Abstract
Cyclooxygenase 2 (COX-2) is an inflammatory enzyme involved in the pathogenesis and prognosis of several malignancies. In the present study, we investigated the prognostic value of COX-2 expression in a large (N = 242), uniformly treated Hodgkin lymphoma (HL) population from the Spanish Network of HL using tissue microarrays. Univariate and multivariate analysis was done, including comparing the most recognized clinical variables: the early- and advanced-stage subgroups. COX-2 was expressed on Reed-Sternberg cells in 37% of patients. There were no differences in the distribution of clinical variables according to COX-2 expression. With a median follow-up time of 58 months, PFS at 5 years was 60% and 79% for COX-2+ and COX-2− patients, respectively (P = .003). The overall survival was 73% and 91%, respectively (P < .001). The major impact on prognosis was observed in the early AA stage (I-II) group. In fact, in these low-risk groups the expression of COX-2 defined a group with significantly worse progression-free and overall survival. In conclusion, COX-2 was expressed on Reed-Sternberg cells in one-third of HL patients and was a major independent, unfavorable prognostic factor in early-stage HL. We conclude that COX-2 may be a major prognostic variable in HL and a potential therapeutic target.
Introduction
Hodgkin lymphoma (HL) represents 10%-15% of lymphoma cases.1 At present, approximately 80%-90% of patients in the early and limited stages can be cured.2 In fact, the behavior of the disease is determined by intrinsic features of tumor cells, Reed-Sternberg (RS) cells, and the characteristics of the tumor microenvironment. This microenvironment is basically composed of several other cell populations associated with their respective extracellular matrix compartment, which includes a deregulated cytokine network with secretion of inflammatory cytokines.3,4 Recently, an increased number of tumor-associated macrophages have been related to short survival, representing a new biomarker for risk prediction.5
Cycloxygenase-2 (COX-2) is an inflammation-associated enzyme involved in the pathogenesis and prognosis of several solid malignancies (eg, colorectal, breast, ovarian, and lung).6,7 Its role in hematologic malignancies has been recently recognized.8,9 It has also been reported that COX-2 constitutes an independent prognostic variable in multiple myeloma10 and also that a significant proportion of HL patients express COX-2, because this expression is associated with proliferation and angiogenesis.8 Furthermore, it is claimed that COX-2 up-regulates several key survival and proangiogenic factors that contribute to tumor growth and survival.11
Aside from its prognostic value in multiple myeloma, the prognostic value of COX-2 has not been studied in any other hematologic malignancy. Current prognostic indexes in this disease are based on clinical variables12 ; however, it could be interesting to evaluate biologic markers for both the RS malignant cell and the inflammatory microenvironment that could add prognostic and pathogenic information to the known important clinical variables in this disease.
Given the characteristic proinflammatory milieu of HL, in the present study, we investigated the prognostic value of COX-2 expression in a large series of uniformly treated HL patients with the standard ABVD (Adriamycin, bleomycin, vinblastine, dacarbazine) regimen with or without radiotherapy (RT).
Methods
Patients
We included 242 patients retrospectively who had been uniformly treated with ABVD (2-8 cycles, in function of risk factors) with or without RT (more or less intensive regimens were excluded). All of the samples were collected with the collaboration of the Spanish National Tumor Bank Network, coordinated by the Spanish National Cancer Research Center, following the technical and ethical procedures of the network, including anonymization processes. Approval was obtained from the Spanish National Cancer Research Center institutional review board. Informed consent was obtained in all cases in accordance with the Declaration of Helsinki.
