Induced pluripotent stem cells (iPSCs) can be generated by the expression of defined transcription factors not only from normal tissue, but also from malignant cells. Cancer-derived iPSCs are expected to provide a novel experimental opportunity to establish the disease model. We generated iPSCs from imatinib-sensitive chronic myelogenous leukemia (CML) patient samples. Remarkably, the CML-iPSCs were resistant to imatinib although they consistently expressed BCR-ABL oncoprotein. In CML-iPSCs, the phosphorylation of ERK1/2, AKT, and JNK, which are essential for the maintenance of both BCR-ABL (+) leukemia cells and iPSCs, were unchanged after imatinib treatment, whereas the phosphorylation of signal transducer and activator of transcription (STAT)5 and CRKL was significantly decreased. These results suggest that the signaling for iPSCs maintenance compensates for the inhibition of BCR-ABL. CML-iPSC–derived hematopoietic cells recovered the sensitivity to imatinib although CD34+38−90+45+ immature cells were resistant to imatinib, which recapitulated the pathophysiologic feature of the initial CML. CML-iPSCs provide us with a novel platform to investigate CML pathogenesis on the basis of patient-derived samples.
Introduction
Hematologic malignancies including leukemias are often chemotherapy-resistant, most of which follows an aggressive clinical course.1 Multiple drug therapies are usually required to treat them, although they are occasionally accompanied with many side effects. Thus, the invention of novel targeted therapies based on newly revealed molecular pathogenesis is expected to overcome the current situation.2 However, previous approaches to understanding pathogenesis involve several limitations. Many mouse models of human diseases have been established, but they may not fully recapitulate many aspects of original human diseases.3 Many kinds of cell lines are also available for research. However, they do not cover all diseases, because it is usually difficult to establish a cell line from a primary patient sample. Furthermore, additional gene mutations may be accumulated in cell lines. Theoretically, primary patient samples should be used for research, but the amount of obtained cells may be inadequate for various analyses.
Induced pluripotent stem cells (iPSCs) can be generated from various types of cells by the transduction of defined transcription factors.4,,,,,–10 In addition to the regenerative medicine,11 iPSCs have been used for studies of the pathogenesis of inherited genetic diseases.12,,,–16 Recently, it was reported that iPSCs were generated not only from normal tissue cells, but also from malignant cells.17,,–20 In those cases, cancer cells themselves must have been the origins of iPSCs. However, in most published data, established cell lines were used as the source material of cancer cells, including chronic myelogenous leukemia (CML),17 gastrointestinal cancers,18 and melanoma,19 except for the JAK2-V617F mutation (+) polycythemia vera (PV) patient.20
CML is a myeloproliferative neoplasm that originates from hematopoietic stem cells transformed by the BCR-ABL fusion gene. The initial indolent chronic phase (CP) is followed by aggressive stages, the accelerated phase (AP), and the blast crisis (BC), in which immature leukemic cells expand.21 CML is now initially treated with one of several tyrosine kinase inhibitors (TKIs) including imatinib, dasatinib, and nilotinib, which have dramatically improved the long-term survival rate of CML patients up to approximately 90%. However, even TKIs are not able to eradicate the CML clone completely, which is demonstrated by the fact that discontinuation of TKIs in molecular remission CML patients usually leads to the recurrence of the BCL-ABL clone. Therefore, many studies are performed to elucidate the mechanisms of TKI-resistance in CML stem cells and to overcome the resistance.
In this study, we established iPSCs from primary CML patient samples, redifferentiated them into hematopoietic lineage and showed the recapitulation of the pathophysiologic features of the initial disease.
Methods
Cell and cell culture
Primary samples of CML bone marrow cells were obtained after informed consent. All studies using human cells were reviewed and approved by the institutional review boards (IRBs) of University of Tokyo. Mononuclear cells (MNCs) were isolated by centrifugation through a Ficoll gradient. CD34+ cells were isolated by an immunomagnetic separation technique (auto magnetic-activated cell sorting; MACS). They were cultured with α-minimum essential medium (MEM) containing 20% fetal calf serum (FCS) supplemented with 100 ng/mL stem cell factor (SCF; Wako), 10 ng/mL thrombopoietin (TPO; Wako), 100 ng/mL FL3L (Wako), 10 ng/mL IL3 (Wako), and 100 ng/mL IL6 (Wako).
Normal iPSCs established from cord blood (CB) CD34+ cells or fibroblasts22 and CML-iPSCs were maintained in Dulbecco modified Eagle medium-F12 (Invitrogen) supplemented with 20% knockout serum replacement (KSR; Invitrogen), 0.1mM 2-mercaptoethanol (Sigma-Aldrich), MEM nonessential amino acids (Invitrogen), and 5 ng/mL recombinant human basic fibroblast growth factor (FGF; Peprotech) on mitomycin C (MMC)–treated mouse embryo fibroblast (MEF) feeder cells.23 Imatinib (LC Laboratories) was added to the culture medium at the various concentrations (1-10μM). U0126 and LY294004 (LC Laboratories) were used to inhibit ERK and AKT, respectively.
The mouse C3H10T1/2 cells were cultured as previously described.24
Production of VSV-G pseudotyped retroviral particles
Construction of pMXs vectors encoding Oct3/4, Sox2, Klf4, and c-myc were performed as previously described.22 Highly concentrated VSV-G–pseudotyped retroviral supernatant was prepared using reported procedures. The 293GPG cells were kind gifts from Dr R. C. Mulligan (Children's Hospital Boston, Harvard Medical School, Boston, MA).25 Stable 293GPG cell lines, each capable of producing VSV-G–pseudotyped retroviral particles on induction were established as previously described.22,25 Retroviral supernatants were concentrated by centrifugation for 16 hours at 6000g.
