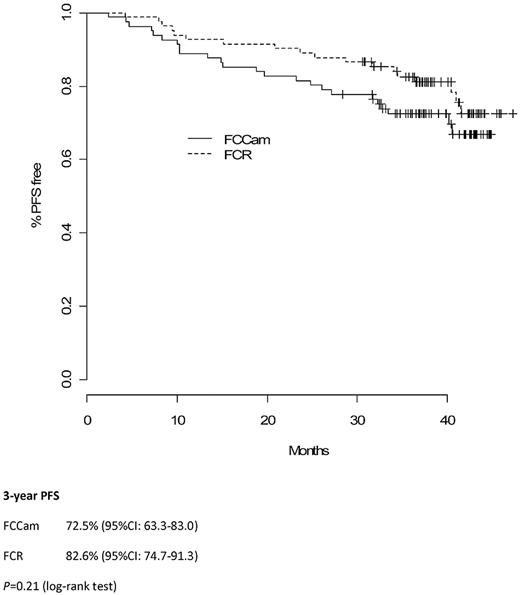
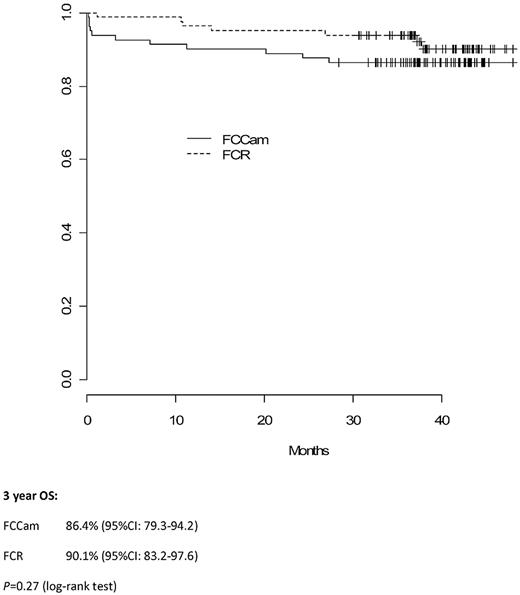
A French and Belgian multicenter phase 3 trial was conducted in medically fit patients with untreated chronic lymphocytic leukemia. Of 178 patients enrolled in the study, 165 were randomly assigned to receive 6 courses of oral fludarabine and cyclophosphamide (FC) in combination with rituximab (FCR; 375 mg/m2 in cycle one, 500 mg/m2 in all subsequent cycles) or alemtuzumab (FCCam; 30 mg subcutaneously injected on cycle days 1-3); each cycle was 28 days. Recruitment was halted prematurely because of excess toxicity; 8 patients died in the FCCam group, 3 from lymphoma and 5 from in-fection. Overall response rates were 91% with FCR and 90% with FCCam (P = .79). Complete remission rates were 33.75% with FCR and 19.2% with FCCam (P = .04). Three-year progression-free survival was 82.6% with FCR and 72.5% with FCCam (P = .21). Three-year overall survival was similar between the 2 arms at 90.1% in the FCR arm and 86.4% in the FCCam arm (P = .27). These results indicate that the FCCam regimen for the treatment of advanced chronic lymphocytic leukemia was not more effective than the FCR regimen and was associated with an unfavorable safety profile, representing a significant limitation of its use. This study is registered with www.clinicaltrials.gov as number NCT00564512.
Introduction
Chronic lymphocytic leukemia (CLL) is the most frequent leukemia in Western countries. For medically fit patients requiring therapy, the goal of treatment is to improve survival by achieving complete remission (CR) and, when possible, eradicating minimal residual disease (MRD). In the German group CLL8 trial, patients with symptomatic CLL were randomized to receive treatment with fludarabine and cyclophosphamide (FC) with (FCR) or without rituximab. Response rates were improved in the FCR group, with longer progression-free survival (PFS) and overall survival (OS). Patients who achieved CR survived longer.1 Therefore, FCR became the standard first-line regimen for fit CLL patients without chromosome 17p deletion. The mAb alemtuzumab (Cam), a humanized Ab against the pan-lymphocytic Ag CD52, is one of the most active single agents for CLL treatment,2 making it a good candidate for combination studies. Cam combined with fludarabine (FluCam) is effective in patients with relapsed or refractory CLL.3 To evaluate the use of Cam in combination with FC in untreated patients with advanced CLL, the French Cooperative Group on CLL and Waldenstrom Macroglobulinemia (FCGCLL/MW) and Groupe Ouest Est des Leucémies et Autres Maladies du Sang (GOELAMS) conducted a multicenter phase 3 trial, CLL2007FMP, comparing FCCam and FCR. Due to investigator decision, recruitment was ceased in January 2009 because of excess mortality in the FCCam arm of the study. We report herein a first analysis of this trial, including response rates, survival, and toxicities.
