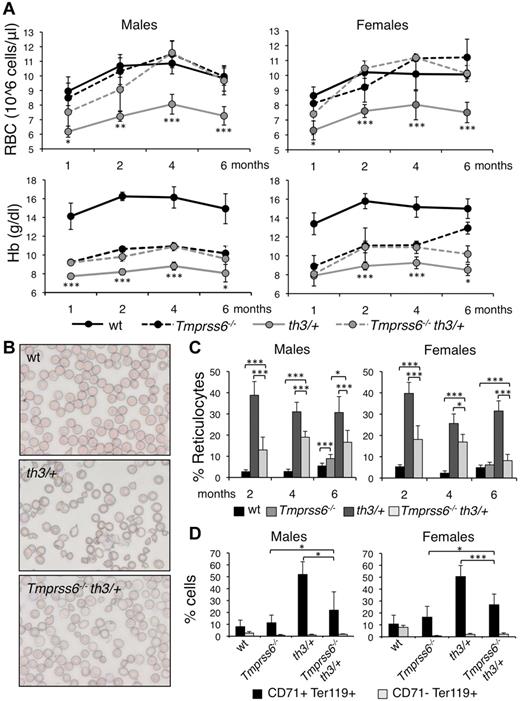
We generated double mutant animals by breeding and screening Tmprss6 and Hbbth3/+ knockout mice. First, we analyzed the effect of homozygous loss of Tmprss6 on the phenotype of Hbbth3/+ mice. We analyzed the hematologic parameters at 1, 2, 4, and 6 months of age, to explore possible age-dependent variation. In addition, because in wild-type (WT) males Hb levels are higher than in females at 1 (P = .039), 2 (P = .044), and 4 months (P = .017), we performed sex specific analysis. Consistent with previous studies, both male and female Hbbth3/+ mice harboring 2 WT Tmprss6 alleles have lower RBC count, Hb levels (Figure 1A), mean corpuscular volume (MCV), and mean corpuscular Hb (MCH, not shown) than WT littermates and all these parameters decrease at 6 months of age.
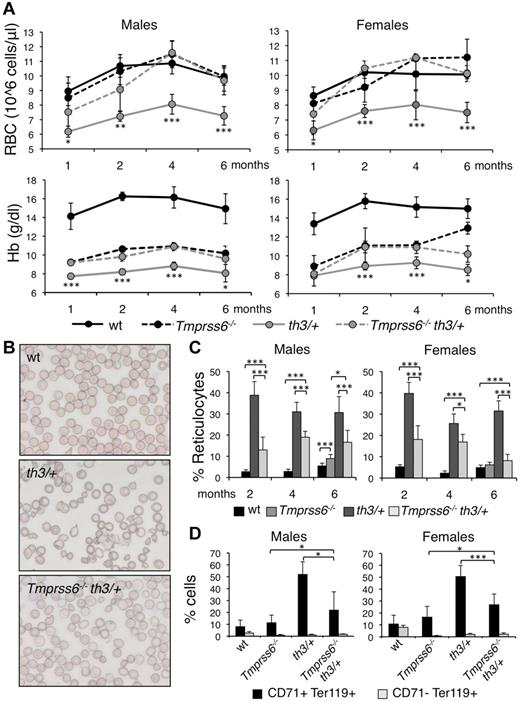
Effect of Tmprss6 inactivation on hematologic parameters and erythroid maturation of thalassemic mice. (A) Time course (1, 2, 4, and 6 months of age) analysis of RBC count and Hb levels of male and female WT, Tmprss6−/−, Hbbth3/+ (th3/+), and Tmprss6−/−Hbbth3/+ (Tmprss6−/−th3/+) mice. Asterisks refer to a statistically significant difference between Hbbth3/+ and Tmprss6−/−Hbbth3/+ mice. (B) Blood smears stained with May-Grunwald-Giemsa showing the morphology of RBC of representative WT, Hbbth3/+ (th3/+), and Tmprss6−/−Hbbth3/+ (Tmprss6−/−th3/+) mice (Original magnification 40×). (C) Percentages of reticulocytes in peripheral blood of 2-, 4-, and 6-month-old male and female WT, Tmprss6−/− (only 6 months old), Hbbth3/+ (th3/+), and Tmprss6−/−Hbbth3/+ (Tmprss6−/−th3/+) mice. (D) FACS analysis performed on splenic erythroid cells of 6-month-old male and female WT, Tmprss6−/−, Hbbth3/+ (th3/+), and Tmprss6−/−Hbbth3/+ (Tmprss6−/−th3/+) mice using CD71 (transferrin receptor 1) and Ter119 (erythroid specific) costaining. The graphs indicate the percentages of early erythroid precursors (CD71+Ter119+), which correspond to basophilic erythroblasts and late basophilic and chromatophilic erythroblasts, and those of mature erythroid cells (CD71− Ter119+), which correspond to orthocromatophilic erythroblasts. Anucleated cells were excluded from analysis. Mean values of 4 to 8 animals for sex and genotype are graphed and error bars indicate SD. Asterisks refer to a statistically significant difference (*P < .05; **P < .01; ***P < .005). For complete statistical analysis see supplemental Table 2.
