Abstract
Familial hemophagocytic lymphohistiocytosis (FHL) is a genetically determined hyperinflammatory syndrome caused by uncontrolled immune response mediated by T-lymphocytes, natural killer (NK) cells, and macrophages. STXBP2 mutations have recently been associated with FHL5. To better characterize the genetic and clinical spectrum of FHL5, we analyzed a cohort of 185 patients with suspected FHL for mutations in STXBP2. We detected biallelic mutations in 37 patients from 28 families of various ethnic origins. Missense mutations and mutations affecting 1 of the exon 15 splice sites were the predominant changes detectable in this cohort. Patients with exon 15 splice-site mutations (n = 13) developed clinical manifestations significantly later than patients with other mutations (median age, 4.1 year vs 2 months) and showed less severe impairment of degranulation and cytotoxic function of NK cells and CTLs. Patients with FHL5 showed several atypical features, including sensorineural hearing deficit, abnormal bleeding, and, most frequently, severe diarrhea that was only present in early-onset disease. In conclusion, we report the largest cohort of patients with FHL5 so far, describe an extended disease spectrum, and demonstrate for the first time a clear genotype-phenotype correlation.
Introduction
Familial hemophagocytic lymphohistiocytosis (FHL) is a genetically heterogeneous hyperinflammatory syndrome caused by an uncontrolled and ineffective proliferation and activation of T lymphocytes, natural killer (NK) cells, and macrophages that infiltrate multiple organs, including liver, spleen, lymph nodes, and CNS, and produce massive amounts of cytokines.
This overwhelming hyperimmune status results in a clinical picture characterized by persistent fever, hepatosplenomegaly, pancytopenia, hemophagocytosis in various tissues, coagulation abnormalities, and inflammatory CNS disease. In the absence of specific symptoms or laboratory values, the Histiocyte Society has developed diagnostic criteria to facilitate diagnosis.1 In 70%-80% of patients with FHL onset of disease is during infancy2 ; untreated it is typically rapidly fatal.3,4
Treatment, including immunosuppressive drugs and chemotherapy, can resolve signs and symptoms in most cases, but the only curative therapy is HSCT.
Disease-causing mutations have been identified in the genes encoding perforin (PRF1; FHL2), Munc13-4 (UNC13D; FHL3), and syntaxin 11 (STX11; FHL4).5-7 Furthermore, immunodeficiencies such as Griscelli syndrome type 2 (RAB27A), Chédiak-Higashi syndrome (CHS1; LYST), Hermansky-Pudlak syndrome type 2 (AP3B1), and X-linked lymphoproliferative syndrome 1 and 2 (SH2D1A, XIAP) have been found to be associated with hemophagocytic lymphohistiocytosis (HLH).8-15 The genes mutated in FHL2-4, Griscelli syndrome type 2, and Chédiak-Higashi syndrome encode proteins that are involved in the cytotoxic granule pathway of CTLs and NK cells.16,17 These defects are thought to contribute to the ineffective elimination of (viral) triggers and down-regulation of immune responses that result in a sustained hyperinflammatory state.4,18,19 Two groups have described STXBP2 mutations as being causative for FHL5.20,21 STXBP2 is part of the Sec/Munc proteins that are important for the assembly and disassembly of the SNARE (soluble N-ethylmaleimide–sensitive factor attachment protein receptor) complex and the control of membrane fusion. The role of STXBP2 in the cytotoxic granule pathway is currently not understood in detail, but it is known from previous studies that the protein is also involved in the regulation of intracellular granule trafficking in epithelial cells, neutrophils, and mast cells.22-24 RNA expression was also observed in different hematopoietic cells, including T cells and monocytes.20 Immunoprecipitation and Western blot experiments found a direct interaction between Munc18-2 and syntaxin 11 that is absent in patients with missense mutations in STXBP-2. These data strongly suggest that both proteins are involved in the same granule exocytosis pathway in vivo.20,21
So far, another 4 reports described mutations in STXBP2 in small numbers of patients only.25-28
We present detailed genetic and clinical analyses of a large cohort of 37 patients with FHL with biallelic STXBP2 mutations from diverse ethnic origins and describe an extended clinical spectrum of the disease together with a clear geno-phenotype correlation.
