Abstract
In this phase 1/2 study, we explored the feasibility and activity of an oral regimen of lenalidomide with low-dose dexamethasone and low-dose oral cyclophosphamide (RdC) in patients with primary systemic light chain amyloidosis. RdC was given for up to 12 cycles in prespecified cohorts at escalated doses: 13 patients were treated in phase 1 and 24 in phase 2; 65% were previously untreated, and most had renal and/or cardiac involvement and elevated cardiac biomarkers. Lenalidomide 15 mg/d and cyclophosphamide 100 mg/d were further evaluated in phase 2. On intention to treat, 20 (55%) patients achieved a hematologic response, including 3 (8%) complete remissions. Hematologic responses were seen at all dose levels and in 4 of 5 patients who had received bortezomib previously. An organ response was recorded in 22% of patients on intention-to-treat and in 40% of patients who survived at least 6 months. The median time to progression was 10 months and the 2-year survival was 41%. Fatigue, nonneutropenic infections, and rash were the most common toxicities. The results of the present study show that RdC is an oral regimen with activity in primary systemic light chain amyloidosis and may be an additional treatment option, especially for patients with preserved organ function or for patients who cannot receive or who relapse after bortezomib. This study is registered at www.clinicaltrials.gov as NCT00981708.
Introduction
Current treatments for primary systemic light chain (AL) amyloidosis are based on alkylating agents, for example, the combination of standard-dose melphalan with dexamethasone (Mel/Dex) or, for some selected patients, high-dose melphalan with autologous stem cell transplantation.1,2 For patients who fail initial therapy and are either refractory or relapsed, there is no standard therapy. Novel agents such as thalidomide, bortezomib, and lenalidomide offer new treatment options for patients with AL amyloidosis. However, because of multiorgan involvement, many patients with AL cannot tolerate standard doses of novel agents. Therefore, thalidomide at standard doses is not well tolerated,3-5 and bortezomib is active in AL amyloidosis6,7 but is associated with peripheral neuropathy, orthostatic hypotension, constipation, or diarrhea.
Lenalidomide with dexamethasone has been shown to be active in AL amyloidosis in 2 phase 2 trials, although the standard doses were poorly tolerated and dose reductions were required in most patients.8,9 In patients with newly diagnosed myeloma, lenalidomide in combination with low-dose dexamethasone was better tolerated than its combination with high-dose dexamethasone.10 Combining lenalidomide with alkylating agents is feasible and effective in both newly diagnosed11 and relapsed myeloma.12 Based on the above data, we initiated a phase 1/2 study to explore the feasibility of, define doses, and evaluate the activity of an oral regimen based on the combination of lenalidomide with low-dose dexamethasone and low-dose oral cyclophosphamide (RdC) in patients with AL amyloidosis.
Methods
Patients
The present study included pretreated or previously untreated patients with biopsy-confirmed AL amyloidosis, at least 1 involved organ, and adequate renal function, defined as a serum creatinine ≤ 2.5 mg/dL (Table 1). This cutoff was used based on the recommendations that were effective at the time when this protocol was designed and approved (2007). Other criteria included absolute neutrophil count ≥ 1.5 × 109/L, platelet counts ≥ 100 × 109/L, adequate liver function (aspartate aminotransferase and alanine aminotransferase ≤ 2 × the upper limit of normal or ≤ 5 × the upper limit of normal if hepatic involvement was present and total bilirubin ≤ 1.5 mg/dL) and an Eastern Cooperative Oncology Group (ECOG) performance status ≤ 3. Patients had to be > 18 years of age, have evaluable or measurable disease defined by either measurable serum free light chains (≥ 100 mg/L, κ or λ, provided the κ/λ ratio was abnormal), or monoclonal protein in the serum ≥ 10 g/L.