Methods
Patients
CLL was diagnosed according to the criteria established in 1996 by a National Cancer Institute–sponsored working group.4 Treatment-naive patients in Binet stage B or C (with no limitation regarding time from diagnosis), between the ages of 18 and 65 years were eligible for the trial. Additional inclusion criteria were a Matutes score of 4 or 5, the absence of chromosome 17 deletion (as determined by FISH analysis), a life expectancy longer than 6 months, and signed informed consent. High comorbidity was considered grounds for exclusion and was defined as any of the following: a cumulative illness rating scale (CIRS)5 of up to 6, an Eastern Cooperative Oncology Group (ECOG) performance status of 2 or higher, the presence of clinically apparent autoimmune cytopenia, HIV seropositivity, hepatitis virus B or C seropositivity (unless clearly because of vaccination), active second malignancy currently requiring treatment, transformation to aggressive B-cell malignancy, abnormal renal function with creatinine clearance < 60 mL/min (as calculated according to the formula of Cockcroft and Gault), or total bilirubin, γ glutamyltransferase, or transaminase levels > 2 times the upper limit of normal. The ethics committee of Rouen University Hospital approved the trial in 2007.
Study objectives
The primary objective of this study was to improve PFS at 36 months. PFS was defined as the time between randomization and the date of first documented disease progression or death from any cause. The initial hypothesis was that PFS in the FCCam arm would reach 75% at 36 months and that this would be 15% greater than the 60% 3-year PFS reported by Keating et al with FCR.6 Secondary objectives were to analyze global or complete response rates, OS, event-free survival time to next treatment, toxicity, and MRD in the various treatment groups. The computation of sample size was based on the main end point of PFS within the first 3 years after randomization; it was determined that 155 patients should be recruited in each randomized group to demonstrate such a difference with a type I error of 5% and a statistical power of 80% based on a 2-tailed test. Therefore, the total number of patients scheduled to be recruited was 310, with 155 assigned to each randomized group.
Study design and treatment
After informed consent was obtained from each patient in accordance with the Declaration of Helsinki, investigators faxed the registration form to the GOELAMS/FCGCLLWM study central office in Tours, France. Analyses of genomic aberrations using FISH and of IGHV mutational status using DNA sequencing were centralized in 4 reference laboratories and performed within 1 month. Patients were then randomly assigned to treatment groups (FCR or FCCam) through a central dynamic randomization process using IGHV mutational status and 11q deletion as stratification factors. The FCR treatment arm consisted of 6 monthly courses of oral FC (40 mg/m2 F per day and 250 mg/m2 C per day; administered on days 1-3 every 28 days) in combination with R (375 mg/m2 intravenously on day 0 in the first cycle and 500 mg/m2 on day 1 for all subsequent cycles every 28 days). FCCam treatment consisted of 6 monthly courses of oral FC (40 mg/m2 F per day on days 1-3 and 250 mg/m2 C per day on days 1-3 every 28 days) in combination with Cam (30 mg subcutaneously on days 1-3 every 28 days). Patients with obvious disease progression (based on clinical or biological criteria) after 2 cycles of FCR or FCCam were removed from the study. Patients with stable disease after 2 cycles continued the study according to investigator decision. Infection prophylaxis included trimethoprim-sulfamethoxazole and valacyclovir given until the CD4+ lymphocyte count reached 0.2 × 109/L. In the FCCam group, CMV status was monitored monthly and the use of G-CSF was recommended as needed.
Assessment of response and survival
The response evaluation, or so-called “end evaluation,” was performed either 3 months after the end of treatment when 6 courses were performed or at the ninth month from the beginning of treatment if the 6 courses were not completed for any reason (eg, toxicity or cessation of the trial). This evaluation was performed according to the 1996 guidelines of a National Cancer Institute–sponsored working group.4 In addition, an interim response assessment was performed after 2 courses of treatment. Patients with progressive disease discontinued the study treatment and received a different treatment at the discretion of the investigator. Response assessments included clinical examination, computed tomography scan of lymph node regions involved at baseline, and peripheral blood evaluation. CR was confirmed by BM biopsy; however, if BM biopsy was not performed, patients were considered to be in partial response (PR). At the end of the evaluation, MRD was assessed in blood and BM.
