Abstract
EBV-associated T/NK–cell lymphoproliferative disease (T/NK-LPD) is defined as a systemic illness characterized by clonal proliferation of EBV-infected T or NK cells. We prospectively enrolled 108 nonimmunocompromised patients with this disease (50 men and 58 women; median onset age, 8 years; age range, 1-50 years) evidenced by expansion of EBV+ T/NK cells in the peripheral blood; these were of the T-cell type in 64 cases and of the NK-cell type in 44, and were clinically categorized into 4 groups: 80 cases of chronic active EBV disease, 15 of EBV-associated hemophagocytic lymphohistiocytosis, 9 of severe mosquito bite allergy, and 4 of hydroa vacciniforme. These clinical profiles were closely linked with the EBV+ cell immunophenotypes. In a median follow-up period of 46 months, 47 patients (44%) died of severe organ complications. During the follow-up, 13 patients developed overt lymphoma or leukemia characterized by extranodal NK/T-cell lymphoma and aggressive NK-cell leukemia. Fifty-nine received hematopoietic stem cell transplantation, 66% of whom survived. Age at onset of disease (≥ 8 years) and liver dysfunction were risk factors for mortality, whereas patients who received transplantation had a better prognosis. These data depict clinical characteristics of systemic EBV+ T/NK-LPD and provide insight into the diagnostic and therapeutic approaches for distinct disease.
Introduction
EBV-associated lymphoproliferative diseases (LPDs) have a vast spectrum from reactive to neoplastic processes in the transformation and proliferation of lymphocytes spanning B, T, and NK cells,1-3 and are clinically complicated by the interaction between the biologic properties of EBV+ lymphocytes and the host immune status. Our understanding of these diseases is now evolving and has led to the recognition of a variety of EBV+ diseases, including Burkitt lymphoma,3 age-related EBV+ B-cell LPD,4 extranodal NK/T-cell lymphoma of nasal type (ENKL),5 aggressive NK-cell leukemia (ANKL),6 classic Hodgkin lymphoma,3 and immunodeficiency-associated lymphoproliferative disorders.1 EBV-associated T- and NK-cell LPD (T/NK-LPD) was first incorporated into the 4th World Health Organization (WHO) classification of tumors of hematopoietic and lymphoid tissues, in which systemic EBV+ T-cell LPD of childhood and hydroa vacciniforme–like lymphoma are proposed as distinct entities.7,8 Historically, based on their broad clinical manifestations, these diseases have been described under various nosological terms from indolent (eg, severe mosquito bite allergy9 and hydroa vacciniforme10 ) to aggressive or fulminant forms (eg, EBV-associated hemophagocytic lymphohistiocytosis [HLH],11 chronic active EBV disease [CAEBV] of the T/NK–cell type,12 fulminant EBV+ T-cell LPD of childhood,13 and fatal infectious mononucleosis3 ).
CAEBV originally referred to chronic or recurrent infectious mononucleosis-like symptoms.14-16 A severe form of CAEBV was found to be prevalent in east Asian countries and was characterized by clonal expansion of the EBV-infected T or NK cells,12,17,18 whereas in Western countries CAEBV is mostly associated with EBV-infected B cells.19,20 The term EBV-associated HLH was coined to describe hemophagocytosis involving BM or other organs and resulting in pancytopenia in the peripheral blood. This disease is also frequently seen in east Asian countries,11 and involves a clonal expansion of EBV+ T or NK cells, which produce inflammatory cytokines that induce the activation of macrophages and hemophagocytosis.21-23 Apart from these systemic diseases, accumulating evidence indicates that 2 cutaneous diseases, hydroa vacciniforme and severe mosquito bite allergy, are closely associated with EBV+ T or NK cells. Hydroa vacciniforme is characterized by recurrent vesiculopapules usually occurring on sun-exposed areas and seen in children and adolescents.10 In some of these patients, systemic symptoms including fever, wasting, lymphadenopathy, and hepatosplenomegaly have been recorded.24-26 Severe mosquito bite allergy was determined to be associated with EBV+ NK cells, but rarely with EBV+ T cells, and to progress into overt lymphoma or leukemia in the long-standing clinical course.9,27 These EBV+ cutaneous diseases had the same geographic distribution as the other EBV+ T/NK–cell lymphomas and LPDs among east Asians and Native Americans in Central and South America and Mexico,8 and were encountered as a part of the initial and accompanying symptoms of the systemic EBV+ T/NK-LPDs.28-30 However, the mutual relationship and clinicopathologic distinctiveness of these EBV+ T/NK-LPDs are unfounded, posing diagnostic and therapeutic problems for pathologists and hematologists, respectively. These patients appear to exist in the gray zone between systemic EBV+ T-cell LPD of childhood and hydroa vacciniforme–like lymphoma according to the 4th WHO classification. The former encompasses CAEBV of T-cell type, EBV+ HLH, and EBV+ T-cell lymphomas with prodromal phase, whereas the latter may include all cases with EBV+ hydra vacciniforme despite the presence or absence of the systemic disease in the patient's history.