Design of the study
After approval by National Organization of Medicines and the National Ethics Committee of Greece, patients were enrolled in this phase 1/2, single-arm, open-label study (www.clinicaltrials.gov identifier NCT00981708; European Union Drug Regulating Authorities Clinical Trials number 2006-007082-36) in a single center between February 2008 and February 2011. The study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. Lenalidomide was provided by Celgene. The primary objective of phase 1 of the study was the determination of the maximum tolerated dose (MTD) of RdC and the primary objective of phase 2 of the study was the assessment of the hematologic response rate. Secondary objectives were the determination of hematologic and organ progression-free survival, overall survival, and safety.
Patients received dexamethasone 20 mg on days 1-4 (80 mg per cycle), oral cyclophosphamide on days 1-10, and lenalidomide on days 1-21 every 28 days for a planned duration of 12 cycles. Table 2 shows the dose levels in phase 1 of the study. The initial design of the study included also lenalidomide at dose levels of 20 and 25 mg; however, based on data from other studies11,13,14 that were made available during phase 1 of our study and our experience with RdC, we decided to use a maximum dose of 15 mg for lenalidomide. In phase 1 of the study, patients were observed for 2 cycles of therapy for the determination of dose-limiting toxicity (DLT). A standard 3 + 3 design was followed. If no DLT was encountered in the first 3 patients at a certain dose level, 3 patients were enrolled at the next dose level. If > 1 of 3 patients experienced a DLT, then MTD was considered to have been exceeded. If 1 of 3 patients experienced a DLT, 3 more patients were enrolled at the same dose level (total 6 patients). If no more patients experienced a DLT (1 of 6), 3 patients were enrolled at the next dose level. In case of ≥ 2 of 6 patients experiencing a DLT, MTD was considered to have been exceeded (for study flow, see supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Patients had to have completed at least 2 cycles of treatment in the previous dose cohort before patients being enrolled at the next higher dose level. In the phase 2 arm of the study, patients received protocol treatment with MTD, as defined in the phase 1 arm. All patients received low-dose aspirin (100 mg) as prophylactic antithrombotic treatment throughout the treatment course. If low-dose aspirin was contraindicated or was not considered adequate because of other conditions (eg, atrial fibrillation, previous deep vein thrombosis, or heavy proteinuria), patients received another form of antithrombotic therapy according to our institutional guidelines. Standard supportive care also included a proton-pump inhibitor, trimethoprim/sulfamethoxazole, and valacyclovir. Efficacy was evaluated at the beginning of each cycle or whenever there was a delay of > 14 days in the beginning of a new cycle. All patients were followed for survival and disease progression. Consensus criteria were used for the definition of organ involvement and for the assessment of hematologic and organ response.15 Adverse events were recorded throughout the study until 30 days after the last dose of lenalidomide. Patients who received at least 1 dose of treatment with lenalidomide were assessable for safety. The severity of adverse events was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events Version 3.0.
Statistical considerations
According to the Simon minimax 2-stage design,16 in the phase 2 study, 24 patients were required to test at α = .05 the null hypothesis that the rate of hematologic response is less than 5% versus the alternative hypothesis that the rate of hematologic response is at least 25%. Following this design, this phase 2 trial had a power of 90%.
Progression-free survival was defined as being the time from the date of initiation of RdC until the date of hematologic or organ progression or death by any cause. Overall survival was calculated from the date of first dose of RdC until the date of death by any cause or the date of last contact. Survival curves were plotted with the method of Kaplan-Meier and compared by the use of the log-rank test.
Results
Thirty-seven patients (13 in phase 1 and 24 in phase 2) received at least 1 dose of RdC. One patient withdrew consent after she had received RdC for a few days and was excluded from the efficacy analysis but included in the safety analysis. Table 1 shows patient characteristics: 65% were previously untreated, 57% had cardiac involvement and 65% had renal involvement, 64% had at least 2 organs involved, and 38% had ECOG performance status ≥ 2. Concerning cardiac function, 51% had symptoms of congestive heart failure, and 64% were Mayo Clinic stage II or III according to their cardiac biomarkers.17
Phase 1 results
Table 2 presents the results of phase 1 of the study. One DLT was recorded at the dose level 1 (an episode of DVT in a patient receiving aspirin as thromboprophylaxis). No DLT was recorded at dose level 2, and this dose (lenalidomide 15 mg/d and oral cyclophosphamide 100 mg/d) was further evaluated in phase 2 of the study.