Generation of iPSCs from CML samples
Two days before infection, cells were stimulated with cytokines as mentioned in “Cell and cell culture.” For infection, each well of a 24-well dish coated with a fibronectin fragment CH296:RetroNectin (Takara-Bio) was covered with virus-containing supernatants. After the adhesion of viruses according to the manufacture's recommendation, 1 × 105 cells of CD34+ CML cells or CB cells were inoculated into each well and filled with the culture medium supplemented with cytokines. The next day, concentrated viral supernatant was added to the culture. On day 3 after infection, cells were harvested with vigorous pipetting, washed by phosphate-buffered saline (PBS), and cultured with the same fresh medium for next 3 days. On day 6, cells were seeded on MMC treated MEF cells. Two to 4 days after, the medium was replaced with human ES medium as previously described with 0.5mM valproic acid (VPA; Sigma-Aldrich).26 Subsequently, medium was changed every other day. After 20 days, ES-like colonies appeared. Using live cell imaging technology with Tra-1-60 antibody as previously described,27 each fully reprogrammed colony was distinguished from deficiently reprogrammed colonies, and was picked up to be reseeded on new MEF feeder cells. Cloned ES-like colonies were subjected to further analysis.
Antibodies, FACS analysis, and immunocytochemistry
The following fluorescent conjugated antibodies were used for fluorescence-activated cell sorter (FACS) analysis and immunocytochemistry: anti–human stage specific embryonic antigen (SSEA)–4 conjugated with Alexa Fluor 488 (BD Bioscience), anti–human tumor related antigen (TRA)–1-60 conjugated with Alexa Fluor 555 (BD Bioscience), anti-CD34 phycoerythrin (PE) conjugated (Beckman Coulter), and anti-CD45 fluorescein isothiocyanate (FITC) conjugated (Beckman Coulter).
Cells were sorted with a FACSAria, and analysis was performed on FACS LSRII (BD Bioscience).
For immunocytochemistry, cells were fixed with 4% paraformaldehyde in PBS, after which they were labeled with an antibody against human SSEA-4 and antibody against human TRA-1-60 antibody and observed using a confocal microscope (Carl Zeiss).
Methylation profiling
Genomic DNA was extracted using the QIAamp DNA Mini Kit (QIAGEN) according to the manufacture's instruction. Methylation status was evaluated as previously reported.28 Methylation status was analyzed using HumanMethylation27 BeadChip (Illumina). Genomic DNA for methylation profiling was quantified using the Quant-iT dsDNA BR assay kit (Invitrogen). Five-hundred nanograms of genomic DNA was bisulfite-converted using an EZ DNA methylation kit (Zymo Research). The converted DNA was amplified, fragmented and hybridized to a beadchip according to the manufacturer's instructions. The raw signal intensity for both methylated (M) and unmethylated (U) DNA was measured using a BeadArray Scanner (Illumina). The methylation level of the each individual CpG is obtained using the formula (M)/(M) + (U) + 100 by the GenomeStudio (Illumina).
Microarray analysis
Gene expression analysis was carried out as previously described29 with the use of the Human Genome U133 Plus 2.0 Array (Affymetrix). The hierarchical clustering techniques classify data by similarity and their results are represented by dendrograms. Previously reported data of human embryonic stem (ES) cells (GSM449729) and CML CD34+ cells (GSM366215, 366216, 366221, and 366222) were used to compare the gene expression profile. The microarray data are available on the Gene Expression Omnibus (GEO) database under accession number GSE37982.
Hematopoietic differentiation of iPSCs
To differentiate iPSCs into hematopoietic cells, we used the same protocol previously used with ES cells and iPSCs.22,24 In brief, small clusters of iPSCs (< 100 cells treated with PBS containing 0.25% trypsin, 1mM CaCl2, and 20% KSR) were transferred onto irradiated 10T1/2 cells and cocultured in hematopoietic cell differentiation medium, which was refreshed every third day. Differentiation medium consists of Iscove modified Dulbecco medium supplemented with a cocktail of 10 μg/mL human insulin, 5.5 μg/mL human transferrin, 5 ng/mL sodium selenite, 2mM l-glutamine, 0.45mM α-monothioglycerol, 50 μg/mL ascorbic acid, and 15% highly filtered FBS in the presence of 20 ng/mL human vascular endothelial growth factor (VEGF).24 On days 14 to 15 of culture, the iPS-sacs were collected into a 50-mL tube, gently crushed with a pipette tip and passed through a 40-μm cell strainer to obtain hematopoietic progenitors. Hematopoietic progenitors were collected by sorting with CD34 and CD45 antibodies, Giemsa stained, and then examied under a microscope. Hematopoietic progenitors were cultured in the α-medium plus 20% FCS supplemented with 100 ng/mL SCF, 10 ng/mL TPO, 100 ng/mL FL3L, 10 ng/mL IL3, and 100 ng/mL IL6.
Hematopoietic colony-forming cell (CFC) assay
CFC assays were performed in MethoCult H4434 semisolid medium (StemCell Technologies). Ten thousand hematopoietic progenitors harvested from an iPS-Sacs were plated in 1.5 mL of medium and cultivated for 14 days.
RT-PCR and quantitative real-time PCR analysis
After extraction of total RNA with RNAeasy reagents (QIAGEN), reverse transcription was performed with SuperScript III (Invitrogen). Primer sequences used for the detection of stem cell genes were as previously described.9
Quantitative real-time PCRs (qPCRs) were carried out in the ABI-7000 sequence detection system with SYBR Green PCR Core reagents according to the manufacturer's instructions (Applied Biosystems). We analyzed expression levels of BCR-ABL fusion transcript as previously described.30 Each assay was performed in triplicate and the results were normalized to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) levels.