Effect of Tmprss6 inactivation on hematologic parameters and erythroid maturation of thalassemic mice. (A) Time course (1, 2, 4, and 6 months of age) analysis of RBC count and Hb levels of male and female WT, Tmprss6−/−, Hbbth3/+ (th3/+), and Tmprss6−/−Hbbth3/+ (Tmprss6−/−th3/+) mice. Asterisks refer to a statistically significant difference between Hbbth3/+ and Tmprss6−/−Hbbth3/+ mice. (B) Blood smears stained with May-Grunwald-Giemsa showing the morphology of RBC of representative WT, Hbbth3/+ (th3/+), and Tmprss6−/−Hbbth3/+ (Tmprss6−/−th3/+) mice (Original magnification 40×). (C) Percentages of reticulocytes in peripheral blood of 2-, 4-, and 6-month-old male and female WT, Tmprss6−/− (only 6 months old), Hbbth3/+ (th3/+), and Tmprss6−/−Hbbth3/+ (Tmprss6−/−th3/+) mice. (D) FACS analysis performed on splenic erythroid cells of 6-month-old male and female WT, Tmprss6−/−, Hbbth3/+ (th3/+), and Tmprss6−/−Hbbth3/+ (Tmprss6−/−th3/+) mice using CD71 (transferrin receptor 1) and Ter119 (erythroid specific) costaining. The graphs indicate the percentages of early erythroid precursors (CD71+Ter119+), which correspond to basophilic erythroblasts and late basophilic and chromatophilic erythroblasts, and those of mature erythroid cells (CD71− Ter119+), which correspond to orthocromatophilic erythroblasts. Anucleated cells were excluded from analysis. Mean values of 4 to 8 animals for sex and genotype are graphed and error bars indicate SD. Asterisks refer to a statistically significant difference (*P < .05; **P < .01; ***P < .005). For complete statistical analysis see supplemental Table 2.
Six-month-old Tmprss6−/− mice have lower Hb levels, but RBCs comparable with those of WT littermates (Figure 1A), because of severe microcytosis. In thalassemic mice the homozygous loss of Tmprss6 significantly increases RBC count of approximately 30% and Hb levels of approximately 15% at all ages analyzed (Figure 1A).
As expected, the erythropoiesis of 6-month-old Hbbth3/+ mice was highly compromised, with all the hallmarks of ineffective erythropoiesis: abnormal RBC morphology (Figure 1B), increased percentage of reticulocytes (Figure 1C), high proportion of immature (CD71+Ter119+) erythroid progenitor cells in the spleen (Figure 1D), splenomegaly (Figure 2A) and remarkably high serum Epo levels (Figure 2B). Notably Hbbth3/+ male mice have higher (1977.15 ± 780.40 pg/mL versus 1122.44 ± 253.36 pg/mL, P = .022) serum Epo levels than females in the presence of similar Hb levels (compatible with a greater difference in Hb levels between Hbbth3/+ and WT mice in males than in females). Serum Epo levels are elevated as expected in the anemic Tmprss6−/− mice (Figure 2B). Genetic loss of Tmprss6 significantly improves erythropoiesis in Hbbth3/+ mice, ameliorating the erythrocytes morphology (Figure 1B), reducing the percentage of reticulocytes (Figure 1C), of immature cells of approximately 50% (Figure 1D) and spleen size (Figure 2A) without significantly changing serum Epo concentration (Figure 2B). In Tmprss6−/−Hbbth3/+ Epo levels persist higher in males than in females (P = .014).
Effect of Tmprss6 deletion on spleen size, serum EPO levels, and iron parameters of thalassemic mice. In the figure are graphed: spleen weights normalized to body weight (A); serum EPO levels (B); splenic (C); and hepatic (D) non-heme iron content (SIC and LIC); liver mRNA expression of hepcidin (Hamp; E), Hamp normalized on LIC (Hamp/LIC; F), Bmp6 (G), and inhibitor of DNA binding 1 (Id1; H). mRNA expression ratio was normalized to a male WT mean value of 1. Mean values of 4 to 8 animals for sex and genotype (WT, Tmprss6−/−, Hbbth3/+ [th3/+], and Tmprss6−/−Hbbth3/+ [Tmprss6−/−th3/+]) are graphed and error bars indicate SD. Asterisks refer to a statistically significant difference. (*P < .05; **P < .01; ***P < .005). For complete statistical analysis see supplemental Table 2.
Effect of Tmprss6 deletion on spleen size, serum EPO levels, and iron parameters of thalassemic mice. In the figure are graphed: spleen weights normalized to body weight (A); serum EPO levels (B); splenic (C); and hepatic (D) non-heme iron content (SIC and LIC); liver mRNA expression of hepcidin (Hamp; E), Hamp normalized on LIC (Hamp/LIC; F), Bmp6 (G), and inhibitor of DNA binding 1 (Id1; H). mRNA expression ratio was normalized to a male WT mean value of 1. Mean values of 4 to 8 animals for sex and genotype (WT, Tmprss6−/−, Hbbth3/+ [th3/+], and Tmprss6−/−Hbbth3/+ [Tmprss6−/−th3/+]) are graphed and error bars indicate SD. Asterisks refer to a statistically significant difference. (*P < .05; **P < .01; ***P < .005). For complete statistical analysis see supplemental Table 2.
Homozygous loss of Tmprss6 reduces tissue iron and increases Hamp mRNA levels of Hbbth3/+ mice
In Hbbth3/+ mice the erythropoietic abnormalities are accompanied by splenic (SIC; Figure 2C) and hepatic (LIC; Figure 2D) iron overload, which is more severe in females (LIC P = .016 vs males). Both parameters are reduced to levels similar to those in WT mice by loss of Tmprss6 (Figure 2C-D).