Hematologic and organ responses
Hematologic responses were recorded in all 3 dose cohorts (Table 2). Table 3 shows the hematologic and organ responses. On intention to treat, a hematologic response was achieved by 20 (55%) patients, including 3 (8%) with hematologic complete remission. In addition, according to recently proposed criteria, 4 additional patients (rated as partial responses by standard criteria15 ) could be rated as a very good partial responses (ie, difference of involved to uninvolved free light chain [dFLC] < 40 mg/L).18 Hematologic responses were observed in 40% of patients with stage II and in 54% of patients with stage III disease compared with 64% of patients with stage I disease (P = .505). Hematologic responses were seen in 58% of patients who received at least 2 cycles of RdC, in 88% of patients who received at least 6 cycles of RdC, and in all patients who completed the planned 12 cycles of RdC. The median time to hematologic response for all patients was 2.54 months (95% confidence interval [95% CI], 1-4.1) and for patients treated at the maximum tolerated dose it was 1.9 months (2 cycles of RdC).
The response rates were similar for previously treated or previously untreated patients (58% and 54%, respectively). Of 4 patients who had previously received thalidomide, 1 achieved a response, and of 5 patients who had received bortezomib previously, 4 achieved a response.
Fourteen patients (38%) received further therapy after failure to respond to RdC or after relapse: 11 (79%) were given bortezomib with dexamethasone and 6 (43%) achieved a hematologic response (all were patients who were treated with bortezomib).
An organ response was recorded in 8 (22%) patients, including 1 cardiac and 8 renal responses (1 patient achieved both cardiac and renal response). Organ responses were recorded in both pretreated and previously untreated patients (Table 3). Because significant improvement in organ function may need several months to occur, organ responses were recorded only in patients who survived long enough to achieve a response; therefore, 8 of 20 patients (40%) who survived at least 6 months had an organ response.
Cardiac biomarkers and renal function
An increase in N-terminal pro brain natriuretic peptide (NTproBNP) ≥ 30% and ≥ 300 ng/L after the first cycle of RdC was observed in 21 (69.5%) of evaluable patients (n = 30) patients, but only 1 patient had also a concomitant increase of troponin T levels (Figure 1). These increases were observed in 5 stage I patients (of 12 evaluable), 7 stage II patients (of 8 evaluable), and 9 stage III patients (of 10 evaluable). However, a reduction of NTproBNP toward the baseline levels was observed after the third cycle (Figure 1A-B). A transient increase of NTproBNP was observed both in patients with no overt cardiac involvement and in patients with cardiac involvement. Most patients with very high levels of NTproBNP went off study before the sixth cycle of RdC, partly explaining the decrease in NTproBNP after cycle 3; however, even among patients with significantly elevated NTproBNP who received at least 6 cycles, an increase of NTproBNP followed by a decrease toward baseline levels was also observed (Figure 1B). The increase in NTproBNP was not associated with fluctuations of estimated glomerular filtration rate (eGFR; Figure 1) and was in discordance with free light chain levels, which either decreased or remained stable (supplemental Figures 3-5). The increase in NTproBNP was associated with inferior survival (P = .02) with a hazard ratio of 1.013 (95% CI, 1.0029-1.023) per 100 pg/mL of increase over baseline. No patient had a decrease of eGFR above 50% during treatment with RdC. However, a transient decrease ≥ 25% was recorded in 15 (42%) patients; in 2 patients, this reduction was associated with an increase in proteinuria, followed by progression of renal diseases. A decrease in the eGFR was associated with the use of diuretics and in most patients, eGFR returned to near or above baseline levels. Furthermore, 1 patient with renal and cardiac involvement who achieved a cardiac and renal response had a significant increase of eGFR > 50%.