The aim of the present study was to clarify the clinicopathologic characteristics of these EBV+ T/NK-LPDs and the biologic properties of the proliferating cells by analyzing a large number of patients. We previously performed a nationwide survey for CAEBV of T/NK–cell type and determined its prognostic factors.29 Similarly, a nationwide study for HLH was recently performed in Japan.31 However, these studies were retrospective and lacked the precise diagnosis of the current level because of their study design. In 1998, we established an EBV-DNA quantification system using real-time PCR,32,33 which allowed for the determination of the phenotype of EBV-infected cells in the peripheral blood with the combination of fractionation to the lymphocyte subset.12,34,35 More recently, we developed the simultaneous staining method for surface antigens and nuclear EBV-encoded small RNA (EBER) to more precisely determine EBV-infected cell phenotypes.36 Using these techniques, we enrolled and prospectively followed patients with definitive cases of EBV+ T/NK-LPDs in 1998. In this study, 108 nonimmunocompromised patients with EBV+ T/NK-LPDs were analyzed for clinical and virological characteristics to obtain an understanding of their pathogenesis and for refining their classification. Furthermore, prognostic factors and the efficacy of therapeutic interventions including hematopoietic stem cell transplantation (HSCT) were analyzed.
Methods
Eligibility criteria
Informed consent was obtained from all participants or their guardians in accordance with the Declaration of Helsinki. This study was approved by the institutional review board of Nagoya University Graduate School of Medicine. From 1998 to 2010, patients whose samples were sent to Nagoya University Graduate School of Medicine for determination of the EBV-infected cell phenotype and who fulfilled the following criteria were prospectively enrolled in this study: (1) EBV-associated T/NK-LPD suspected or diagnosed based on clinical and/or histopathological findings; (2) high EBV load detected in PBMCs by quantitative PCR (≥ 102.5 copies/μg of EBV-DNA)12,32 ; and (3) EBV infection in T or NK cells in the peripheral blood confirmed by either immunobead sorting followed by quantitative PCR34,35 or FISH.36 Exclusion criteria were: (1) pathologically defined ENKL,5 ANKL,37 or peripheral T-cell lymphoma (PTCL)38 ; (2) congenital immunodeficiency; (3) HIV positivity; and (4) other immunodeficiencies requiring immunosuppressive therapies or underlying diseases with potential immunosuppression. Patients were recruited through an announcement by the Japanese Association for Research on Epstein-Barr Virus and Related Diseases and on the homepage of our institute's website. Approximately 240 hematology units and 400 departments of pediatrics were included in the association.
On entry into the study, peripheral blood was collected and sent to Nagoya University Graduate School of Medicine to examine EBV-DNA quantification and EBV-infected cell determination along with detailed clinical data. Clonality analyses were also performed at this time if possible. Primary EBV infection was determined based on serological findings, detection of antiviral capsid Ag-IgM, and seroconversion of either antiviral capsid Ag-IgG or anti-EBV nuclear Ag. A total of 108 patients from 40 hospitals were enrolled in the study (25 from Nagoya University Hospital, 13 from Osaka Medical Center and Research Institute for Maternal and Child Health, 9 from Fukushima Medical University, and 61 from other hospitals). Each patient enrolled in the study was treated according to physician decision at each hospital. The physicians completed questionnaires regarding the administered treatment and outcome every 3 years (2001, 2004, and 2007); the final questionnaire was sent and collected in December 2010. Compared with data provided by previous national surveys for CAEBV and HLH,29,31 we estimated that approximately 15%-20% of systemic EBV+ T/NK-LPD cases during the study period were recruited by this registry.