All patients were treated with curative intent, pretreatment specimens were adequate, and information was available about staging and main clinical prognostic factors. Based on this information, an International Prognostic Score (IPS) could be determined for all patients. For early-stage HL patients, we included European Organization for Research and Treatment of Cancer (EORTC) prognostic factors defining favorable and unfavorable subsets.13 Response assessment and follow-up was based on Cheson criteria.14
TMA design
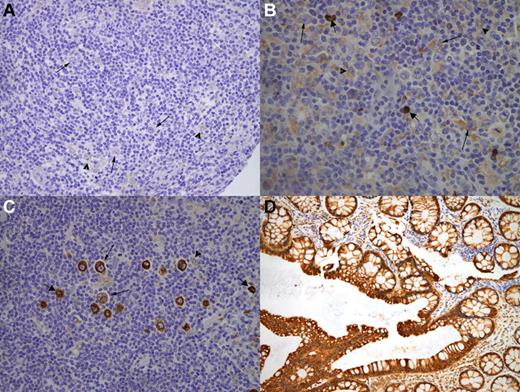
Immunohistochemical expression of COX-2 was assessed using tissue microarray (TMA) technology. We used a Tissue Arrayer device (Beecher Instrument) as described previously.15 Briefly, all HL cases were reviewed histologically and the richest areas of RS cells were marked in the paraffin blocks. In each case, 2 selected 1-mm-diameter cylinders from 2 different areas were included, along with different controls to ensure the quality, reproducibility, and homogenous staining of the slides. A total of 6 different TMA blocks were constructed, each containing 120-140 cylinders. Included in each TMA was a representation of reactive lymphoid tissue of tonsil as an internal control and normal colon tissue as a positive control for COX-2 expression (Figure 1D).
Immunohistochemistry
TMA blocks were sectioned at a thickness of 3 μm and dried for 16 hours at 56°C before being dewaxed in xylene and rehydrated through a graded ethanol series and washed with PBS. Antigen retrieval was achieved by heat treatment in PT LINK with pH High buffer (DAKO).
Immunohistochemical staining for COX-2 was performed on these sections with prediluted rabbit monoclonal clone SP21 (RM-9121-R7; Neomarkers). The complete immunodetection system was performed on an Autostainer PLUS with EnVision FLEX kit using diaminobenzidine chromogen as a substrate. Sections were counterstained with hematoxylin.
The staining of the TMA sections was evaluated by the consensus of 2 pathologists using uniform criteria. To guarantee the reproducibility of this method, we decided to use straightforward and clear-cut criteria. The pattern of staining for COX-2 was recorded as positive or negative taking into account the cytoplasmic/membranous staining, being positive when more than 10% of RS cells expressed COX-2 (Figure 1). To consider a case evaluable for the study, it had to have at least 10 RS cells in at least 1 of the 2 core cylinders analyzed in each patient. The reproducibility of the results obtained was confirmed by comparing them with those from whole sections in 34 randomly selected cases that were stained using the same procedure.
Uniform criteria for evaluation of RS COX-2 staining. Representative immunohistochemistry cases from the tissue microarrays of negative RS COX-2 staining (A), low positive RS COX-2 staining (B), or high positive RS COX-2 staining (C). All cases are presented at a 40× magnification. Thin arrows indicate RS cells; arrowheads, histiocytes; thick arrows, plasma cells. Colon mucosa was used as a positive control for COX-2 expression (D).
Uniform criteria for evaluation of RS COX-2 staining. Representative immunohistochemistry cases from the tissue microarrays of negative RS COX-2 staining (A), low positive RS COX-2 staining (B), or high positive RS COX-2 staining (C). All cases are presented at a 40× magnification. Thin arrows indicate RS cells; arrowheads, histiocytes; thick arrows, plasma cells. Colon mucosa was used as a positive control for COX-2 expression (D).
Statistical methods
To study the relationship between COX-2 and clinical variables, we stratified the whole population into 2 cohorts (early and advanced HL). The Pearson χ2 test and Fisher exact test were used where appropriate. Overall survival (OS) and progression-free survival (PFS) were measured from the date of diagnosis and were estimated according to the Kaplan-Meier method. Comparisons between the variables of interest were performed by the log-rank test. Multivariate analysis with the variables that appeared to be significant in the univariate analysis was performed according to the Cox proportional hazard regression model. All P values reported were 2-sided and statistical significance was defined at P < .05.