Fluctuations of NTproBNP levels during RdC. (A) NTproBNP (median) and eGFR per cycle for all patients (the number of evaluable patients at each cycle is indicated). (B) NTproBNP (median) and eGFR per cycle only for patients who completed at least 6 cycles (n = 17). An increase in NTproBNP ≥ 30% and ≥ 300 ng/L after the first cycle of RdC was observed. A reduction of NTproBNP toward the baseline levels was observed after the third cycle. This transient increase of NTproBNP was observed in patients with no overt cardiac involvement and in patients with cardiac involvement. The increase in NTproBNP was not associated with fluctuations of eGFR and was in discordance with FLC levels, which were either dropping or stable.
Fluctuations of NTproBNP levels during RdC. (A) NTproBNP (median) and eGFR per cycle for all patients (the number of evaluable patients at each cycle is indicated). (B) NTproBNP (median) and eGFR per cycle only for patients who completed at least 6 cycles (n = 17). An increase in NTproBNP ≥ 30% and ≥ 300 ng/L after the first cycle of RdC was observed. A reduction of NTproBNP toward the baseline levels was observed after the third cycle. This transient increase of NTproBNP was observed in patients with no overt cardiac involvement and in patients with cardiac involvement. The increase in NTproBNP was not associated with fluctuations of eGFR and was in discordance with FLC levels, which were either dropping or stable.
Progression and survival
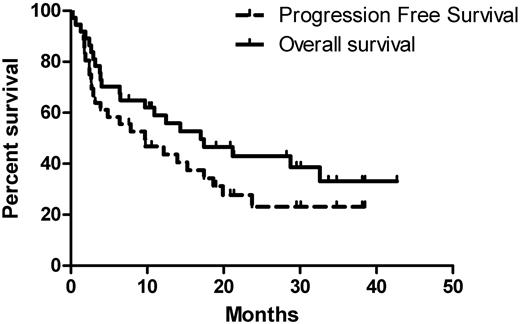
After a median follow-up time of 13 months (range, 0.3-43) for all patients, 26 (72%) patients had progressed (hematologic or organ progression or death) and 22 (60%) patients had died (Figure 2), most due to progressive cardiac amyloidosis. Median follow-up time for surviving patients was 29 months (range, 8-43). No patients were lost to follow-up. The median time to progression for all patients was 10 months (95% CI, 1.8-18) and the median survival time was 17 months (95% CI, 6-28), with a 1-year survival rate of 58% and a 2-year survival rate of 41%. Early deaths (within the first 3 months after initiation of therapy) occurred in 7 (19%) patients; all patients who died early had significant cardiac involvement (median NTproBNP, 6315 ng/L; range, 2023-9197). The median progression-free survival for previously untreated patients was 3 months and for previously treated patients 17 months; the respective median overall survival were 6.5 and 29 months, respectively. Early deaths (< 2 months from the initiation of therapy) occurred in 4 of the previously untreated and in none of the previously treated patients. These differences may be explained by the “preselection” of previously treated patients (ie, they survived long enough to receive a second chance because their organ dysfunction was less severe).
Progression-free and overall survival for all patients. The median progression-free survival was 10 months and the median overall survival was 17 months. Seven patients (19%) died within the first 3 months after initiation of therapy; all patients who died early had significant cardiac involvement.
Progression-free and overall survival for all patients. The median progression-free survival was 10 months and the median overall survival was 17 months. Seven patients (19%) died within the first 3 months after initiation of therapy; all patients who died early had significant cardiac involvement.