Methods
Patients
Patients with suspected FHL (n = 185) were included in this study. Patients with biallelic mutations in PRF, UNC13D, and STX11 were excluded from analysis. The patients had either been registered at the national HLH reference center in Hamburg, Germany, or material for genetic studies had been sent from various other countries, mainly Turkey. In patients with confirmed biallelic STXBP2 mutations clinical data, including age at onset, presenting features, treatment, and outcome, were extracted from documentation forms and medical letters. Missing data were collected by contacting the treating physicians. CNS involvement was assumed in the presence of increased cerebrospinal fluid white blood count and/or protein, neurologic symptoms, or abnormalities in magnetic resonance imaging, consistent with HLH. All families gave their written informed consent to the genetic analysis, immunologic studies, and clinical and laboratory data collection. The study was conducted according to the guidelines of the Declaration of Helsinki and has been approved by the local institutional review board. Thirteen patients have been published in our original study,20 3 of which plus 3 additional patients appear in the study of Rohr et al (patients P1-6).27 Five patients have been described clinically before the detection of the genetic defect.29-31
Genetic analysis
Genomic DNA was isolated from peripheral blood by standard methods. PCR was performed with specific primers for exon 1-19 of STXBP2, including the adjacent intronic sequences for identification of splice-site variants. The primers and general PCR conditions have been published elsewhere.20 The PCR products were directly sequenced bidirectional with the use of BigDye Terminator Version 1.1 (Applied Biosystems), and the reactions were genetically analyzed on an ABI PRISM 3100. We confirmed the mutations in the parents or by repeated experiments. For missense mutations, healthy German/Turkish blood donors were used as controls to exclude a polymorphism.
Immunoprecipitation
Immunoprecipitation experiments for the characterization of Munc18-2 missense mutations were performed as described previously.20 All missense mutations and 2 different products of the exon 15 splice-site mutation were cloned into the eukaryotic expression vector pEGFP-C2 (BD Bioscience): a cDNA that harbors the c.1247-1356 deletion of the complete 110 nucleotides of exon 15, resulting in a premature stop of the protein sequence and an artificial in-frame deletion of exon 15 that deletes nucleotide c.1246-1356 (p.416_452) of the Munc18-2 protein.
Degranulation and cytotoxicity assays
NK-cell and CTL degranulation and cytotoxicity assays were performed according to the protocols described previously.32 Normal values were established in that study by prospective evaluation of these assays in 90 healthy donors and a large cohort of patients with primary and secondary HLH. On the basis of these data, for the present study resting NK-cell degranulation < 5% was considered to be abnormal, and values > 10% were considered to be normal. For IL-2–stimulated NK cells, values < 20% were considered as abnormal and values > 30% as normal. Any values in between were classified to be reduced. CTL degranulation > 15% was classified as normal, < 10% as abnormal, and between 10% and15% as reduced. Values for NK-cell and CTL cytotoxicity were read from titration curves at an NK/target ratio of 3:1 or a CD8 T cell/target ratio of 10:1, respectively. For both tests, values < 40% lysis (ie, the 10th percentile of 90 healthy donors) were considered to be reduced; values < 10% were considered to be abnormal. In an additional 6 patients, NK-cell activity was performed in another laboratory. Because variable NK cell/target ratios were used, decreased NK-cell cytotoxicity in these patients could not be clearly categorized according to the definitions above and was therefore classified as impaired.
Statistics
Statistical analysis was performed with PASW statistics 18 (SPSS Inc). Significance of differences between patient groups was calculated with the χ2 test for nominal variables and Welch test for metric variables. Differences were considered significant at a P value < .05.
Results
Genetic features
We identified biallelic mutations in STXBP2 in 37 patients from 28 unrelated families. In addition to the 16 patients who were recently published, we found homozygous or compound heterozygous mutations in another 21 patients from 15 independent families. In one patient no sample for genetic testing was available. He was included despite missing genetic analysis because he had a clear-cut clinical HLH picture, and a biallelic STXBP2 mutation had been identified in the brother (Tables 1 and 2).