Patient criteria
Patients were clinically divided into 4 groups according to the clinical categorization at the 2008 National Institutes of Health meeting: (1) CAEBV of T/NK–cell type, (2) EBV-associated HLH, (3) hydroa vacciniforme, and (4) severe mosquito bite allergy.39 The clinical diagnosis was made at entry into the study. Definitions of each clinical category are listed in Table 1. CAEBV was defined according to previously proposed criteria.16,29 HLH was defined based on the criteria proposed by an international treatment study group.11 Severe mosquito bite allergy and hydroa vacciniforme were applied for cases with only skin symptoms and lacking systemic symptoms. In this study, “severe mosquito bite allergy” and “hydroa vacciniforme” were used as clinical categories, whereas “hypersensitivity to mosquito bites” and “hydroa vacciniforme–like eruptions” were used as terms for symptoms; “hydroa vacciniforme–like lymphoma” was used as a term for pathologic classification.
Patients were also classified according to the 4th WHO classification for tumors of hematopoietic and lymphoid tissues.7 The definitions of pathologic classification are listed in Table 1. The classification was made both at the diagnosis and at the last follow-up or death. Patients diagnosed with ENKL, ANKL, or PTCL were excluded from the study, but some developed these diseases during the follow-up period. Of 108 patients, 54 were biopsied (liver, n = 15; skin, n = 15; lymph nodes, n = 10; intestine, n = 3; spleen, n = 2; muscle, n = 2; others, n = 7), and 6 were autopsied. For differential diagnosis, BM examination was performed in most patients (79%), even though there were no hematologic abnormalities of the peripheral blood. When abnormal findings were detected in BM or peripheral blood, EBER/immunohistochemical staining was performed. Histopathology was reviewed by the Central Pathology Review Board (Shigeo Nakamura, Nagoya University and Koichi Ohshima, Kurume University).
Disease status was defined as follows: stable disease, partial remission (PR), and complete remission (CR). Patients with PR had no symptoms but had significant EBV loads in PBMCs (EBV-DNA ≥ 102.5 copies/μg of DNA).12,32 CR patients had no symptoms and continuously low or no EBV loads in PBMCs (EBV-DNA < 102.5 copies/μg DNA). Disease activity was assessed before HSCT and was classified as either active or inactive as described previously.40 Active disease was defined by the existence of symptoms and signs such as fever, persistent hepatitis, lymphadenopathy, hepatosplenomegaly, pancytopenia, or progressive skin lesions along with an elevated EBV load in the peripheral blood. Liver dysfunction was defined as an increase in alanine transaminase levels to 2 times above the upper limit of normal on at least 2 consecutive occasions.
Analyses of EBV and determination of EBV-infected cells
DNA was extracted from 1 × 106 PBMCs or 200 μL of plasma and real-time quantitative PCR was then performed as described previously.12,32 EBV clonality was assessed by Southern blotting with a terminal repeat probe, as described previously.12,41 To determine which cell population harbored EBV, either immunobead sorting followed by quantitative PCR or FISH assay was performed. For the former method, PBMCs were fractionated into CD3+, CD4+, CD8+, CD16+, CD19+, CD56+, TCRαβ+, and TCRγδ+ cells using an immunobead method (IMag Cell Separation System; BD Biosciences) that resulted in 97%-99% purity.34,35 Purified cells were analyzed by real-time quantitative PCR. The infected-cell phenotypes were determined in comparison with unfractionated (whole) PBMCs, as described previously.34,35 For example, patients were defined as CD3+ when CD3+ cells contained higher amounts of EBV DNA than whole PBMCs. The FISH assay was performed as described previously.36 Briefly, PBMCs were stained with fluorescence labeled mAbs against surface marker, fixed, permeabilized, and hybridized with EBER-specific PNA Probe/FITC (Y5200; Dako). After enhancing fluorescence, stained cells were analyzed using a FACSCalibur flow cytometer and CellQuest Version 5.1.1 software (BD Biosciences). More than 0.1% of EBER+ cells was considered to be significant and such subset was designated EBV+. This frequency was chosen based on previous data using EBV+ cell lines.36
TCR gene rearrangement
TCR gene rearrangement was determined by multiplex PCR using the T-cell Gene Rearrangement/Clonality assay (InVivoScribe Technologies), which was developed and standardized in a European BIOMED-2 collaborative study.42
Histopathology
Immunostaining was performed using an avidin-biotin peroxidase complex method with mAbs against CD3 (Dako), CD56 (Novocastra Laboratories), perforin (Novocastra Laboratories), T cell–restricted intracellular Ag 1 (TIA-1; Immunotech), and granzyme B (Monosan).43 FISH was performed using the EBER probe (Dako) as described previously.43 Hybridization was detected using mouse monoclonal anti-FITC Ab (Dako) and a Vectastain ABC kit (Vector).