Laboratory assessments
Lymphocyte immunophenotype was determined by flow cytometry using the cell-surface markers CD5, CD20, CD19, CD23, CD52, CD79b, FMC7, and SmIg to establish a diagnosis of CLL. IGHV gene mutational status was examined at baseline. Genetic analyses evaluated for the CLL-related chromosome abnormalities del13q, trisomy 12, del11q, and del17p. MRD evaluation was performed by 6-color flow cytometry analysis in an oligocentric manner following a common predetermined protocol. Immunophenotypic response results were expressed according to the European Research Initiative on CLL (ERIC) recommendations7 using a threshold of positivity of 10−4.
Safety
Patients were monitored before, during, and after each cycle and throughout the follow-up period for adverse events (AEs) and serious adverse events (SAEs), clinical status, vital signs, and critical laboratory data. Toxicity was graded per the National Cancer Institute Common Terminology Criteria (NCI-CTC). All patients were evaluated before the initiation of protocol treatment for Ab-response status (IgG and IgM) and anti-CMV Ags (antigenemia test). For patients treated in the FCCam group, monitoring included monthly investigation for CMV reactivation (CMV PCR or antigenemia test according to local practice). Patients with symptomatic CMV reactivation discontinued treatment and received oral valganciclovir. Asymptomatic cases showing positive CMV antigenemia were retested 2 days later. If the test result showed increasing positivity, treatment was discontinued and valganciclovir was administered. Treatment resumed at the physician's discretion if antigenemia cleared and symptoms resolved. EBV PCR tests were not performed routinely.
Statistical analysis
Analyses were performed according to the intent-to-treat principle. Baseline characteristics recorded at randomization were compared by nonparametric tests: either the Fisher exact test for qualitative variables or the Kruskal-Wallis test for quantitative variables. Censored end points (time to death and PFS) were estimated by the nonparametric Kaplan-Meier method and then compared between randomized groups by the log-rank test after checking for proportionality of hazard functions. Treatment comparisons were adjusted for imbalances or prognostic covariates using a multivariable Cox model. The binary outcomes (response rates and MRD) were crudely compared between randomized groups with the Fisher exact test and then adjusted for prognostic covariates using the logistic regression model. Statistical analysis was performed using the SAS Version 9.2 and R (http://www.R-project.org) software packages. All tests were 2-sided, using 0.05 as the type I error rate. This study is registered with www.clinicaltrials.gov as number NCT00564512.
Results
Baseline demographics and clinical characteristics
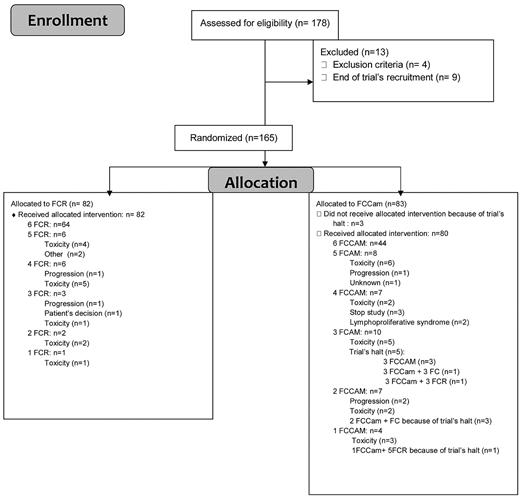
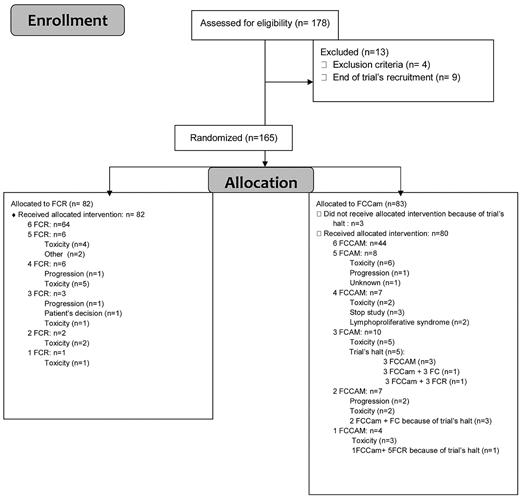
Between November 2007 and January 2009, 178 medically fit patients who were less than 65 years of age and without 17p deletion were enrolled. Thirteen patients were enrolled but not randomized, so a total of 165 patients were randomly assigned to receive 6 courses of oral FCR (n = 82) or FCCam (n = 83; Figure 1). Trial recruitment was stopped in January 2009 because of excess mortality in the FCCam arm of the study. At this point, patients in this group could continue to receive FC without Cam, whereas patients in the FCR arm continued their allocated treatment. Before the investigators' decision to stop the trial, the Data and Safety Monitoring Board (DSMB) met twice in a very short time interval in December 2008, the first time after 3 deaths in the FCCam arm (the conclusion was to halt inclusions) and the second time after 4 deaths in the FCCam arm (the conclusion was to reduce the dose of alemtuzumab to 30 mg per cycle for patients who had not reached the fourth cycle and to stop it for others). Detection of EBV by PCR was mandatory. Among the 165 randomized patients (120 male and 45 female), 80% were Binet stage B and 20% were Binet stage C. The median age was 56.7 years (range, 51-64 years). The 2 treatment groups did not exhibit any significant differences in rates of patients with 13q deletion (53%), 11q deletion (20%), unmutated IgVH status (57.6%), CD38 positivity (42.5%), or elevated β2 microglobulin (80%; Table 1).