Results
Patient characteristics
As shown in Table 1, the median age was 31 years for the early HL cohort (n = 143) and 36 for the advanced HL (n = 99) cohort. Comparing the early and advanced HL groups: 30% and 56% presented B symptoms, 30% and 27% had bulky disease, and 6% and 16% had an Eastern Cooperative Oncology Group Performance Score (ECOG PS) higher than 1, respectively. For early HL, the EORTC score was unfavorable in 84% of the patients. The IPS was 3 or higher in 45% of the patients with advanced disease. A total of 124 patients (51%) received RT added to ABVD chemotherapy, with 66% of these patients presenting with AA stage I-II and 30% presenting with AA stage III-IV. Histologic subtypes were as follows: 62% nodular sclerosis classic HL (CHL), 32% mixed cellularity CHL, 3% lymphocyte-depleted CHL, and 3% lymphocyte-rich CHL.
Most patients with high-risk features such as III-IV AA stage (88%), with IPS > 2 (93%) or bulky disease (93%) received 6-8 cycles of chemotherapy with or without involved-field RT. In the localized I-II AA stage group, most of the patients with high-risk features such as IPS > 2 (89%) or bulky disease (95%) were either consolidated with involved-field RT or received more chemotherapy cycles. To control treatment variability, we considered short (2-4 cycles) and long (6-8 cycles) treatments in all outcome statistical analysis. Because treatment was adapted considering stage and risk features, it had no influence on outcome, as shown in Tables 2, 3, and 4.
COX-2 expression
We evaluated COX-2 cytoplasmic/membranous staining in RS cells. An analysis comparing the results obtained using TMAs and the whole sections in 34 randomly selected cases showed a concordance of 88%, a figure that is consistent with previously reported results for other tumoral models.16 In addition, intraobserver and interobserver reproducibility were 97% and 90%, respectively.
COX-2 staining on RS cells was as follows: 89 patients (37%) showed positive staining and 153 (63%) had no staining. COX-2 staining was not related to any of the clinical variables. However, there was a trend toward a higher positivity percentage with a higher AA stage (P = .074) and this trend was also observed in the nodular sclerosis and lymphocyte-depleted subtypes (P = .088).
Outcome and survival analysis of the global group
With a median follow-up time of 58 months (range, 8-199) for patients alive, the variables of age ≥ 60 years, presence of B symptoms, AA stage III-IV, PS ≥ 2, no RT, and COX-2 expression were associated with an unfavorable PFS and OS (Table 2). The PFS at 5 years was 60% and 79% for COX-2+ and COX-2−, respectively (P = .003), whereas the OS at 5 years was 73% and 91% (P < .001), respectively. By multivariate analysis, B symptoms (hazard ratio [HR] = 2.04), COX-2 expression (HR = 1.91), age ≥ 60 years (HR = 1.89), and RT (HR = 0.54) were independently associated with PFS, whereas age ≥ 60 years (HR = 4.34), ECOG PS > 1 (HR = 4), and COX-2 expression (HR = 2.95) were independently associated with OS. RS+ COX-2 staining was not different in patients responding to first-line therapy with complete remission (35%) and less than complete remission (48%; P = .17).