At 6 months of age, Hbbth3/+ and WT mice have similar liver Hamp mRNA levels (Figure 2E), as reported.37 However, these levels are inappropriately low relative to LIC in Hbbth3/+ mice (Figure 2F). The inactivation of Tmprss6 increases Hamp expression in thalassemic mice, reaching levels comparable with those in Tmprss6−/− mutants (Figure 2E). In both sexes Hamp mRNA remains inappropriately high relative to LIC (Figure 2F). It should be noted that Hamp levels vary according to sex, with higher levels in females (Figure 2E), in response to their elevated iron stores (in control mice: Hamp P = 4.31 × 10−5; LIC P = 3.21 × 10−6; SIC P = 3.54 × 10−4; Figure 2C-D). This sex-dependent variation of total body iron was previously reported.38,39
Finally, we analyzed Bmp6, which is transcriptionally regulated by iron and controls both the expression of Hamp and other targets, such as Id1, Smad7, and Atoh8.40 We noticed that Bmp6 mRNA level in Tmprss6−/− overlaps that of WT mice (Figure 2G), in spite of significantly lower values of LIC (Figure 2D). As expected from the abnormal iron stores, and as previously observed41 Bmp6 is increased in thalassemic mice, especially in females (P = .05 vs males; Figure 2G). However, Bmp6 levels are inappropriately low relative to LIC, a finding more evident in males (not shown). The expression of Id1 (Figure 2H), Smad7 (supplemental Figure 1A) and Atoh8 (supplemental Figure 1B) is up-regulated in male Hbbth3/+ compared with controls. Surprisingly, this is not the case among females. Compared with Hbbth3/+, Tmprss6−/−Hbbth3/+ males have reduced Bmp6 mRNA levels (Figure 2G), in agreement with the decreased LIC. They have elevated Id1 (Figure 2H), Smad7 (supplemental Figure 1A) and Atoh8 (supplemental Figure 1B) as expected because lack of cleavage of Hjv from the plasma membrane of hepatocytes in the absence of Tmprss6 induces a constitutive activation of the Bmp-Smad pathway. The same pathway is even more active in Tmprss6−/−Hbbth3/+ females that maintain high Bmp6 levels despite a strong LIC reduction.
Tmprss6 haploinsufficiency ameliorates the phenotype of Hbbth3/+ male mice
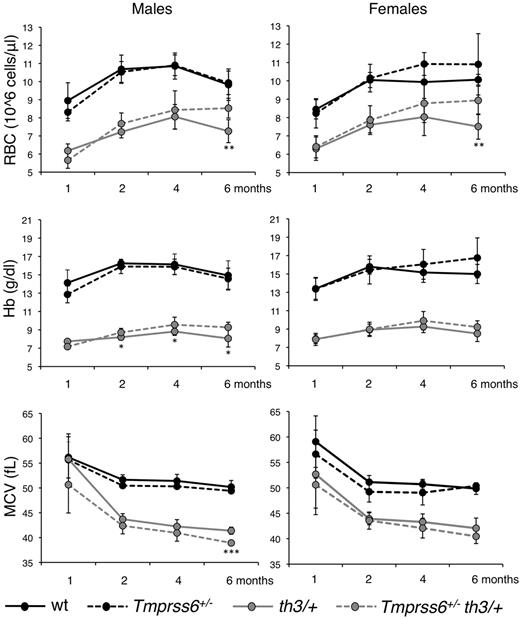
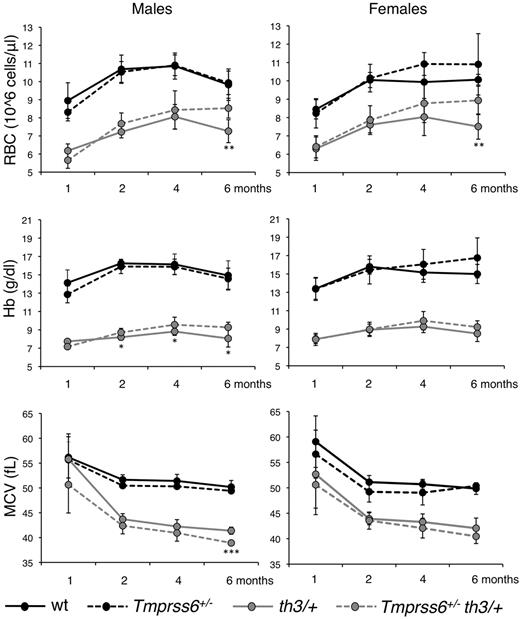
Because Tmprss6 haploinsufficient mice show increased susceptibility to iron deficiency,9,25 we analyzed the effect of the genetic loss of a single Tmprss6 allele in Hbbth3/+ mice. Among thalassemic males heterozygous loss of Tmprss6 increases RBC count and reduces MCV at 6 months, with an even earlier positive effect on Hb (Figure 3 left panels). The increased number of RBC with a decreased size indicates that iron availability for erythropoiesis is restricted.36 In females we observed the same trend documented in males for all parameters, but the difference between Hbbth3/+and Tmprss6+/−Hbbth3/+ mice was statistically significant only for RB count at 6 months of age (Figure 3 right panels).