Toxicity
Of the assigned patients, 79% of the FCR arm and 55% of the FCCam arm received 6 treatment cycles, with the difference being primarily because of the premature halt of treatment in the FCCam arm. When only considering the first 100 patients included before the end of the trial, 71.4% received all 6 cycles in the FCCam arm. Reasons for discontinuation were mainly related to persistent grade 3-4 neutropenia. A total of 197 grade 3-4 AEs were observed during 378 courses of the FCCam arm compared with 192 AEs in 448 courses of the FCR arm (P = .01), corresponding to 69 (83.1%) and 67 (81.7%) patients (P = .84), respectively. Neutropenia (grade 3-4) was the most frequently reported AE, with 38.7% and 29.62% grade 3 (P = .023) and 25.26% and 19.42% grade 4 (P = .13) in the FCCam and FCR arms, respectively. The percentage of grade 3-4 neutropenia was similar during each course of FCCam, suggesting the absence of cumulative toxicity. A similar observation was made in the FCR arm. At the end of treatment, median CD4+ lymphocyte count was 0.127 × 109/L in the FCCam group versus 0.248 × 109/L in the FCR group (P = .001).
A total of 86 SAEs were found (62 events in the FCCam arm and 24 in the FCR arm), consisting mostly of infectious complications (n = 42 and n = 13, respectively; Table 2). The 62 SAEs in the FCCam arm included 24 during the first 3 cycles, 27 after the fourth, fifth, or sixth cycles, and 15 after the end evaluation. Of the 62 SAEs in the FCCam arm, 15 (24%) were responsible for study discontinuation (Table 3), including 7 deaths. Seven patients died during treatment: 3 of diffuse large B-cell lymphoma (2 was EBV+ and 1 was EBV−), 1 of mucormycosis, 1 of septic shock because of Pseudomonas aeruginosa, and 2 of heart failure during neutropenia (unfortunately, no additional information was available on these patients because no autopsy was performed). An eighth patient died later of respiratory failure without associated infection; this death was not attributed to treatment because lung disease preexisted before inclusion. Two EBV-associated lymphoproliferative disorders occurred in FCCam-treated patients. EBV PCR tests were not routinely performed and were not required during the trial. In one case of EBV-negative lymphoma, EBV PCR was strongly positive in blood; of the 2 other cases, 1 was probably present at diagnosis (the patient developed a rectal lymphoma during treatment, but an abnormal thickening of the rectal mucosa was observed on baseline computed tomography scan).
Response
The overall response (OR) rate was 90% in the FCCam arm and 91% in the FCR arm (P = .79; Table 4). For one-third of patients with hematologic CR, BM biopsy was not available at the final evaluation (32.1% in the FCCam arm and 32.9% in the FCR arm). These patients were considered to be in PR. The CR rate among the 78 evaluable patients in the FCCam arm was estimated at 19.2% (95% confidence interval [95% CI], 11.2%-29.7%) compared with 33.75% (95% CI, 23.6%-45.2%) for 80 evaluable patients in the FCR arm (P = .04). For patients who reached CR and benefited from MRD exploration, rates of negative MRD in blood were 62% (8 of 13) in the FCCam arm and 63% (15 of 24) in the FCR arm (P = 1). For BM MRD analyses, rates of negative MRD were 56% (5 of 9) in the FCCam arm and 39% (7 of 18) in the FCR arm (P = .45). Although the number of patients was slightly different, the rates of negative MRD were not significantly different between the 2 arms when considering either blood or BM. For all patients regardless of response, peripheral blood MRD was negative in 64% (35 of 55) in the FCCam arm compared with 70% (42 of 60) in the FCR arm (P = .47), and BM MRD was negative in 44% (19 of 43) in the FCCam arm compared with 51% (24 of 47) in the FCR arm (P = .51).