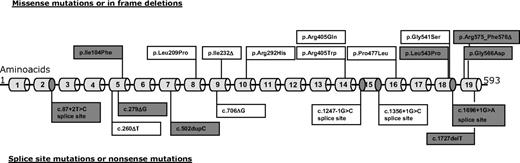
Nine novel mutations were detected (Figure 1). The spectrum of STXBP2 mutations in the entire cohort comprises small deletions or insertions, 9 different missense mutations, and 4 different splice-site mutations. Mutations are scattered over the entire coding region with some mutations being relatively frequent: c.1430C > T (p.Pro477Leu), detected in 5 patients of Arab origin, and c.1621G > A (p.Gly541Ser), found in 7 patients of mainly white origin. The mutation c.1247-1 G > C that affected the splice acceptor site of exon 15 was identified in 12 patients, 5 times homozygous and 7 times in combination with another mutation. One further patient had a compound heterozygous mutation pattern that affected the splice donor site of exon 15 on one allele (c.1356 + 1G > A) together with the c.1621G > A (p.Gly541Ser) missense mutation in exon 18. These splice-site mutations occurred predominantly in German and Turkish patients.
Spectrum of Munc18-2 mutations detected in our cohort. New mutations are shaded in gray. Three mutations were seen in ≥ 5 patients: 13 patients had mutations affecting one of the exon 15 splice sites, 7 patients from central Europe had p.Gly541Ser, and 5 patients of Arab origin had p.Pro477Leu. For details see Tables 1 and 2.
Spectrum of Munc18-2 mutations detected in our cohort. New mutations are shaded in gray. Three mutations were seen in ≥ 5 patients: 13 patients had mutations affecting one of the exon 15 splice sites, 7 patients from central Europe had p.Gly541Ser, and 5 patients of Arab origin had p.Pro477Leu. For details see Tables 1 and 2.
Interaction studies between Munc18-2 and syntaxin 11
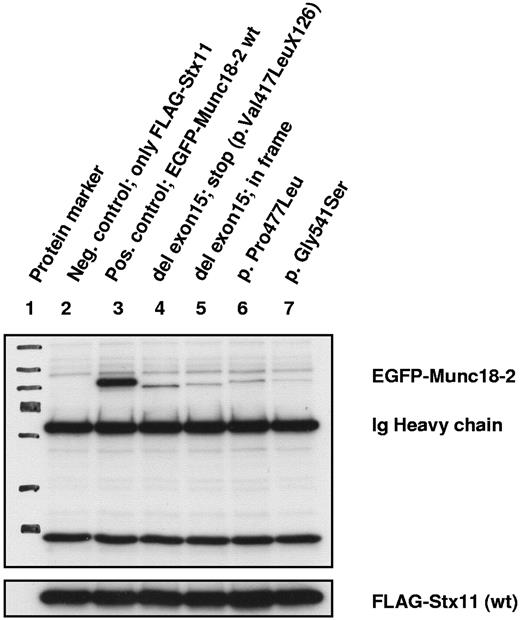
To better characterize the nature of the missense mutations and in-frame deletions we performed an immunoprecipitation assay that allows testing of the interaction capacity of mutated Munc18-2 with wild-type syntaxin 11. A weak-to-absent binding to syntaxin 11, comparable with the results observed for Munc18-2 missense mutations, was found for the 2 exogenous constructs of exon 15 splice-site mutations (Figure 2).
Coimmunoprecipitation analysis for binding between Munc18-2 and syntaxin 11 proteins. All immunoprecipitation reactions were performed with exogenous constructs with the use of either an Ab specific for Flag (syntaxin 11) or for enhanced green fluorescent protein (EGFP; Munc18-2). No endogenous proteins were analyzed. Exposition time of the x-ray film was 15 seconds. Lanes 1 through 3 show control samples, lane 4 shows the Munc18-2 construct as identified as the main RNA product detected in 2 of our patient samples with a homozygous exon 15 splice-site mutation. Lane 5 shows an artificial construct that contains an in-frame deleted exon 15. Lanes 6 and 7 are constructs representing missense mutations with nearly absent syntaxin binding. All Munc18-2 constructs, representing the different kind of mutations, show comparable loss of binding for syntaxin.