PCR primers used for quantitative PCR:
BCR-ABL F TCAGAAGCTTCTCCCTGACATCCGT
BCR-ABL R TCCACTGGCCACAAAATCATACAGT
GAPDH F TGCACCACCAACTGCTTAGC
GAPDH R GGCATGGACTGTGGTCATGAG
Western blotting
Fifty micrograms of cell lysates were subjected to sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis. Antibodies used in immunoblotting were as follows; anti-phospho ERK1/2 (Thr202/Tyr204; Cell Signaling), anti-phospho Akt (Ser473; Cell Signaling), anti-phospho JNK (Thr183/Tyr185; Cell Signaling), anti-phospho-STAT5 (Tyr694; Cell Signaling), and anti-phospho CRKL (Tyr207; Cell Signaling). Enhanced chemiluminescence detection (Amersham) was carried out according to the manufacturer's recommendations.
Results
Generation of iPSCs from primary CML patient samples
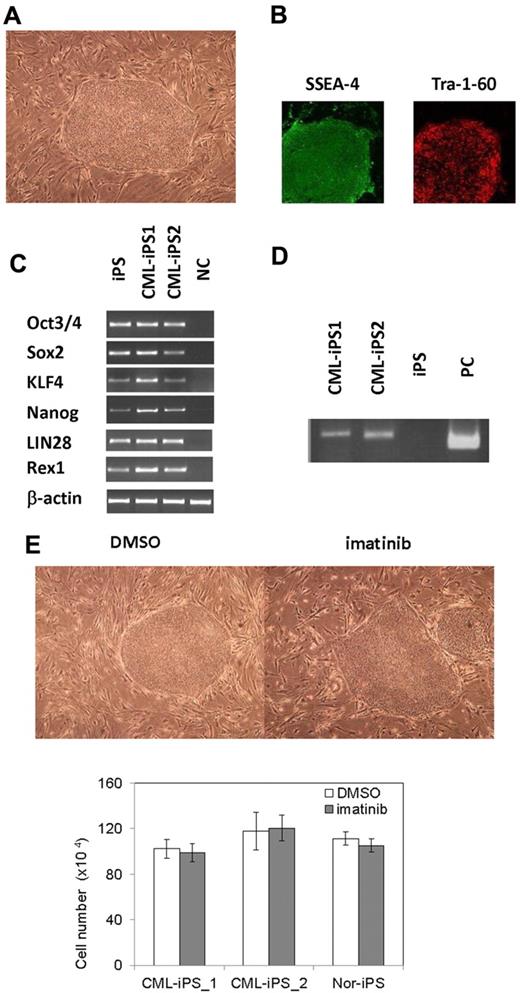
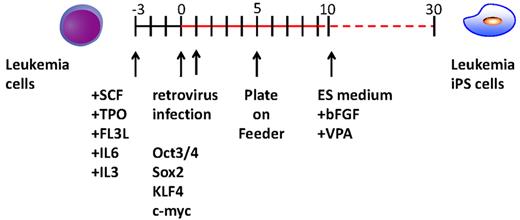
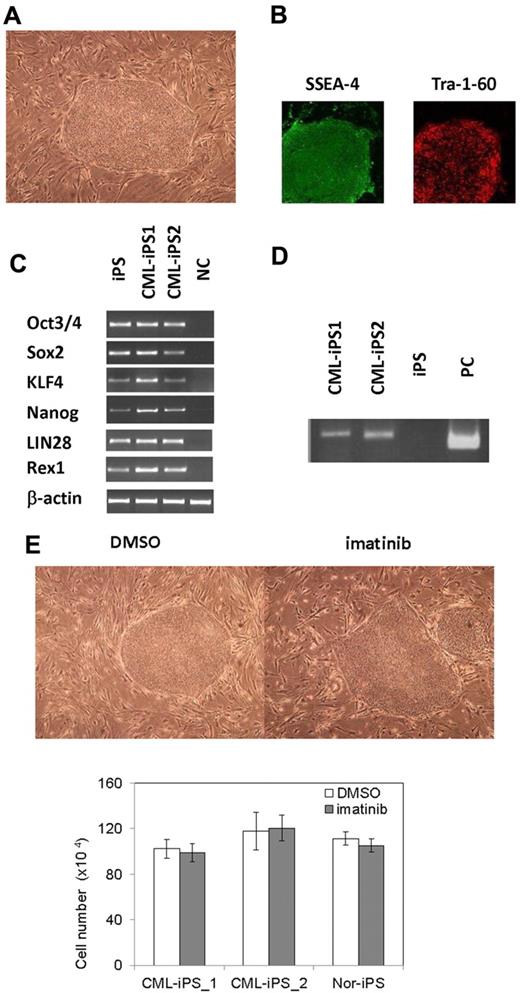
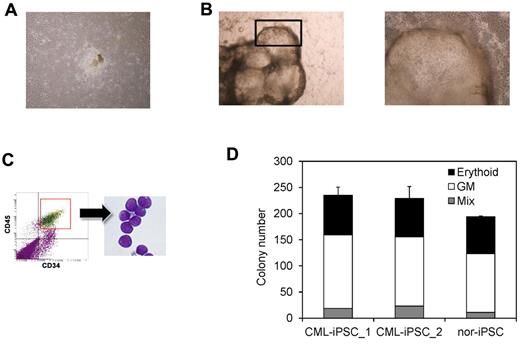
After obtaining informed consent, CD34+ cells were purified from bone marrow mononuclear cells of a CML chronic phase patient. After we stimulated them with cytokines for 2 days, retroviral transduction with the transcription factors OCT3/4, SOX2, KLF4, and MYC was performed. Two days after transduction, we reseeded cells onto MEF cells and cultured them for another 2 days. Then, we replaced the medium with human ES medium supplemented with 5 ng/mL bFGF. To improve the efficiency of the reprogramming, we added VPA,26 a histone deacetylase inhibitor, to the culture (Figure 1). Using a live cell imaging method with Tra-1-60 antibody, bona fide iPSCs were distinguished from deficiently reprogrammed cells.27 As a result, 2 CML-derived iPSCs (CML-iPSCs) were generated, which were derived from independent patients. CML-iPSCs showed the typical morphology as iPSCs (Figure 2A) and expressed the pluripotency markers, such as SSEA-4 and Tra-1-60 (Figure 2B), and the endogenous expression of embryonic stem cell (ESC) characteristic transcripts (OCT3/4, SOX2, KLF4, NANOG, LIN28, and REX1) was confirmed by RT-PCR (Figure 2C). CML-iPSCs also expressed BCR-ABL, which demonstrated that they were truly derived from CML (Figure 2D). Furthermore, fluorescence in situ hybridization with dual color BCR-ABL probes confirmed t(9;22) translocation in CML-iPSCs at the single cell level (supplemental Figure 1A and supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). However, although CML-iPSCs expressed BCR-ABL, they were resistant to imatinib (Figure 2E). Teratoma formation capacity was confirmed, demonstrating the pluripotency of CML-iPSCs (supplemental Figure 2).
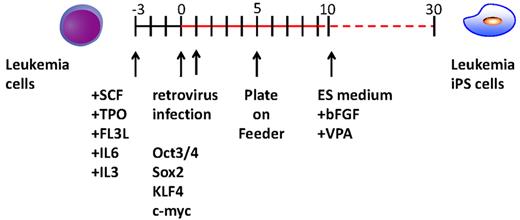
Experimental scheme for generating of iPSCs from the CML patient sample. After cytokine stimulation, CD34+ CML cells were reprogrammed by transduction with Yamanaka factors. To improve the reprogramming, valproic acid was added to the culture.