Outcome and survival subanalysis according to AA stage
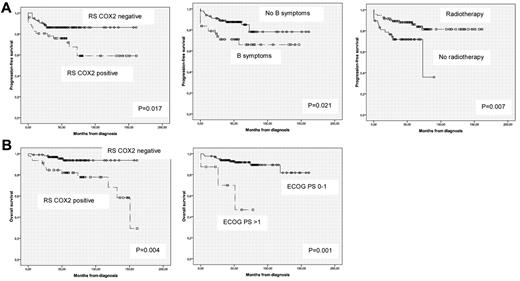
In Tables 3 and 4, we present a subanalysis of PFS and OS of patients with localized (I-II AA stage) and advanced (III-IV AA stage) HL. Interestingly, the major impact in the prognosis was observed in the favorable AA stage (I-II) group. In fact, in these low-risk groups, the expression of COX-2 defined a subgroup with significantly worse prognosis. Accordingly, the 46 (32%) COX-2+ patients of the 143 patients presenting with an AA stage I-II had a PFS at 5 years of 72% versus 86% of COX-2− patients (P = .017). Similarly, the OS at 5 years also differed for both groups, with 82% and 94% for COX-2+ and COX-2−, respectively (P = .004). By multivariate analysis, COX-2 expression (HR = 2.76), B symptoms (HR = 2.34), and administration of RT (HR = 0.32) were independently associated with PFS (Figure 2A), whereas only ECOG PS (HR = 9) and COX-2 RS expression (HR = 4.81) were independently associated with OS (Figure 2B). The EORTC unfavorable subset tended to have a worse PFS and OS, but this did not reach statistical significance, possibly because we could only obtain this information in 83 of the 143 (58%) patients with early HL.
Prognostic factors independently associated with outcome in early-stage HL patients. (A) Prognostic factors associated with PFS. (B) Prognostic factors associated with OS.
Prognostic factors independently associated with outcome in early-stage HL patients. (A) Prognostic factors associated with PFS. (B) Prognostic factors associated with OS.
For patients with advanced HL, only IPS > 2 (HR = 2.88) was independently associated with a worse PFS, whereas age ≥ 60 years (HR = 5.07) and ECOG PS > 1 (HR = 3.38) were independently associated with OS (Figure 3A). In this advanced-stage population, COX-2 RS expression only showed a tendency toward worse PFS and OS (Figure 3B and Table 4).
Prognostic factors independently associated with outcome in advanced-stage HL patients. (A) Prognostic factors associated with PFS and OS. (B) Role of COX-2 RS expression in advanced-stage HL.
Prognostic factors independently associated with outcome in advanced-stage HL patients. (A) Prognostic factors associated with PFS and OS. (B) Role of COX-2 RS expression in advanced-stage HL.
Discussion
More than 70% of patients with HL can be cured with currently available therapeutic strategies.17 Current risk systems are able to properly identify patients with worse prognosis in advanced stages of the disease. However, the use of prognostic factors is much less uniform in early HL. Whereas trials and recommendations in the United States and the United Kingdom often distinguish between nonbulky and bulky early-stage HL, the EORTC defines distinct risk subsets for adapting the intensity of treatment.13 Therefore, in patients with early-stage HL, it is particularly important to find prognostic factors associated with a worse outcome.
As far as we know, our finding of COX-2 expression associated with poor prognosis in HL is the first observation in this disease. In solid tumors (eg, head and neck, ovarian, breast, and lung), this relationship between COX-2 expression and poor prognosis has been associated with pathogenic phenomena such as induction of angiogenesis, chemoresistance through induction of antiapoptotic mechanisms such as up-regulation of bcl-2 or resistance to Fas, or promotion of invasion through induction of some metalloproteinases.18 The role of COX-2 in HL is basically unknown, although it has been associated with higher proliferation and angiogenesis.8 In the present study, we observed that RS cells express this marker in approximately one-third of cases.
It may be that the COX-2 pathway is important in the pathogenesis of this disease, which is characterized by a small tumor cell population in a milieu composed of a deregulated cytokine and chemokine network and several proinflammatory and immune cell populations that contribute to the maintenance of the neoplasia by several antiapoptotic and survival mechanisms.19 Our findings of its value as a key prognostic biomarker in this disease are important, because current risk systems are based on clinical variables and therefore relevant biologic information could be important to implement new and more specific therapies. Moreover, our identification of a sizable group of approximately 30% of patients who have a significantly worse prognosis because they express COX-2 will be valuable in the implementation of new therapeutic strategies.