Coimmunoprecipitation analysis for binding between Munc18-2 and syntaxin 11 proteins. All immunoprecipitation reactions were performed with exogenous constructs with the use of either an Ab specific for Flag (syntaxin 11) or for enhanced green fluorescent protein (EGFP; Munc18-2). No endogenous proteins were analyzed. Exposition time of the x-ray film was 15 seconds. Lanes 1 through 3 show control samples, lane 4 shows the Munc18-2 construct as identified as the main RNA product detected in 2 of our patient samples with a homozygous exon 15 splice-site mutation. Lane 5 shows an artificial construct that contains an in-frame deleted exon 15. Lanes 6 and 7 are constructs representing missense mutations with nearly absent syntaxin binding. All Munc18-2 constructs, representing the different kind of mutations, show comparable loss of binding for syntaxin.
Cytotoxicity and degranulation assays
Data on NK cytotoxicity were available from 12 patients and showed pathologic results (“impaired,” “abnormal,” and “reduced”) in 11 of them (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Detailed functional testing of cytotoxic and degranulation capacity of NK and/or T cells with standardized methods was performed in 16 patients from 14 unrelated families in one reference laboratory (Tables 1–2). NK degranulation was analyzed in all 16 patients and showed a clear deficiency in 13 and reduced degranulation in 3. NK degranulation after IL-2 stimulation was tested in 12 patients and was abnormal in 5 and reduced in 3 patients, all of whom also had abnormal resting NK-cell degranulation. Interestingly, 4 patients with abnormal resting NK-cell degranulation showed normal degranulation of IL-2–activated NK cells. Degranulation of CTLs was tested in 14 patients and was abnormal in 10 and reduced in 4 patients. CTL cytotoxicity was normal in 10 of 13 patients and reduced in the remaining 3 patients.
Clinical characteristics of patients with FHL5
Detailed clinical data of the 37 patients with FHL5 from 28 unrelated families are shown in the supplemental Table 1, a summary of which is presented in Table 3. Fourteen patients originated from Central Europe, 14 from Turkey, 7 from the Middle East, and 2 from Sri Lanka. Consanguinity of the parents was reported in 19 of 35 patients. Twenty-three of 36 patients had a positive family history of HLH. The age at diagnosis ranged from 3 days to 19 years with a median age of 3 months. At diagnosis, most patients fulfilled the HLH-2004 diagnostic criteria; in 5 patients the data on HLH criteria were incomplete. Most patients showed signs of liver involvement such as hepatomegaly, elevation of liver enzymes, hyperbilirubinemia, and/or low protein/albumin. CNS involvement was observed in 55% of the patients. Treatment according to the HLH-1994/2004 study protocols was administered in 26 patients; another 2 were treated with a modified protocol (supplemental Table 1). Five patients received different combinations of corticosteroids, immunoglobulins, antibiotics, or plasmapheresis. In one patient, Hodgkin disease stage III B was diagnosed at the age of 9 years. Retrospective evaluation found the presence of typical HLH features at that time. Both Hodgkin disease and HLH symptoms responded to the treatment according the German Hodgkin lymphoma protocol. At the age of 10 years, still in remission of Hodgkin disease, an HLH relapse occurred.
The course of disease showed a considerable variability in this cohort. Part of the patients displayed an early onset and a severe and rapid progression of disease; others had a late onset and a chronic recurrent course with sometimes long episodes of well-being and absence of HLH symptoms without therapy. In 8 patients with a partial or full HLH reactivation, response to steroids or unspecific treatment only could be observed.