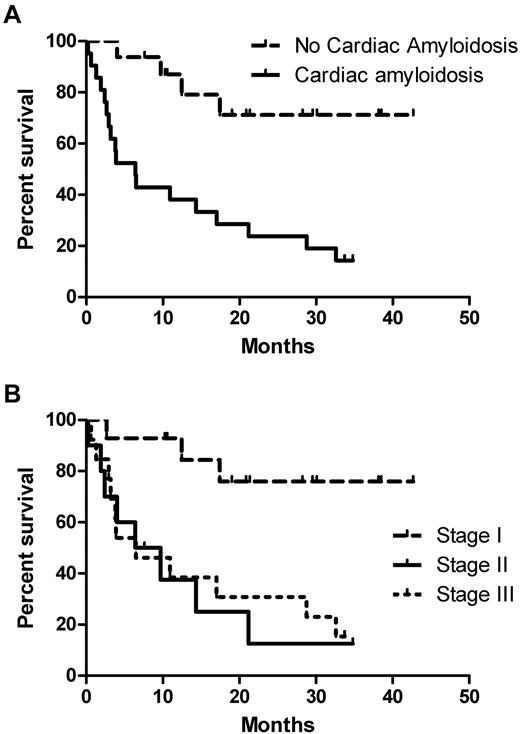
Patients with cardiac involvement had a median survival time of 6.4 months, and this has not been reached for those without cardiac amyloidosis (2-year survival of 68% vs 24% for those with cardiac involvement; P = .001; Figure 3A). According to Mayo Clinic risk stratification by cardiac biomarkers, median survival for stage I has not been reached (the 2-year estimate was 73%), and was 6.5 months for both stage II and III, respectively (P = .004; Figure 3B). However, stage II patients had quite unfavorable characteristics (median NTproBNP, 2167 ng/L; 50% had ECOG performance status ≥ 2; and 40% were New York Heart Association stage II-III).
Survival according to cardiac involvement and Mayo stage. (A) Patients with cardiac involvement had a 2-year survival of 68% versus 24% for those with cardiac involvement, P = .001. (B) Two-year survival estimate was 73% for patients with stage I disease and it was 6.5 months for both stages II and III respectively (P = .004).
Survival according to cardiac involvement and Mayo stage. (A) Patients with cardiac involvement had a 2-year survival of 68% versus 24% for those with cardiac involvement, P = .001. (B) Two-year survival estimate was 73% for patients with stage I disease and it was 6.5 months for both stages II and III respectively (P = .004).
Toxicity
A total of 242 cycles of RdC have been given, 89 in phase 1 and 153 in phase 2 of the study. The median number of cycles that was given in phase 2 of the study was 5, while 47% of patients received at least 6 cycles and 24% received the planned 12 cycles of RdC. Treatment was discontinued before cycle 12 because of disease progression or death in 19 patients, toxicity in 3 patients, and 5 patients' refusal to continue therapy because of reasons other than toxicity. Ten (71%) patients with stage I disease completed at least 6 cycles and 6 patients (43%) completed 12 cycles of RdC. The respective figures for patients with stage II disease were 30% (3 of 10) and 20% (2 of 10 patients) and for stage III were 31% (4 of 13) and (2 of 13) 15%. A dose reduction for lenalidomide was required in 9 (27%) patients. Only 1 patient required reduction of the dose of dexamethasone and 2 patients required dose reduction of cyclophosphamide.
The most common hematologic toxicities included neutropenia and anemia (Table 4); no platelet or RBC transfusions were required. G-CSF support was required in only 1 patient and after a reduction in the dose of lenalidomide, no further G-CSF was required. Fatigue, nonneutropenic infections, and rash were the most common nonhematologic toxicities (Table 4). No significant neurotoxicity was recorded. Fatigue was the most common reason for dose reductions of lenalidomide. Infections were also common; however, no neutropenic infections were recorded. Most of the febrile episodes were associated with symptoms of upper respiratory tract infection and were treated on an outpatient basis with oral antibiotics. Two patients in phase 2 of the study, both of whom had severe nephrotic syndrome, died because of nonneutropenic sepsis. Rash was common (33% of patients), but required dose reduction in only 2 patients. No patient discontinued therapy because of a skin rash.