Statistical analysis
Statistical analysis was performed using SPSS for Windows Version 18.0. For univariate analysis, either the χ2 or the Fisher exact test (single-sided) was used to compare categorical variables. The Mann-Whitney U test was used to compare quantitative variables. Logistic regression analysis was used for multivariate analysis. Comparison between quantities of EBV-DNA in PBMCs and plasma was performed by regression analysis. The Kaplan-Meier method and the log-rank test were used for survival analysis. P < .05 was considered statistically significant for all analyses.
Results
Characteristics of patients with EBV+T/NK-LPD
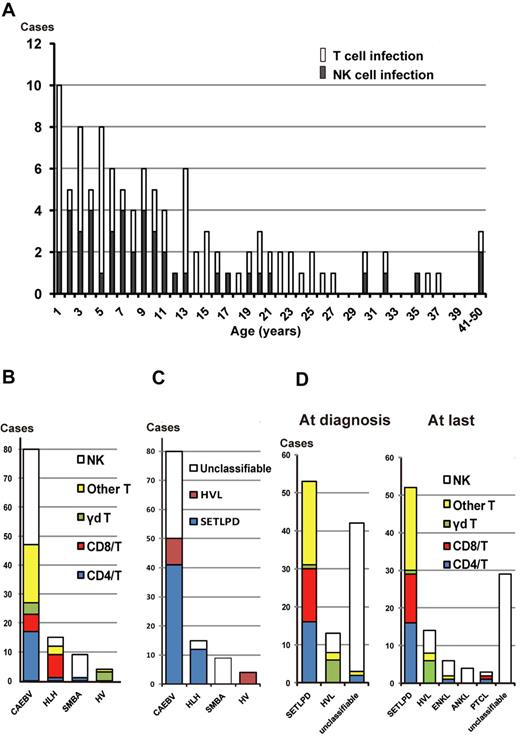
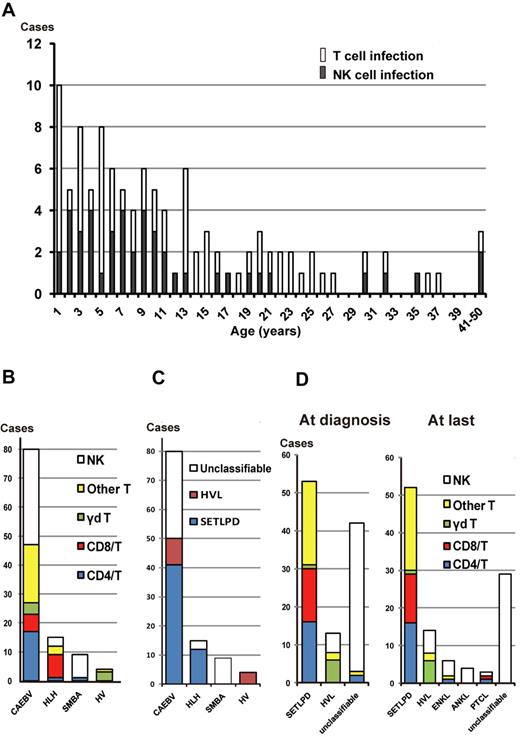
A total of 108 patients (50 men and 58 women) were enrolled in this study. Detailed characteristics of each patient are shown in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Age at diagnosis ranged from 1 to 51 years (median, 14 years). At the time of diagnosis, the main phenotypes of EBV-infected cells in the peripheral blood were T cells and NK cells in 64 and 44 patients, respectively. Onset age ranged from 1 to 50 years (median, 9 years). Most patients (91%) were children and young adults less than 30 years of age, but there were some middle-aged patients (age range, 30-50 years) also existed (Figure 1A). There was no difference in onset age between patients with the T-cell type and those with the NK-cell type. The former were further subdivided into the CD4+ T-cell type (n = 18), the CD8+ T-cell type (n = 14), the γδ T-cell type (n = 7), and other or ill-defined T-cell type (n = 25). In 2 patients (patients 92 and 100, supplemental Table 1), 2 lineages of cells were infected with EBV.