Experimental scheme for generating of iPSCs from the CML patient sample. After cytokine stimulation, CD34+ CML cells were reprogrammed by transduction with Yamanaka factors. To improve the reprogramming, valproic acid was added to the culture.
Generation of CML derived iPSCs. (A) Morphology of CML-iPSCs. (B) Immunofluirescence staining shows expression of pluripotent marker (left: SSEA-4 and right: Tra-1-60) in CML-iPSCs. (C) RT-PCR analysis of ES cell marker genes. Endogenous expression of these stem cell–specific genes in CML-iPSCs was verified. (D) CML-iPSCs expressed the BCR-ABL fusion transcript. (E) Imatinib (10μM) were added to the culture of iPSCs. DMSO (top left panel) and imatinib (top right panel) treated CML-iPSCs were shown. The number of alive CML-iPSCs (CML-iPS_1 and CML-iPS_2) and normal iPSCs (Nor-iPS) after 5 days treatment was calculated (bottom panel). These were the representative data from 3 independent experiments.
Generation of CML derived iPSCs. (A) Morphology of CML-iPSCs. (B) Immunofluirescence staining shows expression of pluripotent marker (left: SSEA-4 and right: Tra-1-60) in CML-iPSCs. (C) RT-PCR analysis of ES cell marker genes. Endogenous expression of these stem cell–specific genes in CML-iPSCs was verified. (D) CML-iPSCs expressed the BCR-ABL fusion transcript. (E) Imatinib (10μM) were added to the culture of iPSCs. DMSO (top left panel) and imatinib (top right panel) treated CML-iPSCs were shown. The number of alive CML-iPSCs (CML-iPS_1 and CML-iPS_2) and normal iPSCs (Nor-iPS) after 5 days treatment was calculated (bottom panel). These were the representative data from 3 independent experiments.
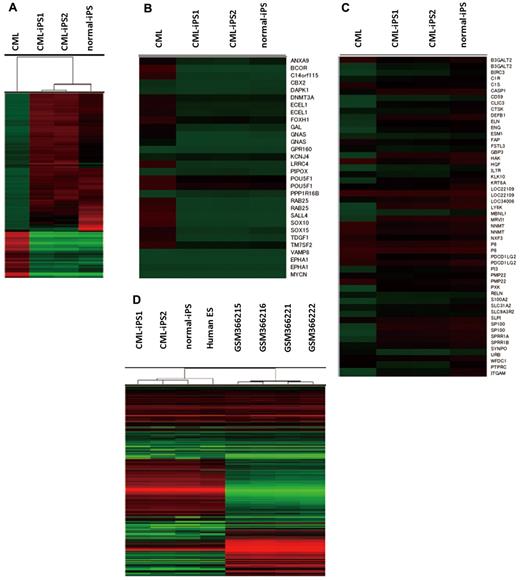
Comprehensive analysis of DNA methylation revealed that methylation pattern of CML-iPSCs was different from that of original CML sample but was very similar to that of normal iPSCs although there were slight differences (Figure 3A). Previously, stem cell–specific differentially methylated regions (SS DMRs) were identified during reprogramming process of iPSCs.31 Hypomethylated SS DMRs (hypo SS DMRs) in the variety of iPSCs were also hypomethylated in the CML-iPSCs including the promoters of OCT4 (Figure 3B). In the same way, hypermethylated SS DMRs (hyper SS DMRs) in the variety of iPSCs were also hypermethylated in the CML-iPSCs (Figure 3C). The promoters of hematopoietic lineage-specific marker genes, such as CD45 and CD11b, were hypermethylated in the CML-iPSCs. Thus, the methylation pattern of CML-iPSCs was confirmed to be not hematopoietic cell-like, but iPSC-like. Next, we compared the gene expression pattern among CML-iPSCs and normal iPSCs (Figure 3D). In a result, CML-iPSCs and normal iPSCs were very similar in regard to global gene expression profile. Furthermore, comparing our results with publicly available expression data of human ES cells and CML CD34+ cells, we found that CML-iPSCs were very similar to human ES cells, whereas they were different from CML CD34+ cells in terms of gene expression patterns (Figure 3D).