HSCT was performed in 26 patients (19 from matched related or unrelated donors) at a median age of 1 year (range, 4 months to 24 years). Conditioning regimens consisted of busulfan, cyclophosphamide with or without etoposide in half of the patients; 10 received fludarabine-based regimens. Most patients had nonactive disease before HSCT. Median follow-up after HSCT is 4 years (range, 5 months to 16 years). Six patients died of transplantation-related complications. Surviving patients have remained free of HLH symptoms except 2 who are alive with mild HLH reactivations after rejection of the graft. Eight patients died of HLH or HLH therapy–related complications without HSCT within the first year of disease onset. Causes of death are listed in supplemental Table 1. Two patients are alive without HSCT, 13 and 2 years, respectively, after onset of disease but are currently evaluated for HSCT because of recent reactivations.
Unusual clinical features
Gastrointestinal symptoms.
In 14 patients, severe chronic diarrhea was described which resulted in failure to thrive even in the first months of life. All patients except one who received feeding by percutaneous endoscopic gastrostomy tube needed long-term parenteral nutrition. Weight was below the third percentile in most of the patients. Height was below the third percentile in all patients who survived long enough for evaluation. Diarrhea was often present before the diagnosis of HLH. Extensive clinical investigations, including biopsies in 6 patients (5 after HSCT), failed to detect a specific cause of their gastrointestinal symptoms. Histology was normal in the patient without HSCT and showed unspecific changes such as discrete inflammation and slight loss of villi or crypts in the other patients. The diarrhea did not respond to HLH or unspecific treatment, even if HLH symptoms had been controlled. In 6 of 8 patients who had undergone HSCT, severe diarrhea persisted even after transplantation. In 1 patient, the information is missing, in the other patients it is not available yet, because HSCT has been performed recently.
Hearing deficit.
Sensorineural hearing loss was documented in 6 patients between 4-17 years of age. Pure tone audiometry found a mild-to-moderate low-frequency hearing impairment. Two children experienced additional conductive hearing loss (maximum 25 and 60 dB, respectively).
Hypogammaglobulinemia.
Hypogammaglobulinemia is another unusual clinical symptom that has been observed in 10 of 17 patients. Three of them have already been included in the report on atypical patients by Rohr et al.27 Most patients had a chronic course that repeatedly required intravenous immunoglobulin infusions.
Bleeding tendency.
Bleeding problems were reported in 8 patients. In 4 patients major bleeding occurred in the presence of disease- or transplant-related abnormalities of coagulation or platelets. In 2 siblings unexplained recurrent bleeding symptoms have been observed. In one further patient pulmonary hemorrhage during the early period after transplantation was judged as atypical and not clearly associated with HSCT by the treating physicians. In 4 patients a specific platelet secretion defect has been found (for details see Sandrock et al33 ). Only 1 of them displayed bleeding symptoms.
Genotype-phenotype correlation
Patients were classified into 2 distinct subgroups: group 1 carrying biallelic missense mutations, in-frame, or frameshift deletions and splice-site mutations other than exon 15 and group 2 with either homozygous or compound heterozygous mutations with 1 allele carrying an exon 15 splice-site mutation (Table 4).
The 24 patients of group 1 had a heterogeneous ethnic background. All of them had a classic course of disease with an early onset before 1 year of age (median, 2 months) and a rapidly fatal course if HSCT could not be performed. This group also comprises all 14 cases with diarrhea. Only one of these patients was diagnosed to have hypogammaglobulinemia. In contrast, the 13 patients of group 2, mainly from central Europe, with exon 15 splice-site mutations on ≥ 1 allele showed a relatively mild and atypical course of FHL. They were diagnosed at a median age of 3 years (range, 1.3-19 years). Most of them developed a chronic recurrent course with long episodes of absent HLH symptoms and recurrent reactivations with spontaneous remissions or response to therapy with steroids only. None of these patients had diarrhea; however, in 9 patients hypogammaglobulinemia was observed. HSCT was performed in 10 patients at a median age of 9 years (range, 2.4-24 years). Two further patients, 11 and 15 years old, are awaiting HSCT. Age at diagnosis, frequency of hypogammaglobulinemia, and frequency of episodes with spontaneous remission or response to steroids only were significantly different in group 2 than in group 1.