EBV-infected cell phenotypes of EBV+ T/NK lymphoproliferative diseases. (A) Age distribution of patients with T-cell and NK-cell types. (B) EBV-infected cells among categories of clinical groups. Infected T cells were further divided into CD4+ T cells, CD8+ T cells, γδ T cells, and “other T cells.“ The 25 cases of “other T cells” were defined as either phenotypically different T-cell subsets (2 patients were CD4−CD8−, 1 patient was CD4+CD8+, and 1 patient had 2 lineages consisting of CD4+CD8− and CD4−CD8+ cells) or ill-defined T cells (n = 21). In the majority of the ill-defined T-cell patients, Abs against CD4 or CD8 could not be used to define their CD4/CD8 phenotype because the number of recovered PBMCs was not sufficient. SMBA indicates severe mosquito bite allergy; and HV, hydroa vacciniforme. (C) The 4th WHO pathologic classification of each clinical group at the time of diagnosis. SETLPD indicates systemic EBV+ T-cell lymphoproliferative disease of childhood; and HVL, hydroa vacciniforme–like lymphoma. (D) EBV-infected cells among categories of the pathologic classification at diagnosis and at the last follow-up or death. Patients in CR were classified according to the data and status before remission.
EBV-infected cell phenotypes of EBV+ T/NK lymphoproliferative diseases. (A) Age distribution of patients with T-cell and NK-cell types. (B) EBV-infected cells among categories of clinical groups. Infected T cells were further divided into CD4+ T cells, CD8+ T cells, γδ T cells, and “other T cells.“ The 25 cases of “other T cells” were defined as either phenotypically different T-cell subsets (2 patients were CD4−CD8−, 1 patient was CD4+CD8+, and 1 patient had 2 lineages consisting of CD4+CD8− and CD4−CD8+ cells) or ill-defined T cells (n = 21). In the majority of the ill-defined T-cell patients, Abs against CD4 or CD8 could not be used to define their CD4/CD8 phenotype because the number of recovered PBMCs was not sufficient. SMBA indicates severe mosquito bite allergy; and HV, hydroa vacciniforme. (C) The 4th WHO pathologic classification of each clinical group at the time of diagnosis. SETLPD indicates systemic EBV+ T-cell lymphoproliferative disease of childhood; and HVL, hydroa vacciniforme–like lymphoma. (D) EBV-infected cells among categories of the pathologic classification at diagnosis and at the last follow-up or death. Patients in CR were classified according to the data and status before remission.
After entry into the study, patients were clinically categorized into 4 groups based on clinical symptoms and diagnostic criteria: CAEBV (n = 80), EBV-associated HLH (n = 15), severe mosquito bite allergy (n = 9), and hydroa vacciniforme (n = 4; Figure 1B). The CAEBV group consisted of 47 patients with the T-cell type (59%) and 33 with the NK-cell type (41%); the former were further subdivided into the CD4+ T-cell type (21%), the CD8+ T-cell type (8%), and the γδ T-cell type (5%). Eight of 15 (53%) EBV-associated HLH patients had EBV-harboring CD8+ T cells, in contrast to their low occurrence in the other clinical groups. In addition, most patients (89%) with severe mosquito bite allergy had EBV-infected NK cells, whereas many (75%) with hydroa vacciniforme had EBV-infected γδ T cells (Figure 1B). Therefore, clinical profiles were closely linked with the EBV+ cell immunophenotype.
Between 1 and 349 months from the onset of disease (median, 46 months), 47 patients had died, whereas 61 patients were alive for follow-up periods of 13-263 months (median, 82 months). The main causes of death were multiple organ failure (n = 10), hepatic failure (n = 6), heart failure (n = 5), pulmonary failure (n = 5), sepsis (n = 5), intracranial hemorrhage (n = 5), intestinal hemorrhage or perforation (n = 3), hemophagocytic syndrome (n = 2), and other (n = 6). Of the 47 patients who died, 20 (42%) died after transplantation. Of the 61 surviving patients, 41 were in CR and 4 were in PR without any symptoms, whereas 16 remained in stable disease at the last follow-up.