Effect of Tmprss6 haploinsufficiency on hematologic parameters of thalassemic mice. Time course analysis of RBC count, Hb levels and MCV of male and female WT, Tmprss6+/−, Hbbth3/+ (th3/+) and Tmprss6+/−Hbbth3/+ (Tmprss6+/−th3/+) mice. Mean values of 4 to 8 animals for sex and genotype are graphed and error bars indicate SD. Asterisks refer to a statistically significant difference between Hbbth3/+ and Tmprss6+/−Hbbth3/+ mice (*P < .05; **P < .01; ***P < .005). For complete statistical analysis see supplemental Table 3.
Effect of Tmprss6 haploinsufficiency on hematologic parameters of thalassemic mice. Time course analysis of RBC count, Hb levels and MCV of male and female WT, Tmprss6+/−, Hbbth3/+ (th3/+) and Tmprss6+/−Hbbth3/+ (Tmprss6+/−th3/+) mice. Mean values of 4 to 8 animals for sex and genotype are graphed and error bars indicate SD. Asterisks refer to a statistically significant difference between Hbbth3/+ and Tmprss6+/−Hbbth3/+ mice (*P < .05; **P < .01; ***P < .005). For complete statistical analysis see supplemental Table 3.
In male mice, the improvement of the hematologic parameters is accompanied by a statistically significant reduction of spleen size (Figure 4A), serum Epo (Figure 4B), and LIC (Figure 4D). SIC, measured as micrograms iron/grams tissue, is not affected (Figure 4C). However, total splenic iron is reduced in Tmprss6+/−Hbbth3/+ mice compared with the Hbbth3/+ after spleen volume reduction (not shown). In females the difference in spleen size, Epo levels, SIC and LIC between Tmprss6+/−Hbbth3/+ and Hbbth3/+ mice is not significant, although the trend is toward an improved phenotype associated with the heterozygous loss of Tmprss6 allele (Figure 4A-D). The different effect of the Tmprss6 haploinsufficiency in thalassemic male and female mice is probably because of the remarkably higher LIC in females (Figure 4D).
Effect of Tmprss6 haploinsufficiency on spleen size, serum EPO levels, and iron-related parameters of thalassemic mice. In the figure are graphed: spleen weights normalized to body weight (A), serum EPO levels (B), splenic (C) and hepatic (D) non-heme iron content (SIC and LIC), liver mRNA expression of Hepcidin normalized on LIC (Hamp/LIC; E), Bmp6 (F), inhibitor of DNA binding 1 (Id1; G), and Tmprss6 (H). mRNA expression ratio was normalized to a male WT mean value of 1. Mean values of 4 to 8 animals for sex and genotype (WT, Tmprss6+/−, Hbbth3/+ [th3/+], and Tmprss6+/−Hbbth3/+ [Tmprss6+/−th3/+]) are graphed and error bars indicate SD. Asterisks refer to a statistically significant difference (*P < .05; **P < .01; ***P < .005). For complete statistical analysis see supplemental Table 3.
Effect of Tmprss6 haploinsufficiency on spleen size, serum EPO levels, and iron-related parameters of thalassemic mice. In the figure are graphed: spleen weights normalized to body weight (A), serum EPO levels (B), splenic (C) and hepatic (D) non-heme iron content (SIC and LIC), liver mRNA expression of Hepcidin normalized on LIC (Hamp/LIC; E), Bmp6 (F), inhibitor of DNA binding 1 (Id1; G), and Tmprss6 (H). mRNA expression ratio was normalized to a male WT mean value of 1. Mean values of 4 to 8 animals for sex and genotype (WT, Tmprss6+/−, Hbbth3/+ [th3/+], and Tmprss6+/−Hbbth3/+ [Tmprss6+/−th3/+]) are graphed and error bars indicate SD. Asterisks refer to a statistically significant difference (*P < .05; **P < .01; ***P < .005). For complete statistical analysis see supplemental Table 3.
The loss of one Tmprss6 allele does not affect liver Hamp mRNA (supplemental Figure 2A). However, the Hamp/LIC ratio is significantly higher in Tmprss6+/−Hbbth3/+ male mice than in Hbbth3/+, whereas among females the difference is not statistically significant (Figure 4E).
Although the Bmp6 expression was unchanged (Figure 4F), the expression of target genes Id1 (Figure 4G), Smad7 (supplemental Figure 2B), and Atoh8 (not shown) was partially reduced in Tmprss6+/−Hbbth3/+ compared with Hbbth3/+ males, mirroring LIC. However, the difference reached statistical significance only for Id1. Among females no difference was observed between Tmprss6+/−Hbbth3/+ and Hbbth3/+ in the expression of genes of the Bmp-Smad pathway.
In attempt to understand why the heterozygous loss of Tmprss6 has a different effect on the phenotype according to sex, we measured Tmprss6 expression in thalassemic mice. Compared with WT, Tmprss6 is up-regulated in Hbbth3/+ males, but not in females, probably because Tmprss6 mRNA is constitutively higher in females than in males both in WT mice (P = 5.95 × 10−5) and in Hbbth3/+ mice (P = 9.06 × 10−6) because of higher LIC and Bmp6 levels (Figure 4H).