Only 2 patients were removed from the study after 2 cycles, both for disease progression as determined by the study design. When considering 31 other patients who discontinued therapy for toxicity (18 in the FCCam arm and 13 in the FCR arm), at the “end evaluation,” 7 patients were in CR (5 in the FCCAm arm and 2 in the FCR arm), 13 in PR (5 in the FCCam arm and 8 in the FCR arm), 6 with stable disease (4 in the FCCam arm and 2 in the FCR arm), and for 5 patients response was not available. These patients discontinued therapy at cycle 1 (n = 4), cycle 2 (n = 5), cycle 3 (n = 8), cycle 4 (n = 4), cycle 5 (n = 7), or cycle 6 (n = 3).
The CR rate was not different in patients with del11q, del13q, mutated or unmutated status or Binet stage B or C (Table 5).
Survival
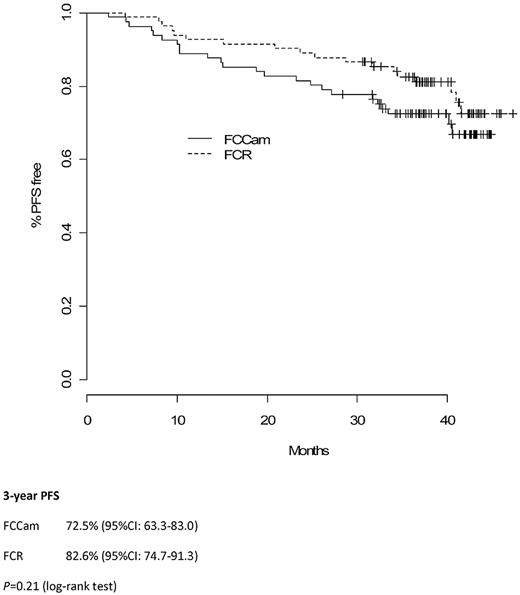
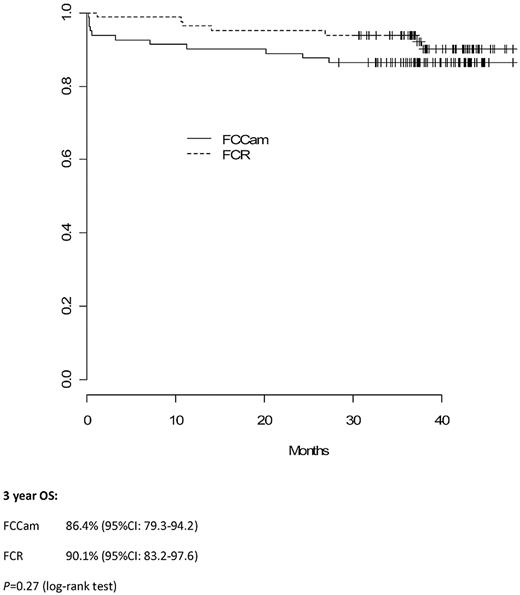
Median follow-up time was 38 months (interquartile range, 36-43). Three-year PFS in the FCCam arm was 72.5% (95% CI, 63.3%-83.0%) versus 82.6% (95% CI, 74.7%-91.3%) in the FCR arm (P = .21; Figure 2). Moreover, the 3-year OS was similar between the 2 arms: 86.4% (95% CI, 79.3%-94.2%) in the FCCam arm and 90.1% (95% CI, 83.2%-97.6%) in the FCR arm (P = .27; Figure 3).