Clonality analysis
At the time of diagnosis, viral clonality was analyzed by Southern blot analysis using EBV terminal repeat. Of 76 patients with available DNA, EBV-infected cells were monoclonal in 64 (84%) and oligoclonal in 8 (11%). Polyclonal EBV-infected cells were detected in only 4 patients (5%). TCR rearrangement was analyzed in 90 patients at the time of diagnosis, 42 of whom had monoclonal rearrangements. Six patients with NK-cell infection demonstrated TCR rearrangement. Because this analysis uses a PCR-based method, erroneous detection of a seemingly clonal cell population (pseudoclonality) or reduced TCR diversity caused by the prevalence of a few Ag-selected subclones, which are often seen in EBV infection, may occur.42 Chromosomal aberrations were detected in the peripheral blood or lymph nodes at diagnosis in 6 patients, whereas an additional 6 patients later developed chromosomal aberrations in their clinical course of 1-9 years (median, 5 years). Patterns of chromosomal aberrations in each patient are shown in supplemental Table 2. These results provided additional support to the assertion that patients with EBV+ T/NK-LPDs had clonality at early stages and subsequently developed overt lymphoma or leukemia with an increase of chromosomal aberrations in their clinical course.
Pathologic categories based on the 4th WHO classification
At the time of diagnosis, based on the 4th WHO classification, 53 and 13 patients were classified into systemic EBV+ T-LPD of childhood and hydroa vacciniforme–like lymphoma, respectively. The proportion of these pathologic categories in each clinical group is shown in Figure 1C. Four patients clinically categorized to hydroa vacciniforme without any cellular atypia or systemic symptoms were classified into hydroa vacciniforme–like lymphoma based on the monoclonality of cells with TCR rearrangements. In systemic EBV+ T-cell LPD, T-cell subsets of EBV-infected cells were variable (Figure 1D). In hydroa vacciniforme–like lymphoma, 6 of 13 patients had γδ T-cell infection. Conversely, 42 patients were not classified into either of these pathologic categories because they failed to correspond to criteria in the current WHO classification. Classification of each patient is shown in supplemental Table 1.
At the last follow-up or death, there were 29 patients who were unclassifiable, most of whom had CAEBV of the NK-cell type and severe mosquito bite allergy with NK-cell infection (Figure 1D). In the clinical course, ENKL developed in 6 patients (patients 2, 5, 20, 34, 60, and 81 in supplemental Table 1) after 9 months to 12 years of follow-up after onset (median, 1.5 years), whereas ANKL developed in 4 patients (patients 8, 43, 66, and 80) after 2-17 years of follow-up (median, 12 years); most of these patients had NK-cell infection. EBV+ PTCL developed in 3 patients after 1 year (patient 83), 5 years (patient 93), and 20 years (patient 53) of follow-up. The EBV+ PTCL patients in this study were characterized by their expression of cytotoxic molecules, nodal manifestation, lack of CD56 expression, and TCR gene rearrangement. These features suggest a pathologic distinction between these EBV+ PTCL and extranasal ENKL.
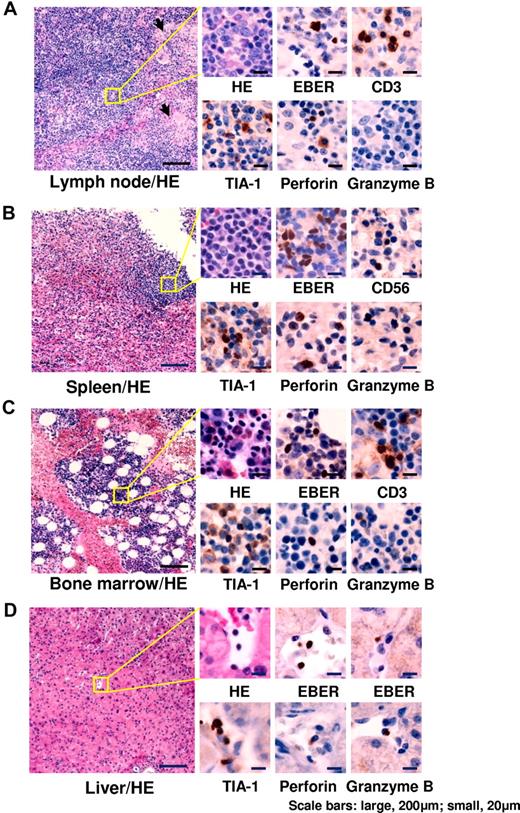
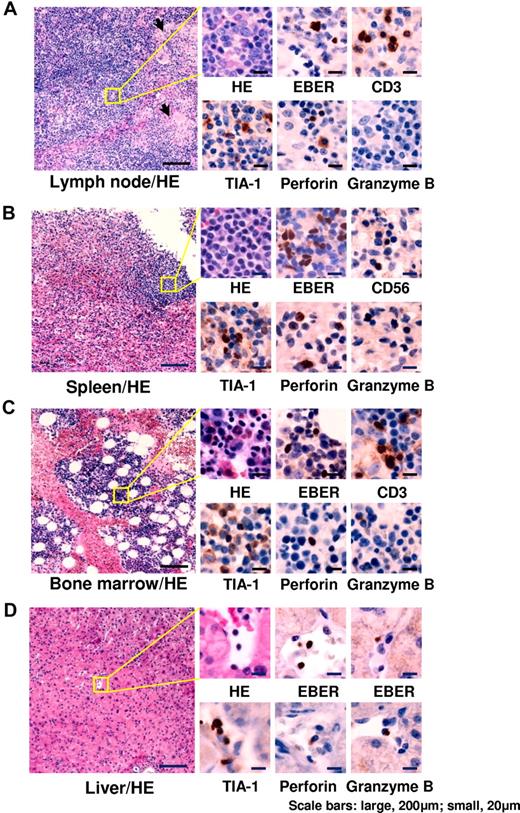