In 8 patients with missense mutations who were tested at the reference laboratory, NK-cell degranulation was abnormal in 7 and showed reduced responses between 5% and 10% in 1 patient. CTL degranulation was abnormal in all tested patients. No patient regained normal NK degranulation on IL-2 stimulation. CTL cytotoxicity was analyzed in 5 patients of this cohort and was abnormal in 3 and normal in 2 of them.
In 8 patients with exon 15 splice-site mutations NK-cell degranulation was abnormal in 6 and reduced in 2 patients. CTL degranulation was abnormal in 4 patients and reduced in the other 4 patients. In contrast to patients with missense mutations, on IL-2 stimulation NK-cell degranulation returned to normal in 4 of 7 patients. CTL cytotoxicity was normal in all 8 patients.
Discussion
This is the largest cohort of patients with FHL5 reported so far with 37 patients from a widespread ethnic origin. In contrast to FHL4, which seems to be more frequent in the Turkish and Arab population, FHL5 does not appear to be restricted to a specific geographic region. STXBP2 mutations were found in a considerable number of patients from Central Europe, thus contributing to the characterization of genetic defects in a population with many previously genetically undefined patients with FHL.
STXBP2 mutations are scattered all over the gene and comprise homozygous and compound heterozygous mutations in 25 and 12 patients, respectively. Nine novel mutations were found. We identified a large group of 13 patients with a splice-site mutation affecting exon 15 (either c.1247-1G > C or c.1356 + 1G > A; group 2). Together with the other published FHL5 cases, the c.1247-1G > C splice-site mutation is the most common mutation detected so far.21,25-28
The predicted effect of this splice-site mutation at the protein level remains unclear. In our cDNA analysis from 2 homozygous patients, we predominantly detected a transcript that results in a truncated protein (c.1247-1G > C; p.Val417LeufsX126) containing a large stretch of 126 out-of-frame amino acids without any residual functional domains. In addition, several other out-of-frame and in-frame constructs were observed; however, they were observed as minor fractions visible only after cloning of the corresponding cDNA fragment.20 Cote et al described for the same splice-site mutation an in-frame product that resulted from an exchange of the first 17 aa of exon 15 by 19 aa from the intron 14.21
Western blot analysis of lymphoblasts showed residual Munc18-2 protein for patients with a homozygous exon 15 splice acceptor site mutation, yet of reduced intensity and probably with a slightly reduced molecular weight. Both research groups also showed a reduced but not absent expression of syntaxin 11, a proven interaction partner of Munc18-2 which itself is mutated in patients with FHL4.20,21 To explain at least in part the nature of this splice-site mutation we tested the in vitro syntaxin 11 binding capacity of our main out-of-frame construct (c.1247-1G > C; p.Val417LeufsX126) and of an artificial construct that completely deletes exon 15 in frame. As in missense mutations none of them showed a clear binding, indicating that other effects cause the phenotypic variation between patients with exon 15 splice-site mutation compared with patients with a missense or nonsense mutation.
Deficient NK-cell degranulation seems to be a uniform finding in all patients with FHL5, and IL-2–mediated reconstitution in some of them has also been described by other groups.21,26 However, in line with a prior observation of our group,27 we could establish a clear association of reconstitution of NK-cell degranulation with distinct mutations. Defective NK-cell degranulation improved after IL-2 stimulation in most patients with exon 15 splice-site mutations (group 2) but not in patients with other mutations (group 1). CTL cytotoxicity was normal in all patients from group 2, whereas it was abnormal in 3 patients from group 1. The results of the immunologic studies in our patients therefore support the assumption of a residual function of exon 15 splice-site mutations compared with a deleterious effect of the other mutations.
Although it remains to be clarified how these in vitro processes can be translated into in vivo pathomechanisms, there seems to be an association with the clinical picture. The median age at diagnosis in our whole cohort was 3 months with a range from 3 days to 19 years.