Most patients (83%) received low-dose aspirin and the rest received either low-molecular-weight heparin (14%) or Coumadin (3%). Two episodes of DVT were recorded, the first occurred in phase 1 of the study, and the second in a patient with heavy proteinuria receiving low-molecular-weight heparin. One patient died because of complications that followed an acute myocardial infarction while on treatment with RdC; coronary angiography showed a 2-vessel disease. Another patient with cardiac involvement and 3-vessel coronary artery disease died suddenly 7 days after the initiation of therapy with RdC. Finally, 1 patient suffered a stroke after the 11th cycle of RdC. In all of these episodes, patients were receiving aspirin.
Discussion
The management of patients with AL amyloidosis requires therapies that effectively target the plasma cell clone but also have a favorable toxicity profile because of the frailty of many patients who have severe organ dysfunction. Within this context, the present study was undertaken to develop an effective oral regimen with acceptable toxicity. We found that the oral RdC regimen has manageable and predictable toxicity with significant activity. We also confirmed that patients with AL amyloidosis can benefit from a lower dose of lenalidomide without the risk of excessive toxicity associated with standard doses of lenalidomide.8,9 Patients with AL amyloidosis are also very sensitive to toxicities associated with high-dose steroids,19 and the use of low-dose dexamethasone in the RdC regimen was associated with improved tolerance, as reflected by the requirement for dexamethasone dose reduction in only 1 patient. Furthermore, the use of low-dose oral cyclophosphamide was accompanied by very low hematologic toxicity. Fatigue was the most common reason to reduce the dose of lenalidomide, but in patients with AL amyloidosis, fatigue may also be related to multisystem involvement, congestive heart failure, diuretics, or significant hypoalbuminemia. Rash was also common in our patients, but was mostly mild, probably because of the lower doses of lenalidomide. What was concerning were the significant rates of infection, which were the cause of 2 deaths despite the use of prophylactic antibiotics. The absence of neurotoxicity should also be acknowledged.
The response rates with RdC were similar to those in phase 2 studies using higher doses of lenalidomide8,9 or lenalidomide with cyclophosphamide and dexamethasone13,20 and were also rapid, within the first 3 cycles of RdC. The use of lower doses of dexamethasone and the lack of a maintenance phase for lenalidomide, because the treatment was given for a maximum of 12 months, may explain these low rates of complete remissions. We should also acknowledge that our patients had characteristics (ie, age, organ dysfunction, cardiac biomarkers, and performance status) that are typical of nonselected patients with AL amyloidosis. These characteristics may also explain to a certain extent the lower response rates that were observed with RdC compared with regimens such as the recently published MLD (lenalidomide, melphalan, and dexamethasone) regimen.14 That study enrolled patients with an ECOG performance status of 0-1 and the 2-year survival was 80%; however, in the present study, the median survival of patients with ECOG performance status of 0-1 was 60%, but 40% of our patients had an ECOG performance status > 1. It is also difficult to compare RdC with Mel/Dex, which has been used widely and is still the standard therapy for AL amyloidosis. In patients with high-risk features (ie, cardiac involvement and elevated cardiac biomarkers), response rates are similar for oral Mel/Dex,21 Mel/Dex plus thalidomide,22 or the more intensive IV Mel/Dex.23 Bortezomib-based therapy has also shown significant activity in standard-risk AL amyloidosis, but in high-risk patients the results were less favorable24,25 However, we believe that the 2 therapies cannot be compared based only on the results of phase 1 or 2 studies in populations with significantly different characteristics. Nevertheless, an interesting finding of our study was the fact that most patients who had been pretreated with bortezomib achieved a response with RdC. This was not the case for thalidomide-pretreated patients, but because of the small numbers, these results should be interpreted with caution. RdC may be a treatment option for patients who relapse after bortezomib, an increasingly used therapy for patients with AL amyloidosis. Furthermore, RdC may be an option for AL patients who are not eligible for bortezomib-based regimens because of peripheral autonomic neuropathy or other reasons.