Discussion
Iron overload is the primary cause of death in patients with β-thalassemia syndromes, because of heart toxicity.27 Iron overload occurs not only in transfused patients but also in thalassemia intermedia patients who, because of milder genetic defects, survive without the need of blood transfusions.42 In these patients and in the corresponding animal models, increased intestinal iron absorption and recycling occurs despite replete iron stores, driven by a signal from the expanded erythropoiesis that causes low/inappropriate hepcidin production (the so called erythroid regulator).43 Indeed, moderate Hamp increase in β-thalassemia mice limits dietary iron absorption, improving not only iron overload but also ineffective erythropoiesis, and anemia.37
Here we show that the genetic loss of Tmprss6, the most important Hamp inhibitor, results in an impressive improvement of anemia, ineffective erythropoiesis, and splenomegaly in Hbbth3/+ mice. This benefit is present early in life and persists up to 6 months in both sexes. In the Tmprss6−/−Hbbth3/+ mice RBC count and Hb levels are increased compared with Hbbth3/+ mice, with a reduction in the number of immature erythroid cells and reticulocytes. The improved erythropoiesis was accompanied by profound changes in systemic iron homeostasis, with marked reduction of iron stores to levels comparable or even lower than WT mice. Hamp expression in the double-mutant mice is elevated, as in Tmprss6−/− mice, and was inappropriately high considering the LIC values.
Our results strengthen and extend previous findings on the beneficial effect of limiting iron supply in β-thalassemia. Compared with administration of transferrin36 or dietary iron restriction,37 that partially correct the established thalassemic phenotype of adult mice, a constitutionally high Hamp production prevents the full expression of the phenotype of the Hbbth3/+ mouse from the first months of life. The effect is similar to that obtained in the Hamp transgenic mouse transplanted with a thalassemic bone marrow,37 although our model is a more physiologic one. As expected, chronic increase of Hamp prevents iron overload in the Hbbth3/+ mouse. However, the amelioration of anemia is not due to hepcidin increase but to the reduced body iron. The erythrocyte indexes (MCH and MCV) are further reduced in the double mutants compared with those in thalassemic mice, probably as the result of the heme synthesis reduction in the erythroblast. It was demonstrated that heme controls protein translation in erythroid cells through the modulation of the activity of the Heme-regulated eukaryotic initiation factor 2α (eIF2α) kinase (HRI). In conditions of heme deficiency HRI is activated and phosphorylates elF2α, blocking the translation of proteins, in particular of globin chains.44 For this reason it has been proposed that the reduced heme synthesis in iron deficiency activates HRI, partially correcting the globin chain imbalance that characterizes thalassemic syndromes.37 Ours and previous results36,37 indicate that reducing iron availability benefits thalassemic erythropoiesis reducing erythroblast premature death and improving RBC survival.
Our data clearly indicate that the modulation of iron absorption mediated by Tmprss6 is indispensable for the phenotype “iron overload/low hepcidin” observed in β-thalassemia. In addition, our results are relevant to define the erythroid regulator pathway. Anemia and some degrees of ineffective erythropoiesis, as shown by the proportion of immature red cell precursors compared with Tmprss6 null mice, persist in the double-mutant mice and the Epo level is maintained high in accordance with similar findings in Hbbth3/+ mice overexpressing Hamp.37 Tmprss6−/−Hbbth3/+ animals seem resistant to the inhibitory effect of the erythroid regulator. The nature of this regulator is still under debate and how it acts to suppress Hamp remains obscure. The prevalent view is that a role is played by cytokines, such as GDF1545 or TWGS1,46 released by an expanded erythroid marrow. However, liver and spleen Gdf15 mRNA and spleen Twsg1 mRNA of Hbbth3/+ mice were not different from WT mice (data not shown). Independently from the nature of the regulator, we favor the hypothesis that Tmprss6 is a component of the erythroid regulator pathway.
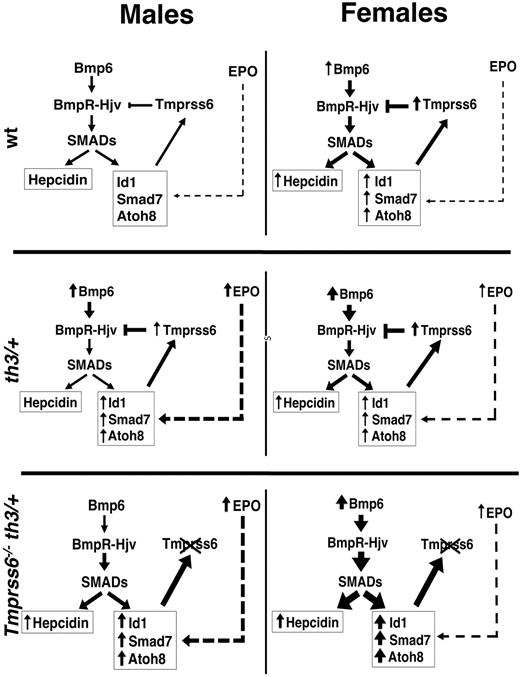
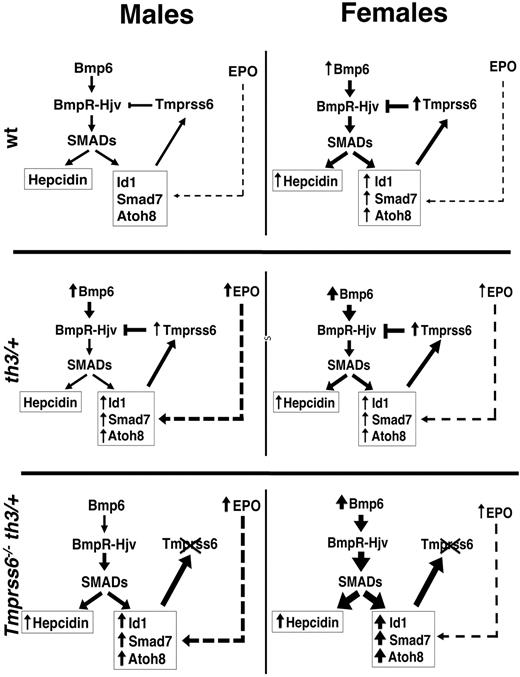