Furthermore, the identification of a subgroup in the limited stages of the disease may facilitate decisions in 2 opposite directions. The COX-2− subgroup with an OS of 94% and a PFS of 82% may be the target of trials focusing on limiting treatment toxicity while maintaining efficacy. Conversely, even the apparently excellent prognostic COX-2+ subgroup with a PFS of 73% may need a more intense therapy than what is performed currently, such as a higher number of ABVD cycles, more intensive regimens such as BEACOPP, or testing the use of novel agents targeting this pathogenic pathway. This is important, because in limited stages or low-risk groups of HL, no prognostic risk factor system published so far can reliably identify patients with worse prognosis. Furthermore, we discarded a potential bias associated with the possibility that some patients requiring more treatment could be undertreated. In fact, outcome was not worse in patients receiving short treatments in either the univariate or multivariate analysis, as shown on Tables 2, 3, and 4, because this treatment decision considered stage and risk features.
However, in the advanced-disease groups, we could only observe a trend toward a lower OS and PFS in patients expressing COX-2 (Figure 3B). Perhaps multiple biologic derangements in these advanced stages of the disease make this single biomarker unable to provide meaningful independent information. Another potential explanation is that the increased therapies that these patients are receiving may overcome the COX-2+ phenotype. For this reason, these patients could also benefit from more intense regimens such as BEACOPP, frontline autologous stem cell transplantation, or novel agents.
We cannot determine why COX-2 expression is important in this disease based on the results of the present study. The pathogenic importance of COX-2 has been reported in the more comprehensively studied solid tumors. In fact, COX-2 expression has been associated with several antiapoptotic pathways, such as induction of bcl-2 and subsequent survival mechanisms, or by induction of ATM and ATR in some solid tumors.20 Furthermore, COX-2 has been associated with chemoresistance and radiation resistance by inducing neoangiogenesis and also because of its strong correlation with the expression of the MDR gene.21
Nevertheless, the specific role of the COX-2 pathway in the pathogenesis of hematologic malignancies is not known. For example, Ladetto et al reported the association of COX-2 expression and poor prognosis in multiple myeloma and suggested a reciprocal interaction of COX-2 and IL-6, a major biomarker involved in the pathogenesis of multiple myeloma.10 In fact, both COX-2 and IL-6 up-regulate each other reciprocally, suggesting the possibility of a biologic loop. Interestingly, IL-6 is reported to be an unfavorable prognostic marker of HL.22 In the same way that COX-2 induces VEGF indirectly through induction of HIF-α, an association of COX-2 and promotion of angiogenesis has been proposed. Moreover, the adverse prognosis that markers of neoangiogenesis confer to patients with HL is much more established.23-26
Given the importance of COX-2 as a prognostic biomarker and given the probability that it plays an important pathogenic role in this disease, one wonders whether it could be used as a therapeutic target in HL, as it is already used in solid tumors with promising efficacy.27,28 However, the presently available COX-2 inhibitors have serious potential toxicity, making their introduction difficult in the clinical practice of HL.29,30 Interestingly, Fujita et al recently reported inhibition of COX-2 by thalidomide and other immunomodulatory drugs.31 Lenalidomide has demonstrated promising efficacy in HL,32 and therefore may be associated with COX-2 inhibition, among multiple other effects, and COX-2 may be a predictor of efficacy for this drug. This is another example of the new paradigm of tumor treatment by targeting the tumor microenvironment. In HL, this therapeutic approach was used by Younes et al, who incorporated rituximab targeting the B-cell component of the tumor into the current ABVD regimen.33,34