Organ responses were recorded in 22% of our patients, a figure that is similar to those reported by other investigators for lenalidomide-based therapies.8,9 Organ responses may take several months to occur, sometimes occurring more than a year after a hematologic response has been achieved. Furthermore, patients with severe cardiac dysfunction may die early because of complications of heart disease before any organ response can be achieved. Therefore, the organ response rate in patients who survived at least 6 months in the present study was 40%, which is significant. The major prognostic impact of elevated cardiac biomarkers17 was also seen in our patients. The outcome of patients who were Mayo Clinic stage II and III was poor, whereas that for patients with Mayo Clinic stage I disease was significantly better. The median survival of our patients with stage II and III disease was similar; however, the numbers are too small for meaningful comparisons. Furthermore, stage II patients had high-risk features (50% had poor ECOG performance status, the median levels of NTproBNP were 2167 ng/L, and 40% were NYHA stage II-III). These facts confirm what has been considered as a “predetermined fate” for patients with AL amyloidosis with severe organ dysfunction. The management of patients with severe cardiac amyloidosis is very challenging, and both conventional21,23 and novel therapies22,25 have been associated with poor results. Our data also indicate that RdC is not able to change the fate of patients with severe cardiac dysfunction. However, even among patients with elevated cardiac biomarkers, there is a subset who may benefit from effective treatment.
In the present study, we observed an increase of NTproBNP in our patients after the first cycle of RdC; this has been well described in patients with AL treated with immunomodulatory drugs, including thalidomide, at a similar frequency.26-28 The increases in the level of NTproBNP were not associated with poor survival and did not seem to be related to deterioration of renal function.28 We observed that an increase in NTproBNP was associated with shorter survival times in univariate analysis, but the small number of patients did not allow further analysis. Because we did not observe an increase of cardiac troponins, it is difficult to consider this increase of NTproBNP as a result of a direct cardiotoxic effect of lenalidomide, with or without cyclophosphamide. We have also observed that even patients without evidence of cardiac involvement (ie, patients with Mayo Clinic stage I disease) had significant increases in NTproBNP while they were receiving RdC, which returned to baseline after a few cycles. These patients did not have any signs of cardiac involvement during or after therapy with RdC and we did not observe an increase in cardiac troponins. We cannot rule out that this increase was not because of fluid retention. Whether the addition of cyclophosphamide may amplify a putative cardiotoxicity of lenalidomide needs further investigation. We cannot consider these increases innocuous and physicians should be careful when they use lenalidomide with cyclophosphamide in AL amyloidosis and follow patients closely for signs of deterioration of cardiac function. In contrast to a previous study,29 in the present study, we did not observe any major decrease of eGFR during treatment with RdC despite fluctuations in eGFR probably related to factors such as the use of diuretics and hydration status.
In conclusion, the oral combination of lenalidomide with low-dose steroids and low-dose cyclophosphamide is feasible and results in significant response rates with a manageable toxicity profile. RdC could be an additional option, especially for patients with preserved organ function and low levels of cardiac biomarkers who relapse after bortezomib or autologous stem cell transplantation or melphalan with dexamethasone. For patients at moderate or high risk, RdC may not be able to alter outcome.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.K. designed the study, managed the patients, collected and analyzed the data, and wrote the manuscript; E.T. analyzed the data and critically reviewed the manuscript; M.R. managed the patients, collected and analyzed the data, and critically reviewed the manuscript; M.G. collected and analyzed the data and critically reviewed the manuscript; C.P. performed the cardiac studies, collected and analyzed the data, and critically reviewed the manuscript; I.B., S.M., T.A., N.N., G.G., E.M., and S.D. managed the patients, analyzed the data, and critically reviewed the manuscript; and M.A.D. designed the study, managed the patients, collected and analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: M.A.D. has received honoraria from Celgene and OrthoBiotech. The remaining authors declare no competing financial interests.
Correspondence: Prof Meletios A. Dimopoulos, Department of Clinical Therapeutics, University of Athens School of Medicine, 80 Vas Sofias Avenue, Athens 11528, Greece; e-mail: mdimop@med.uoa.gr.