Discussion
The FCR combination resulted in unacceptable toxicity. When the FCGCLL/MW built this trial, limited data were available regarding this combination. Most previous studies concerned heavily pretreated patients in relapse or refractory settings. The FCam combination was evaluated by Engert et al in a phase 3 trial comparing FCam and F in pretreated CLL patients (CAM314). Grade 3-4 neutropenia occurred in 66% of patients, and only 2% of patients died during treatment.8 The FCCam schedule was reported by the GCLLSG in a phase 2 CLL2L study of relapsed CLL patients.9 Five patients (9%) died from treatment-related toxicity. The FCR-Cam combination (CFAR) was evaluated as a frontline treatment and in relapsed high-risk CLL patients.10,11 In the latter group, 92% of patients experienced at least one grade 3 or 4 episode of neutropenia, and 46% of patients developed grade 3-4 infections. As a frontline treatment, 11 patients (18%) died, including 2 from Richter transformation; grade 3-4 neutropenia occurred in 33% of the patients. In the relapse and frontline groups, 18% and 42% completed their 6 cycles, respectively. Recently, an Italian phase 2 trial tested the combination FCCam (alemtuzumab was given at lower dose of 10-20 mg subcutaneously for 3 days) in relapsed CLL patient, and this was also associated with high toxicity (46% grade 3-4 neutropenia and 28% major infection) but not with high mortality.12 More recently, the HOVON group reported results of a randomized phase 2 trial comparing FC with FCCam (with alemtuzumab 30 mg each cycle) in a high-risk population (del 11q and del 17p) of 281 patients.13 OS with FCCam and FC were 88% and 80% (nonsignificant), respectively, with 57% and 47% CR (P = .049), respectively; 29% and 17% showed undetectable MRD, respectively (P < .02). The median PFS was 37 months in the FCCam arm compared with 30 months in the FC arm (P = .04). Grade 3-4 toxicity was more prominent in the FCCAm arm, with 145 events, including 27 fever-related Abs, 25 opportunistic infections, and 34 other complications. Mortality rate was 4% in the 2 arms.
In the present study, a lower CD4+ lymphocyte count may have contributed to the unexpected toxicity (0.127 × 109/L in the FCCam group vs 0.248 × 109/L in the FCR group at the end evaluation, P = .001). Comparing the FCR and FCCam arms is difficult because we had to prematurely close the FCCam arm; many patients were underevaluated because they did not receive the 6 planned FCCam courses. However, by comparing the results of patients who received 4, 5, or 6 cycles of FCR or FCCam, we observed no differences in terms of CR, PR, OR, or MRD. Among patients in both groups who received 6 courses of treatment, the clinical and immunophenotypic response rates were similar to those observed in the entire cohort and were not significantly different between the 2 arms. Eighty patients were available for evaluation in the FCR arm and OR and CR rates were 91% and 33.75%, respectively. In a phase 3 trial comparing FC with FCR (the CLL8 trial), 44% of the 408 FCR patients achieved CR.1 Response criteria were certainly less accurate in the present study, because one-third of CR patients in our trial were not subjected to a medullar evaluation so the CR rate is probably underestimated (26 patients were counted as PR because they did not have a BM biopsy). The dose and routing of FC were also different in the present trial, which used oral administration; IV administration was used in the CLL8 study for FCR. Several studies have confirmed the equivalence of dose between oral and IV administration of fludarabine.14,15 The oral combination of FC as a frontline treatment in 75 Binet stage B and C patients resulted in 80% OR and 53.3% CR.16 Finally, another difference between the present study and the CLL8 trial is that we excluded patients with chromosome 17p deletion; 7% of patients included in CLL8 had a 17p deletion, and among them only 5% reached CR.
In conclusion, the FCCam regimen for the treatment of advanced CLL is not more effective than FCR and is associated with an unfavorable safety profile, representing a significant limitation of its use. Other combinations with Cam should be studied with caution. The FCR combination with being FC given orally was well tolerated and provided a very good response rate.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Schering AG, Genzyme Corporation, and Amgen SA for supporting this trial.
Authorship
Contribution: S.L., T.A., B.M., B. Cazin, O.T., A.D., V.L., R.D., E.V.D.N., M.C.B., R.L., F.C., and P.F. designed the study; S.L. and P.F. co-chaired the study; S.L., T.A., B.M., B. Cazin, O.T., H.M., O.C., A.D., V.L., B.R., B. Corront, E.V.D.N., and P.F. recruited the patients; M.C.B., R.L., and F.C. performed the central laboratory tests; S.L., P.F., S.C., R.D., and M.C.B. analyzed the study data; and all authors critically reviewed the manuscript.
Conflict-of-interest disclosure: S.L., V.L., and P.F. are consultants or on the boards of Roche and MundiPharma and received travel grants and payment for educational presentations for Roche. The remaining authors declare no competing financial interests.
Correspondence: Pierre Feugier, Pôle Hématologie CHU de Nancy, Allée du Morvan 54500 Vandoeuvre, France; e-mail: p.feugier@chu-nancy.fr.