How Tmprss6 might be related to the erythroid regulator activity? Recently it was demonstrated that increased Id1 expression by increased Bmp6 may up-regulate Tmprss6 transcription (and activity) in a negative feedback loop.47 We noticed a striking sex difference in the activation of this pathway that could be informative to the Tmprss6/erythroid regulator relationship. In our model the Bmp-Smad pathway is properly regulated in the Hbbth3/+ male mice with the only exception being Hamp expression. As expected from the high concentration of tissue iron, Bmp6 and its target genes (Id1, Smad7, and Atoh8) are all up-regulated. On the contrary, in Hbbth3/+ female mice Bmp6 is correctly up-regulated in response to iron-loading, whereas Hamp and the other targets are all expressed at levels similar to those of WT mice. In an effort to understand the cause of the observed sex difference, we noticed that Hbbth3/+ female mice are “less anemic” than males. Although male and female Hbbth3/+ mice have similar Hb levels, WT females have lower levels than WT males. Thus, the difference between female Hbbth3/+ mice and their WT counterparts is significantly smaller than the difference between Hbbth3/+ and WT male mice. Accordingly, serum Epo levels, that roughly correspond to the degree of anemia and may be used as a surrogated signal of the expanded erythropoiesis, are significantly lower in female than in male Hbbth3/+ mice. Because Epo is inversely related to Hamp levels, this is consistent with the higher levels of Hamp expression in females that have both increased Bmp6 (high iron stores) and reduced activity of the erythroid regulator. According to the negative feedback loop, Tmprss6 mRNA is higher in females than in males, secondary to high LIC and Bmp6 levels. However, in our mixed background mice, Tmprss6 is up-regulated in Hbbth3/+ males (Figure 4H) compared with WT, but not in females, which have higher basal expression. These results are in accordance with the data of Id1 mRNA expression and with previous reports47. With the limitation of the mixed genetic background of our Hbbth3/+ mice and of expression level data obtained by RT-PCR, we propose a model for the interpretation of our findings, summarized in Figure 5. We hypothesize that 2 pathways control Id1: one is Bmp6-dependent within the negative feedback of Hamp regulation47 ; we speculate that the second is erythroid (Epo)–dependent and increases Id1 and Tmprss6 independently of Bmp6 expression. In our model the former is prevalent in females with higher tissue iron, the second in males with lower Bmp6. Further studies will explore whether increased Tmprss6 transcription corresponds to enhanced Tmprss6 activity, as proposed.47
Schematic model of the proposed mechanism of hepcidin regulation. Representation of the proposed pathways of Id1 control: the first Bmp6-dependent and the second EPO-dependent (see Discussion for details). The thickness of the arrows is proportional to the intensity of the signal. WT mice: low activation of the Bmp-Smad pathway causes basal transcription of Hamp (higher in females, because of their more abundant iron stores) and of the other targets (Id1, Smad7, and Atoh8). The latters are modulated also by the erythroid regulator through a still unknown pathway. Id1 induces transcription of Tmprss6, which by cleaving Hjv, inhibits Hamp transcription, activating a negative feedback loop.47 Hbbth3/+ (th3/+) mice: in males there is a significant iron overload which increases Bmp6 production and a concurrent strong ineffective erythropoiesis which up-regulates Id1 and consequently Tmprss6 transcription. The balance between the positive (Bmp6) and the negative (Tmprss6) regulators of the Bmp-Smad pathway, leaves Hamp mRNA levels unchanged compared with WT mice. In the less anemic females the erythropoietic effect is lower and probably irrelevant on the Id1 transcription, overcome by the strong up-regulation of Bmp6 and resulting in Hamp levels similar to WT mice. Tmprss6−/−Hbbth3/+mice: the loss of Tmprss6 increases the activation of the Bmp-Smad pathway. The effect is more pronounced in females, because of their higher Bmp6 levels. In the absence of Tmprss6, the erythroid factor is ineffective and Hamp transcription remains up-regulated.
Schematic model of the proposed mechanism of hepcidin regulation. Representation of the proposed pathways of Id1 control: the first Bmp6-dependent and the second EPO-dependent (see Discussion for details). The thickness of the arrows is proportional to the intensity of the signal. WT mice: low activation of the Bmp-Smad pathway causes basal transcription of Hamp (higher in females, because of their more abundant iron stores) and of the other targets (Id1, Smad7, and Atoh8). The latters are modulated also by the erythroid regulator through a still unknown pathway. Id1 induces transcription of Tmprss6, which by cleaving Hjv, inhibits Hamp transcription, activating a negative feedback loop.47 Hbbth3/+ (th3/+) mice: in males there is a significant iron overload which increases Bmp6 production and a concurrent strong ineffective erythropoiesis which up-regulates Id1 and consequently Tmprss6 transcription. The balance between the positive (Bmp6) and the negative (Tmprss6) regulators of the Bmp-Smad pathway, leaves Hamp mRNA levels unchanged compared with WT mice. In the less anemic females the erythropoietic effect is lower and probably irrelevant on the Id1 transcription, overcome by the strong up-regulation of Bmp6 and resulting in Hamp levels similar to WT mice. Tmprss6−/−Hbbth3/+mice: the loss of Tmprss6 increases the activation of the Bmp-Smad pathway. The effect is more pronounced in females, because of their higher Bmp6 levels. In the absence of Tmprss6, the erythroid factor is ineffective and Hamp transcription remains up-regulated.