Patients with either homozygous or compound heterozygous exon 15 splice-site mutations had a late onset of disease > 1 year of age and frequently courses with long episodes of nonactive disease or mild reactivations. In contrast, patients in group 1 carrying mutations other than at exon 15 splice sites (mainly missense mutations) had a typical course of FHL with a significantly earlier diagnosis < 1 year of age and a severe course that required early HSCT or resulted in fulminant death. This phenotype/genotype correlation has already been suggested previously in a smaller number of patients.21
Compared with other FHL types, median age of diagnosis in the whole FHL5 cohort seems to be similar to that found in patients with FHL2 (3 months) and is slightly less than in patients with FHL3 (4 months). In patients with FHL4 the age of onset varies widely with a median of 14 months. Interestingly, patients with FHL2 and patients with FHL3 with missense mutations have a significantly later onset of disease than patients with nonsense mutations.34-36
As has been described for patients with FHL5 before, our patients also displayed a large spectrum of features that are not part of the typical HLH clinical picture.26,27 Previous studies have reported an ubiquitous Munc18-2 RNA expression not restricted to hematopoietic cells, suggesting a role of the protein in vesicle transport in many mammalian tissues.37 In mouse embryonic tissues Munc18-2 protein expression has been found in the apical surface of epithelial cells of the intestine, kidney, lungs, spleen, and testes.38 Unless there are alternative pathways in these cell types, as it is for the case for rab27a in platelets, Munc18-2 deficiencies would therefore be expected to cause diverse clinical manifestations.
The most frequent symptom in 14 patients of our cohort was severe diarrhea that often affected the patients before they developed classic HLH symptoms. Most of these patients needed parenteral feeding. Diarrhea persisted during HLH treatment and even after HSCT, often resulting in failure to thrive and always in growth retardation. Because other causes of chronic bowel disease have been ruled out in our patients, we assume that diarrhea is a feature of the underlying genetic defect. In addition, we show a clear association with patients in group 1, indicating that patients in group 2 might have a residual function of the protein.
The pathophysiology of the diarrhea is not yet understood. Colocalization of Munc18-2 with syntaxin 3 was shown to be involved in the regulation of apical membrane transport in murine intestinal cells and in a colon carcinoma cell line.39 Defective Munc18-2 expression in epithelial cells of the human gut may therefore lead to disturbed regulation of secretory pathways.
There is evidence of a role of STXBP2 in platelet function. Four of our patients were tested for functional defects, and a distinct secretion defect could be found.33 The abnormality was not restricted to a group of patients with specific mutations or course of disease. Only one of them developed a major hemorrhage, questioning the clinical significance of this finding.
However, Meeths et al have also described an increased bleeding tendency in some of their patients with FHL5.26 Episodes of abnormal bleeding have been observed in 7 patients of our study. In a setting associated with different coagulation disorders and a lot of comorbidities that also can result in hemorrhage, it is difficult to identify the main underlying cause. We therefore recommend platelet function testing in all patients with FHL to better define the association with specific genetic defects. Closer observation of affected patients might then contribute to a more profound understanding of the clinical consequences.
Hearing impairment is a feature that so far has not been reported in patients with FHL5. The only report on sensorineural hearing loss in FHL describes a patient with a perforin defect who had hearing loss that affected all frequencies.40 We have observed low-frequency hearing impairment in 6 of 17 patients with FHL for whom clinical information or test results were available.
Sensorineural hearing impairment affecting low frequencies is a rare event in early childhood. Typical causes of hearing loss in critically ill patients are toxic effects of drugs which result in high-frequency hearing impairment.
Low-frequency hearing is located at the cochlear apex. Known causes for low-frequency hearing loss include rare hereditary disorders,41 Menière's disease, autoimmune processes, viral infection, allergy, or ischemia. Low-frequency hearing impairment can easily remain undetected because it does not affect listening to the frequencies of normal speech. It might therefore become obvious only in later life, that is, in long-term survivors of HLH. Specific testing would be necessary to determine the low-frequency hearing threshold42 and to better characterize the specific defect within the inner ear.43 It therefore remains speculative if the underlying pathomechanism in patients with FHL5 is associated with defective signal transduction mechanisms in the inner ear or rather is the result of an immunologic or inflammatory process. Future detailed studies in patients with FHL5 and those with other genetic background are recommended to understand this phenomenon and its possible relation with specific genetic defects.