In summary, for the first time to our knowledge, in the present study, we report the value of RS COX-2 expression as a prognostic factor in HL. We have identified a hidden subgroup of patients with theoretically excellent prognosis who have a significantly worse prognosis associated with the expression of this marker. The use of COX-2 as a meaningful prognostic variable and potential therapeutic target needs to be confirmed in other independent studies. If both facts are confirmed, trials incorporating drugs that inhibit its expression are warranted.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The following centers and investigators participated in the Spanish Hodgkin Lymphoma Study Group and contributed to these studies with tumor samples and clinical data: R. Ramos, A. Gutiérrez, J. Rodríguez, and F. Mestre (Hospital Son Dureta, Palma de Mallorca, Spain); P. Domínguez and C. Jara (Fundación Hospital Alcorcón, Madrid, Spain); M. J. Mestre, R. Quibén, M. Méndez, and L. Borbolla (Hospital de Móstoles, Madrid, Spain); M. A. Martínez and C. Grande (Hospital 12 de Octubre, Madrid, Spain); M. García-Cosío, C. Montalbán, and J. García-Laraña (Hospital Ramón y Cajal, Madrid, Spain); M. Canales and J. Alves (Hospital La Paz, Madrid, Spain); C. Bellas and M. Provencio (Hospital Puerta de Hierro, Madrid, Spain); A. Castaño and P. Sánchez-Godoy (Hospital Severo Ochoa, Leganés, Madrid, Spain); C. Martín and R. Martínez (Hospital Clínico Universitario San Carlos, Madrid, Spain); J. Menárguez, P. Sabín, and E. Flores (Hospital Gregorio Marañón, Madrid, Spain); J. González-Carrero and C. Poderós (Hospital Xeral-Cies, Vigo, Spain); A. Salar and S. Serrano (Hospital del Mar, Barcelona, Spain); T. Álvaro and L. Font (Hospital Verge de la Cinta, Tortosa, Spain); V. Romagosa and A. Fernández de Sevilla (Hospital Duran i Reynalds, Institut Catala d'Oncologia, Barcelona, Spain); M. Mollejo and M. A. Cruz (Hospital Virgen de la Salud, Toledo, Spain); A. Cánovas and C. Camarero (Hospital de Cruces, Baracaldo, Spain). H. Álvarez-Arguelles and M. Llanos (Hospital Universitario Canarias, Tenerife, Spain); R. Arranz and A. Acevedo (Hospital La Princesa, Madrid, Spain); R. García-Sanz and T. Flores (Hospital Universitario de Salamanca, Spain); C. Morante (Hospital Cabueñes, Gijón, Spain); A. Marín, E. Ríos (Hospital Virgen del Rocío, Seville, Spain); F. Mazorra and E. Conde (Hospital Marqués de Valdecilla, Santander, Spain); M. F. Fresno, C. Rayón, and C. Nicolás (Hospital Central de Asturias, Oviedo, Spain); C. Santonja and J. L. López (Fundación Jiménez Díaz, Madrid, Spain); T. Flores and R. García-Sanz (Hospital Universitario de Salamanca, Salamanca, Spain); J. Guma (Hospital Sant Joan, Reus, Spain); P. Gonzalvo (Hospital Comarcal de Jarrio, Coaña, Spain); G. Fernández (Hospital Alvarez Buylla, Mieres, Spain); J. Forteza, M. Fraga, and J. L. Bello (F Med Santiago de Compostela, Spain); A. Bas (Hospital Universitario Virgen de la Arrixaca, Murcia, Spain); J. R. Méndez (Hospital Valle de Nalón, Asturias, Spain); J. F. Tomás and M. Estevez (M.D. Anderson España, Madrid, Spain); C. Ruiz-Marcellán and A. López (Hospital Vall d′Hebron, Spain); and J. F. García, M. M. Morente, and M. A. Piris (Centro Nacional de Investigaciones Oncológicas, Madrid, Spain).
This work was supported by grant number DGAVAL_PI_044/10 from Govern Illes Balears and by a research project grant from Asociación Española Contra el Cáncer-Junta Balears (7/2010).
Authorship
Contribution: F.M., A.G., J.M.-S., T.R., and J.R. collected and analyzed the data; A.G. and J.R. designed the research and wrote the manuscript; and R.R., L.S., G.M., and J.F.G. provided expert histopathologist analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr J. Rodríguez, Department of Internal Medicine. University Hospital Severo Ochoa, Avenida de Orellana s/n 28911 Leganes, Madrid, Spain; e-mail: joseguez89@hotmail.com.
References
Author notes
F.M. and A.G. contributed equally to this work.