Representative results of histological examinations are shown in Figure 2. Histological findings and the number of EBER+ cells varied among patients. EBER+ lymphocytes were detected at various frequencies. Infiltrating cells (presumably EBV-infected) expressed cytotoxic molecules such as TIA-1, perforin, and granzyme B. BM aspirations showed various findings, but most patients had normocellular BM without any abnormal findings. Patients with EBV-associated HLH showed normoplastic or hyperplastic BM with mild or moderate hemophagocytosis. In all patients, however, BM findings showed an absence of hematologic malignant disorders at the time of diagnosis.
Histopathological findings of representative patients. (A) Cervical lymph node from a 6-year-old boy with chronic active EBV disease with T-cell infection (patient 3). Follicles and paracortical hyperplasia including a mild increase in transformed lymphocytes were seen. Focal epithelioid reactions were detected (arrows). Medium-sized transformed lymphocytes in the paracortex were positive for EBER. TIA-1 and perforin were positive, but granzyme B was negative. (B) Spleen from a 13-year-old boy with chronic active EBV disease with NK-cell infection (patient 6). White pulp was atrophic and red pulp showed congestion. Small lymphocytes infiltrating in the red pulp were positive for EBER. TIA-1 and perforin were positive, but granzyme B was negative. (C) BM from a 25-year-old female with chronic active EBV disease with T-cell infection (patient 17). In the mild hyperplastic BM, small lymphocytes were positive for EBER. TIA-1, perforin, and granzyme B were positive. (D) Liver from a 42-year-old female with chronic active EBV disease with NK-cell infection (patient 60). Small lymphocytes infiltrating in vessels and sinusoid were positive for EBER. TIA-1, perforin, and granzyme B were positive. HE indicates H&E staining. Images of sections were obtained by a microscopy (BX50, Olympus Corp) with CCD camera (D5-5M-L1, Nikon Corp). Each micrograph was represented at either a 100× or 400× magnification using 10× or 40× objective lens (UPlanFL, Olympus Corp), respectively.
Histopathological findings of representative patients. (A) Cervical lymph node from a 6-year-old boy with chronic active EBV disease with T-cell infection (patient 3). Follicles and paracortical hyperplasia including a mild increase in transformed lymphocytes were seen. Focal epithelioid reactions were detected (arrows). Medium-sized transformed lymphocytes in the paracortex were positive for EBER. TIA-1 and perforin were positive, but granzyme B was negative. (B) Spleen from a 13-year-old boy with chronic active EBV disease with NK-cell infection (patient 6). White pulp was atrophic and red pulp showed congestion. Small lymphocytes infiltrating in the red pulp were positive for EBER. TIA-1 and perforin were positive, but granzyme B was negative. (C) BM from a 25-year-old female with chronic active EBV disease with T-cell infection (patient 17). In the mild hyperplastic BM, small lymphocytes were positive for EBER. TIA-1, perforin, and granzyme B were positive. (D) Liver from a 42-year-old female with chronic active EBV disease with NK-cell infection (patient 60). Small lymphocytes infiltrating in vessels and sinusoid were positive for EBER. TIA-1, perforin, and granzyme B were positive. HE indicates H&E staining. Images of sections were obtained by a microscopy (BX50, Olympus Corp) with CCD camera (D5-5M-L1, Nikon Corp). Each micrograph was represented at either a 100× or 400× magnification using 10× or 40× objective lens (UPlanFL, Olympus Corp), respectively.