Overlapping symptoms with chronic variable immunodeficiency such as hypogammaglobulinemia have already been described in patients with FHL5.26,27 Ten patients of our cohort were reported to have persistent hypogammaglobulinemia. Most of them belonged to group 2, harboring an exon 15 splice-site mutation as did the patients reported by Meeths et al.26 The finding that patients with FHL3 also may be affected and that hypogammaglobulinemia was not preexistent but developed during the course of the disease argues against a specific causative role of STXBP2 mutations. The demonstration of an impaired B-cell maturation in some patients favors a role of a defective B- to T-cell interaction for the development of hypogammaglobulinemia in the persistent hyperinflammatory condition of HLH. It might therefore become only evident in patients with a long-term course of the disease as is the case in patients with FHL5 with splice-site mutations.
Acquired HLH in association with malignant diseases, especially lymphomas, is a well-known entity. Our cohort includes one patient with Hodgkin disease associated with incomplete HLH symptoms at diagnosis. Genetic HLH was suspected when the HLH symptoms reactivated. Subsequently, a homozygous exon 15 splice-site mutation was identified. So far, it is not known whether carriers of STXBP2 mutations have a higher risk of developing malignant diseases, as has been suggested for patients with PRF1 or STX11 mutations.44,45
In conclusion, we present the so far largest cohort of patients with FHL5 of various ethnic origins with a wide spectrum of mutations and report a clear genophenotype correlation. Patients with FHL5 who carry missense mutations have a higher risk of early onset, severe disease and development of chronic diarrhea. In contrast, patients with exon 15 splice-site mutations are characterized by a later onset and, if HSCT is not performed, frequent mild reactivations that, however, may eventually result in permanent consequences and death. FHL5 should be considered as a differential diagnosis in patients with incomplete HLH symptoms in the presence of additional symptoms, including abnormal bleeding, hearing deficit, or overlapping features with other immunodeficiencies such as hypogammaglobulinemia. In such atypical cases functional analyses that can guide subsequent genotyping are recommended as well. A murine model of STXBP2 deficiency is needed for identifying the underlying pathomechanisms.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Julia Strauß and Manuela Adao for the excellent technical assistance. They thank the patients and their families for their participation and the following physicians for their generous cooperation in this study: Ulrich Glöckel, Regina Wieland, Michael Frühwald, Lisa Lassay, Klaus Kapelari, Wilhelm Wössmann, Jeanette Greiner, Barbara Schütz, and Anne Marie Gerdes.
This work was supported by grants from the Bundesministerium für Bildung und Forschung (BMBF; grant BMBF 01 EO 0803, S.E.), the European Union (EU FP7 grant agreement HEALTH-F2-2008-201461), and Fördergemeinschaft Kinder-Krebs-Zentrum Hamburg e.V.
Authorship
Contribution: J.P. performed genetic analyses, collected clinical data, and wrote manuscript; K.B. analyzed and interpreted clinical data and wrote manuscript; K.L. collected data and performed statistical analyses; F.K., A.M.-P., and S.E. performed research and analyzed and interpreted data; G.J. and U.z.S. designed research and analyzed and interpreted data; A.-K.R. analyzed and interpreted clinical data; A.A.-J., R.B., L.B.O., K.E., U.G.-W., N.J., B.K., A.P., M.S.-S., E.M., and A.W. cared for patients and provided clinical data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gritta Janka, Pediatric Hematology and Oncology, University Medical Center Hamburg-Eppendorf, Martinistrasse 52, 20246 Hamburg, Germany; e-mail: janka@uke.uni-hamburg.de.
References
Author notes
J.P. and K.B. contributed equally to this study.
U.z.S. and G.J. contributed equally to this study.