Comprehensive analysis of DNA methylation and gene expression. (A) Unsupervised hierarchical clustering based on differentially methylated CpGs is shown on the dendrogram. The accompanying heatmap shows the methylation status across 5001 differentially methylated CpGs. In the heatmap, red indicates a CpG methylation more than 50%, and green less than 50%. The methylation status in hypo SS DMRs (B) or hyper SS DMRs (C) was shown in the heatmap. (D) Unsupervised hierarchical clustering based on global gene expression data are shown on the dendrogram. The accompanying heatmap shows the normalized log2 transformed expression values (Z-scores) for each probe. In the heatmap, red indicates expression more than mean, and green less than mean.
Comprehensive analysis of DNA methylation and gene expression. (A) Unsupervised hierarchical clustering based on differentially methylated CpGs is shown on the dendrogram. The accompanying heatmap shows the methylation status across 5001 differentially methylated CpGs. In the heatmap, red indicates a CpG methylation more than 50%, and green less than 50%. The methylation status in hypo SS DMRs (B) or hyper SS DMRs (C) was shown in the heatmap. (D) Unsupervised hierarchical clustering based on global gene expression data are shown on the dendrogram. The accompanying heatmap shows the normalized log2 transformed expression values (Z-scores) for each probe. In the heatmap, red indicates expression more than mean, and green less than mean.
Hematopoietic differentiation of CML-iPSCs
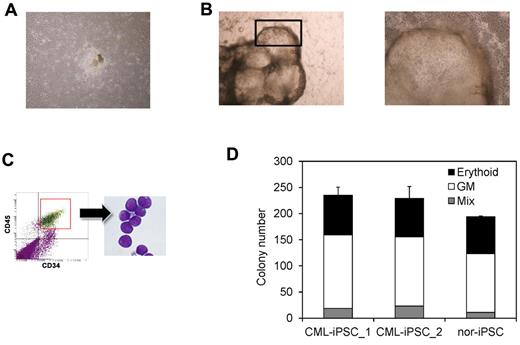
Then we differentiated them into hematopoietic progenitors within the “unique sac-like structures” (iPS-sacs; Figure 4A-B). This method was reported to be able to produce the hematopoietic progenitors with higher efficiency than the usual embryoid body formation method using human ESCs and iPSCs.22,24 On day 15 of culture, iPSCs sacs contained round hematopoietic-like cells (Figure 4B). Then we picked up iPS-sacs with a pipette tip and dissociated them mechanically and obtained the inner round cells. Round cells, positive for a hematopoietic lineage marker CD45 and an immature marker CD34, proved to be hematopoietic progenitors (Figure 4C).
Hematopoietic differentiation of CML-iPSCs. CML-iPSCs were differentiated on the 10T1/2 cells. On day 7 (A), iPSCs began to mount. On day 14 of culture (B: left panel), inflated sac-like structures appeared. These sac-like structures contained the round hematopoietic cells (B: right panel: higher magnification). (C) These hematopoietic cells expressed immature marker CD34 and CD45. (D) CFC activity was estimated using 1 × 104 3CD34+ CD45+cells. Erythroid colonies (black bars), granulocyte-monocyte (GM) colonies (white bars), and mixed GM colonies with erythtoid cells (mix; gray bars) were plotted.
Hematopoietic differentiation of CML-iPSCs. CML-iPSCs were differentiated on the 10T1/2 cells. On day 7 (A), iPSCs began to mount. On day 14 of culture (B: left panel), inflated sac-like structures appeared. These sac-like structures contained the round hematopoietic cells (B: right panel: higher magnification). (C) These hematopoietic cells expressed immature marker CD34 and CD45. (D) CFC activity was estimated using 1 × 104 3CD34+ CD45+cells. Erythroid colonies (black bars), granulocyte-monocyte (GM) colonies (white bars), and mixed GM colonies with erythtoid cells (mix; gray bars) were plotted.
Then we characterized the CML-iPSCs derived hematopoietic cells, comparing with those derived from normal iPSCs. CFC activities were measured using the same number of CD34+ cells (Figure 4D). Hematopoietic progenitors derived from CML-iPSCs and normal iPSCs produced colonies of mature erythroid, granulocyte-macrophage, or mixed of these hematopoietic cells in growth factor-supplemented methyl cellulose medium with a similar distribution of colony size, morphologies, and kinetics of growth and maturation. The colony forming cells expressed BCR-ABL (supplemental Figure 1B and supplemental Table 2).
Next, we tested the engraftment potential of these cells. nonobese diabetic/severe combined immunodeficiency IL2Rg deficient (NOG) mice serve as a superior host for engraftment of human normal and malignant hematopoietic cells.32 One million CD34+ cells were intravenously transplanted into NOG mice with minimal irradiation (2 Gy; supplemental Figure 3A). Only transient engraftment was observed and the recipient mice never showed CML phenotype in vivo (supplemental Figure 3B).