In the double-mutant mice, the Id1 pathway was greatly induced especially in females, which have higher Bmp6 expression secondary to higher LIC, but it is inefficient because of the lack of the Tmprss6 target.
Our interpretation is further supported by the sex differences in the phenotype of Tmprss6+/−Hbbth3/+ mice. We and others9,25 previously demonstrated an increased susceptibility to iron deficiency in Tmprss6 haploinsufficient mice. For this reason, we explored the effect of the genetic loss of a single Tmprss6 allele in Hbbth3/+ mice. Again we observed a striking sex difference in the response to Tmprss6 haploinsufficiency. In male mice the loss of one Tmprss6 allele partially rescued the thalassemic phenotype, improved anemia and erythropoiesis, reduced iron-loading, and increased Hamp/LIC ratio. In female mice we observed a trend toward the same behavior, but the differences between Hbbth3/+ and Tmprss6+/−Hbbth3/+ mice did not reach statistical significance for all parameters examined (with the exception of RBC count at 6 months of age). We hypothesize that the different response observed was related to a more active Bmp6 pathway because of higher tissue iron loading in females compared with males. The genetic loss of a single Tmprss6 allele has positive effects in males with higher activation of the Epo-dependent signaling, whereas in females with a more active Bmp6 pathway the effect is less evident.
Bmp6 remains remarkably elevated in the double-mutant, notwithstanding the strong LIC reduction. A similar phenomenon was observed in our Tmprss6 null mice of both sexes but not in another report.9 Whether Tmprss6 loss leads to a different threshold for Bmp6 activation by iron or may variably increase the stored iron requires further studies.
In conclusion, our results strengthen the relevance of the balance between BMP6 and TMPRSS6 activities in hepcidin regulation and indicate that manipulating the Hamp pathway is of benefit in β-thalassemia in terms of improvement of anemia and prevention of iron overload. In addition, the elevated Hamp levels in the double-mutant suggest that the erythroid regulator requires Tmprss6 activity to inhibit Hamp in conditions characterized by ineffective erythropoiesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge Prof Carlos Lopez-Otin (Oviedo University, Spain) for the kind gift of Tmprss6−/− mice.
This work was partially supported by the Telethon Foundation Onlus, Rome (Grant GGP08089 to C.C. and Telethon Institute for Gene Therapy grant to G.F.), and e.rare 2009 to C.C.
Authorship
Contribution: A.N. designed the experimental work, performed research, and cowrote the paper; A.P., G.M., M.R.L., and L.S. performed research and analyzed data; G.F. contributed to the experimental design and to write the paper; and C.C. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Clara Camaschella, Vita-Salute San Raffaele University, Via Olgettina, 60, 20132 Milan, Italy; e-mail: camaschella.clara@hsr.it.

![Figure 2. Effect of Tmprss6 deletion on spleen size, serum EPO levels, and iron parameters of thalassemic mice. In the figure are graphed: spleen weights normalized to body weight (A); serum EPO levels (B); splenic (C); and hepatic (D) non-heme iron content (SIC and LIC); liver mRNA expression of hepcidin (Hamp; E), Hamp normalized on LIC (Hamp/LIC; F), Bmp6 (G), and inhibitor of DNA binding 1 (Id1; H). mRNA expression ratio was normalized to a male WT mean value of 1. Mean values of 4 to 8 animals for sex and genotype (WT, Tmprss6−/−, Hbbth3/+ [th3/+], and Tmprss6−/−Hbbth3/+ [Tmprss6−/−th3/+]) are graphed and error bars indicate SD. Asterisks refer to a statistically significant difference. (*P < .05; **P < .01; ***P < .005). For complete statistical analysis see supplemental Table 2.