BCR-ABL dependence is lost in the CML-iPSCs
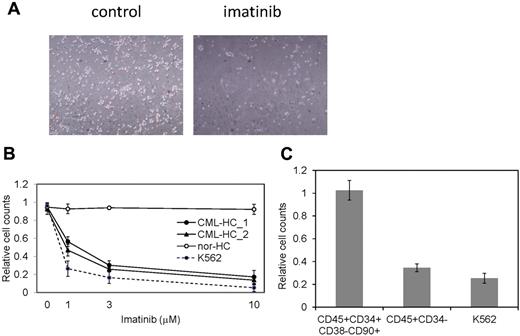
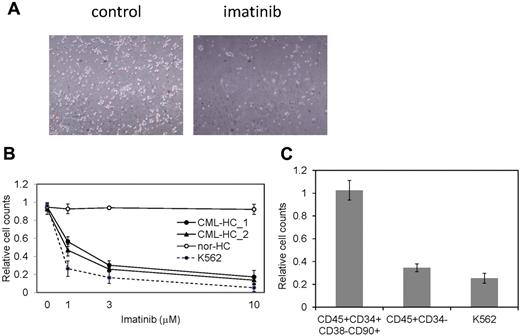
The restricted dependence of BCR-ABL signaling on survival of CML cells enables the disease suppression by imatinib and dramatically changed the CML treatment after the development of imatinib.33 CML patients whose cells were used for the generation of iPSCs effectively responded to imatinib therapy. However, although CML-iPSCs expressed BCR-ABL, they were resistant to imatinib (Figure 2E). Interestingly, CML-iPSC–derived hematopoietic cells recovered the sensitivity to imatinib except CD34+38−90+45+ immature cell population, which recapitulated the feature of initial CML disease (Figure 5A). Various concentrations of imatinib were added to the culture of iPSC derived hematopoietic cells. Similar kinetics of imatinib response between CML-iPSC–derived hematopoietic cells and imatinib sensitive CML cell line K562 was observed (Figure 5B). Furthermore, we generated CD34+CD38−CD90+CD45+ cells from CML-iPSCs. Surprisingly, this fraction of phenotypically immature cells showed the imatinib resistance like CML-iPSCs although more differentiated cells (CD34−CD45+) showed the sensitivity to imatinib (Figure 5C).
CML-iPSC derived hematopoietic cells recovered the sensitivity to imatinib. (A) Imatinib but not the vehicle (DMSO) decreased the growth of hematopoietic cells derived from CML-iPSCs in suspension culture. (B) Various concentrations of imatinib were added to the culture of iPSC derived hematopoietic cells for 4 days. CML-iPSC–derived CD34+ hematopoietic cells (CML-HC_1 and CML-HC_2), normal iPSC-derived hematopoietic cells (nor-HC), and K562 cells were used for analyses. Relative cell counts compared with the vehicle control were plotted. Shown is the mean of a single experiment conducted in triplicate as a representative of 3 independent experiments. (C) Imatinib (10μM) was added to the suspension culture of CML-iPSC–derived hematopoietic cells for 4 days. The immature cell fraction (CD34+CD38−CD90+CD45+) showed resistance similar to CML-iPSCs, although more differentiated cells (CD34−CD45+) showed the sensitivity to imatinib. Relative cell counts compared with the vehicle control was plotted.
CML-iPSC derived hematopoietic cells recovered the sensitivity to imatinib. (A) Imatinib but not the vehicle (DMSO) decreased the growth of hematopoietic cells derived from CML-iPSCs in suspension culture. (B) Various concentrations of imatinib were added to the culture of iPSC derived hematopoietic cells for 4 days. CML-iPSC–derived CD34+ hematopoietic cells (CML-HC_1 and CML-HC_2), normal iPSC-derived hematopoietic cells (nor-HC), and K562 cells were used for analyses. Relative cell counts compared with the vehicle control were plotted. Shown is the mean of a single experiment conducted in triplicate as a representative of 3 independent experiments. (C) Imatinib (10μM) was added to the suspension culture of CML-iPSC–derived hematopoietic cells for 4 days. The immature cell fraction (CD34+CD38−CD90+CD45+) showed resistance similar to CML-iPSCs, although more differentiated cells (CD34−CD45+) showed the sensitivity to imatinib. Relative cell counts compared with the vehicle control was plotted.