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/21/10.1182_blood-2012-01-401885/4/m_zh89991291190002.jpeg?Expires=1770086851&Signature=1ay2uwTDqHCGxwxx~Yo7-divKn~~GsGaxo8Qq0MafFQypsKmPSAlvsUtPW72fVJdpKis55TgK9brUDkjS~NoJpozIMLM1T3Ad~ItW4KowOJVh9~xCJzC7ThFdtfqwDuAVV67hpOscFEF43TAE5n3CMtDZ7MNJyAddbn-tk5Fmz55J~9fVSw8Rs8zudaZn6ZHVNDb7dGgLzfI79UI5uBGY-sOjnZ0Q~pRdF~10j2hTui3bTQxJb0GJI0weLfs~VbYIsBe8A9O2eB9ef9oyqY-GyzVCB1qi~FoOAfJ7gybqsnZGwce5owe6uj1KLoCBHy0VQdaRNuuZfNTjin0EiVY7w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Effect of Tmprss6 haploinsufficiency on spleen size, serum EPO levels, and iron-related parameters of thalassemic mice. In the figure are graphed: spleen weights normalized to body weight (A), serum EPO levels (B), splenic (C) and hepatic (D) non-heme iron content (SIC and LIC), liver mRNA expression of Hepcidin normalized on LIC (Hamp/LIC; E), Bmp6 (F), inhibitor of DNA binding 1 (Id1; G), and Tmprss6 (H). mRNA expression ratio was normalized to a male WT mean value of 1. Mean values of 4 to 8 animals for sex and genotype (WT, Tmprss6+/−, Hbbth3/+ [th3/+], and Tmprss6+/−Hbbth3/+ [Tmprss6+/−th3/+]) are graphed and error bars indicate SD. Asterisks refer to a statistically significant difference (*P < .05; **P < .01; ***P < .005). For complete statistical analysis see supplemental Table 3.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/21/10.1182_blood-2012-01-401885/4/m_zh89991291190004.jpeg?Expires=1770086851&Signature=xNMYWMupbnDXQfM8tJciTDz5KQnGAsaCai~CD70RvEf0Wsyl9Dt-z4eqZ8EIRctK22JrYDMJsFmwD7XCoVL3i9~XZ8Uf560hCLG0Tijq1tgySJLlu1lQVztB55zdWk739USVQn2ikOam5nWnGVK50LalMgHrYNWPMtNCkeOMVzHjK5d9cXrsYtn3DI-v6BmA2~fy7X-~OgSUS1nd9QyIrCDiMlyn2ihJwLLUb0Z9XTp2nJt73ezXdN7w3mKDx-ikmmX7enGPKLFoL5pEIWmxVcLnQvSdJlEV5~R57HtsP~Bzd7IgiVsF~SNUPJxskIxDcfExUdvH8lStsF1CzmVOhQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 2. Effect of Tmprss6 deletion on spleen size, serum EPO levels, and iron parameters of thalassemic mice. In the figure are graphed: spleen weights normalized to body weight (A); serum EPO levels (B); splenic (C); and hepatic (D) non-heme iron content (SIC and LIC); liver mRNA expression of hepcidin (Hamp; E), Hamp normalized on LIC (Hamp/LIC; F), Bmp6 (G), and inhibitor of DNA binding 1 (Id1; H). mRNA expression ratio was normalized to a male WT mean value of 1. Mean values of 4 to 8 animals for sex and genotype (WT, Tmprss6−/−, Hbbth3/+ [th3/+], and Tmprss6−/−Hbbth3/+ [Tmprss6−/−th3/+]) are graphed and error bars indicate SD. Asterisks refer to a statistically significant difference. (*P < .05; **P < .01; ***P < .005). For complete statistical analysis see supplemental Table 2.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/21/10.1182_blood-2012-01-401885/4/m_zh89991291190002.jpeg?Expires=1770086852&Signature=Bangu4o-YPQREuXE-Uemn6N6JLh-qE0M72Uf4IvEkLnZ4GZ88a3QtnuI-O0tkeqMkXYFrdTrkmR2KPtYZ9ZlKyCH2BGF88iiF90DoIdepmWZcqEXknsENiRcjj-c6JTlPF~xtmlsICC~XDXHYJgTrb~BZ1ApNfz0EpjbT86Ya~aDInd~FLhg4jzSMEoqFDxlol6dPkdEHQN9hlZfiZnG~SI6YWGLauBEsJEmuJEUHiZYiedVLkm-mau5epS5h6Jq9ZEOMQDYklw1I6hVPRuIy8rRnDa8PwkKscX3FmUGLrSltiQO9Jd~UTB4AX9mPsAAkLpeAPxx8ScxYFQOSUTtgQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Effect of Tmprss6 haploinsufficiency on spleen size, serum EPO levels, and iron-related parameters of thalassemic mice. In the figure are graphed: spleen weights normalized to body weight (A), serum EPO levels (B), splenic (C) and hepatic (D) non-heme iron content (SIC and LIC), liver mRNA expression of Hepcidin normalized on LIC (Hamp/LIC; E), Bmp6 (F), inhibitor of DNA binding 1 (Id1; G), and Tmprss6 (H). mRNA expression ratio was normalized to a male WT mean value of 1. Mean values of 4 to 8 animals for sex and genotype (WT, Tmprss6+/−, Hbbth3/+ [th3/+], and Tmprss6+/−Hbbth3/+ [Tmprss6+/−th3/+]) are graphed and error bars indicate SD. Asterisks refer to a statistically significant difference (*P < .05; **P < .01; ***P < .005). For complete statistical analysis see supplemental Table 3.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/21/10.1182_blood-2012-01-401885/4/m_zh89991291190004.jpeg?Expires=1770086852&Signature=31FITdKTaJ79rRr6jR9I-NHH5kvqUSJQ2pNI6zu~DK4V7KJI1onugKwCfmnnKcfGScK2w7WioMq76XEIxMAqwilex2R3bFEGI24qRiUnsR2Xw9xStNpuTOGT3KHVPct82UDwf8XBdvmgEcWmdQZiANQOYQPDvBcJh8Fe2PGnIJb8VjnDaLPFpq5zXLf7DdnGDKTslwdcGa5POKhOSdwX0-KlLPaAHJ94BVi10krlS5m2lbOfa6nHStDYOmLOIq8f0~Gxm7fA5ePdHFTWB3YJy-aIWyCCdJ~3g7EIYwq57GSmx51LmZAmaGSmIu1c1D0Uf1wmR5B5fanQwxp9NXvZAQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)