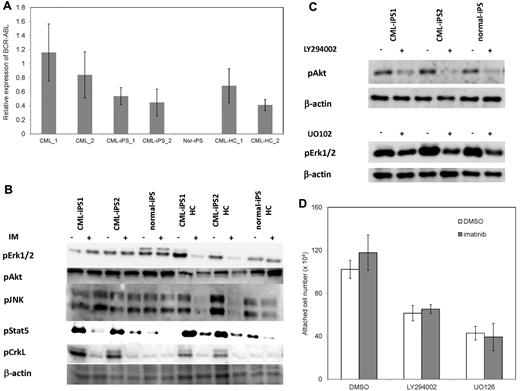
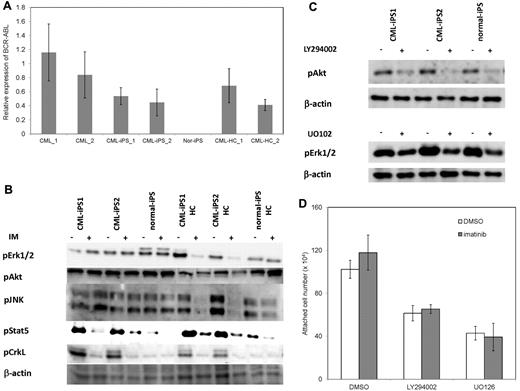
Then, we investigated why CML-iPSCs showed the imatinib-resistance. It was reported that imatinib resistant patients sometimes express higher BCR-ABL transcript than imatinib sensitive patients.34 In addition, CML leukemia stem cells showed higher BCR-ABL expression than differentiated CML cells.35 Therefore, we examined the BCR-ABL mRNA expression levels in the CML-iPSCs, and compared them with the primary CML sample, and CML-iPSC–derived hematopoietic cells. As a result, BCR-ABL expression was not increased in CML-iPSCs compared with the primary CML sample and CML-iPSCs–derived hematopoietic cells. (Figure 6A)
The mechanism of imatinib resistance in the CML-iPSCs. (A) The expression profile of BCR-ABL transcript during hematopoietic differentiation. The expression levels of BCR-ABL in the CML-iPSCs were compared with those of primary CML samples (CML_1 and CML_2), CML-iPSC–derived CD34+ hematopoietic cells (CML-HC_1 and CML-HC_2), and normal iPSC (nor-iPS). The expression level of the mean in the primary CML sample was set at 1. (B) BCR-ABL signaling was estimated in the CML-iPSCs after imatinib (IM) treatment. The phosphorylation state of ERK1/2, AKT, JNK, and STAT5, which are the essential for the survival of BCR-ABL (+) hematopoietic progenitors (CD34+CD45+), were evaluated after imatinib treatment in CML-iPSCs. These were the representative data from 3 independent experiments. (C-D) LY294002 and U0126 (10μM) were added to the culture of CML iPSCs to inhibit AKT and ERK, respectively with or without imatinib. (C) After 4 hours of culture, each inhibitor decreased the phosphorylation of ERK or AKT as expected. (D) The attached cell numbers after treatment with specific AKT or ERK inhibitor were shown. These were the representative data from 3 independent experiments.
The mechanism of imatinib resistance in the CML-iPSCs. (A) The expression profile of BCR-ABL transcript during hematopoietic differentiation. The expression levels of BCR-ABL in the CML-iPSCs were compared with those of primary CML samples (CML_1 and CML_2), CML-iPSC–derived CD34+ hematopoietic cells (CML-HC_1 and CML-HC_2), and normal iPSC (nor-iPS). The expression level of the mean in the primary CML sample was set at 1. (B) BCR-ABL signaling was estimated in the CML-iPSCs after imatinib (IM) treatment. The phosphorylation state of ERK1/2, AKT, JNK, and STAT5, which are the essential for the survival of BCR-ABL (+) hematopoietic progenitors (CD34+CD45+), were evaluated after imatinib treatment in CML-iPSCs. These were the representative data from 3 independent experiments. (C-D) LY294002 and U0126 (10μM) were added to the culture of CML iPSCs to inhibit AKT and ERK, respectively with or without imatinib. (C) After 4 hours of culture, each inhibitor decreased the phosphorylation of ERK or AKT as expected. (D) The attached cell numbers after treatment with specific AKT or ERK inhibitor were shown. These were the representative data from 3 independent experiments.
BCR-ABL activates Ras-MAPK, PI3K-AKT, JAK-STAT pathways. Among them, it was reported that STAT5, ERK1/2, JNK, and AKT are essential for the survival of BCR-ABL–dependent leukemic cells.36,37 In addition, CRKL is another direct target of BCR-ABL.38 The phosphorylation status of ERK1/2, AKT, JNK, and STAT5 in CML-iPSCs, which are essential for the survival of BCR-ABL (+) hematopoietic progenitors, were evaluated after imatinib treatment. The phosphorylation of ERK1/2, AKT, and JNK, which are also essential for the maintenance of iPSCs and ES cells,39,40 were unchanged after treatment in the CML-iPSCs although they were decreased in the CML-iPSCs–derived hematopoietic cells (Figure 6B). The phosphorylation of CRKL and STAT5, which were not activated in the normal iPSCs, was decreased in both CML-iPSCs and CML-iPSCs–derived hematopoietic cells (Figure 6B). These results showed that the signaling for iPSCs maintenance might compensate for the inhibition of BCR-ABL in CML-iPSCs and that BCR-ABL dependence was lost in CML-iPSCs. In addition, the specific inhibitor of ERK or AKT signaling worked as expected, respectively (Figure 6C), resulting in the reduction of attached cells regardless of the addition of imatinib (Figure 6D).
Discussion
Generation of CML-derived iPSCs
We generated iPSCs from primary CML patient samples. Methylation pattern and gene expression of CML-iPSCs were very similar to those of normal iPSCs. Previously, SS DMRs were identified during reprogramming process of iPSCs.31 Hypo SS DMRs were also hypomethylated in the CML-iPSCs (Figure 3B). Among them, some genomic regions, such as the promoter of N-MYC, had already been hypomethylated in the primary CML sample. In the same way, some genes associated with hyper SS DMRs had already been hypermethylated in the primary CML sample (Figure 3C). However, we could not detect the CML-iPSC–specific DMRs in this study. Then, we redifferentiated them into hematopoietic lineage and showed the recapitulation of the features of the initial disease. In addition, although CML-iPSCs expressed BCR-ABL, it was surprising that there were no obvious differences of gene expression profile between normal iPSCs and CML-iPSCs (Figure 3D). The results that inhibition of BCR-ABL by imatinib did not affect CML-iPSC survival indicate that signaling of BCR-ABL might not be important in iPSCs. These results are consistent with the gene expression profile data in which the effect of BCR-ABL signaling was hardly observed. One possibility is that global tyrosine kinase activities and downstream signaling pathways would be so activated in iPSCs irrespective of BCR-ABL that BCR-ABL no longer adds significant effects.
CML is known to be a clonal disorder originated from hematopoietic stem cells caused by BCR-ABL fusion gene. Although BCR-ABL TKI imatinib can reduce CML cells below the detection of molecular level, its discontinuation often results in the rapid relapse of leukemia.41 These results indicate the existence of CML stem cells, which are resistant to the TKI. CML stem cells are thought to be included in the primitive population (CD34+CD38−). According to some published data, they have lost the addiction to BCR-ABL42,43 In addition, CML-iPSCs also have shown resistance to the imatinib.44 Furthermore, in our experiments, immature CD34+38−90+45+ cells differentiated from CML-iPSCs also showed imatinib resistance similar to CML-iPSCs, although more differentiated cells (CD34−CD45+) showed sensitivity to imatinib (Figure 5C). So, these immature cells showed a phenotype of CML stem cells. Imatinib treatment of CML stem cells decreased the phosphorylation of CRKL and STAT5 but not of AKT,42 as shown in the CML-iPSCs described here. There may be some shared mechanism between CML stem cells and CML-iPSCs. For example, Wnt-β-catenin signaling is essential for the maintenance of both CML stem cells and iPSCs.45,46 Using immature cells obtained in our study, the mechanism of imatinib resistance of CML stem cells can be further investigated.
Previously, it was reported that primary CML samples and the CML BC cell line KBM7 were reprogrammed and that primary CML-iPSCs47 and KBM7-iPSCs were established.17 As shown here, KBM7-iPSCs lost the BCR-ABL dependence and became resistant to imatinib, although primary CML-derived iPSCs were not checked for the imatinib sensitivity. Carette et al argued that a specific differentiated epigenetic cell state is needed to maintain BCR-ABL dependence.17 However, they only showed the BCR-ABL expression but did not confirm BCR-ABL activation in the KBM7-iPSCs. We showed BCR-ABL specific phosphorylation of STAT5 and CRKL although they were not necessary for the survival of iPSCs and that imatinib treatment inhibits these signaling. On the other hand, RAS-MAPK and PI3K-AKT signaling were unchanged after imatinib treatment. It was reported that inhibition of caspase-mediated anoikis by bFGF is dependent on activation of ERK and AKT in human ES cells.39 We also showed that the inhibition of ERK or AKT irrespective of the presence of the imatinib resulted in the decrease of the attached cell numbers. Some key molecules essential for the maintenance of iPSCs may compensate for the BCR-ABL inhibition in the CML-iPSCs through downstream ERK and AKT signaling pathways. They may include contact-mediated signaling with stem cell niches, and may be shared with CML stem cells and CML-iPSCs.
The progression of CML from initial indolent CP to the aggressive stages, the AP and BC is caused by additional gene mutations. If we introduce some additional mutation into the CML-iPSCs, the CML BC model may be generated.
Generation of hematologic malignancies derived iPSCs other than CML
Primary samples of hematologic malignancy are usually difficult to be expanded. However, after they are reprogrammed to iPSCs, they can expand unlimitedly. As a result, we can obtain the genetically abnormal hematopoietic cells continuously by redifferentiating them into hematopoietic cells and use them for the studies which require the large number of living cells, such as the analysis for proteome, epigenome, transciptome, leukemia stem cells, or drug screening. Thus, iPSCs technology would be useful for the study of hematologic malignancy based on the patient samples.
However, reprogramming of leukemia cells may be harder than generation of normal iPSCs because of the genetic and epigenetic status of leukemia cells. To overcome the difficulty, application of other factors in addition to the Yamanaka factors may be effective, such as exogenous expression of miRNA-302,48 chemical compounds, such as azacitidine (DNA metyltranferase inhibitor),49 BIX01294 (G9a histone metyltransferase inhibitor),50 VPA (histone deacetylase inhibitor), or TSA (histone deacetylase inhibitor),26 and knockdown of p53, p21, and Ink4/Arf.51,52
In addition, there may be more desirable gene delivery system for iPSC generation for the study of disease pathogenesis. The integration site of retrovirus in the iPSCs may affect the gene expression and change the disease phenotype after redifferentiating them into the original lineages. Recently, efficient induction of transgene free iPSCs, such as using Sendai virus system, was reported53 and will be applicable for the disease derived iPSCs. We could establish the CML-iPSCs by this system. Using the newly established CML-iPSCs with sendai virus and feeder free culture system, we confirmed the same resistance to imatinib (supplemental Figure 4). Furthermore, without feeder cells, the phosphorylation of ERK and AKT were maintained, although the phosphorylation of STAT5 and CRKL were decreased by imatinib treatment.
In addition, the sendai virus system can be applied to the establishment of other disease derived iPSCs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank T. Kitamura for pMXs retroviral vector; and Y. Hokama, M. Kobayashi, and Y. Oikawa for expert technical assistance.
This work was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science and by Health and Labor Sciences Research grants from the Ministry of Health, Labor and Welfare, Japan and a grant-in-aid from Core Research for Evolutional Science and Technology of Japan.
Authorship
Contribution: K.K. designed the research, performed experiments, and wrote the paper; S.A., M.H., K.T., N.T., M.O., G.N., K.U., K.N., and Y.K. performed experiments, K.E., H.A., and H.N. discussed the paper; and M.K. conceived and designed the research, supervised the whole project, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mineo Kurokawa, Deptartment of Hematology and Oncology, Graduate School of Medicine, University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan; e-mail: kurokawa-tky@umin.ac.jp.