Differences between patients with T-cell and NK-cell infection
We compared clinical and virological differences between T- and NK-cell infections (Table 2). T-cell infection was characterized by higher rates of primary EBV infection and TCR rearrangement, whereas a significant number (43%) of patients with NK-cell infection had hypersensitivity to mosquito bites (Table 2). Interestingly, 5 patients had both hypersensitivity to mosquito bites and hydroa vacciniforme–like eruptions; these patients all had NK-cell infection (Table 2). Conversely, 8 of 10 patients with hydroa vacciniforme–like eruptions but without hypersensitivity to mosquito bites had T-cell infections (Table 2).
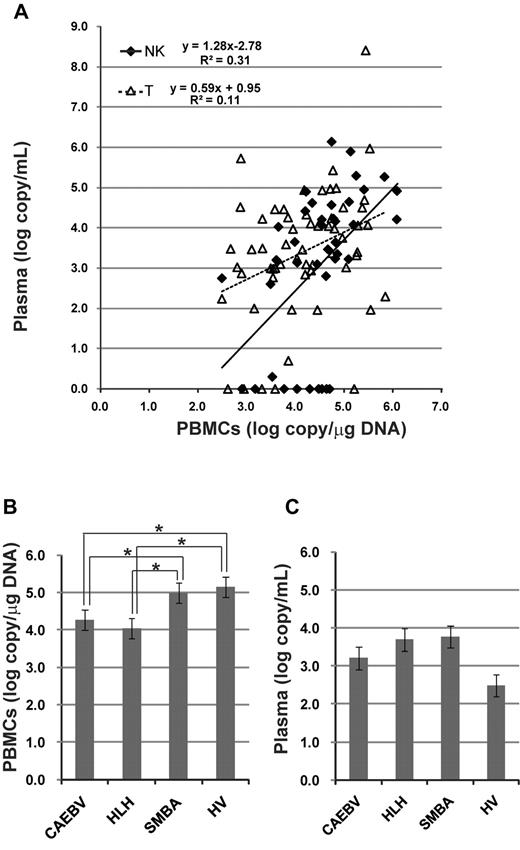
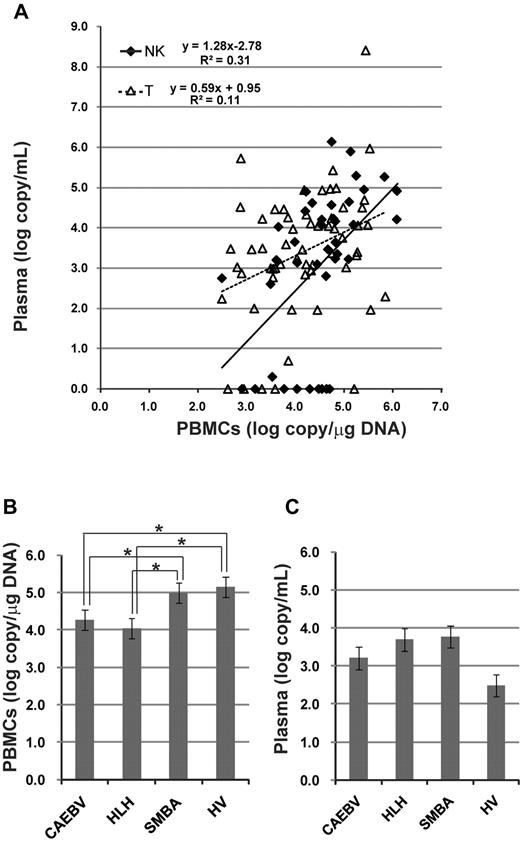
A comparison of viral load in the peripheral blood between patients with T- and NK-cell infections detected similar levels of EBV-DNA in both PBMCs and plasma (Table 2). Correlation of viral loads between PBMCs and plasma was estimated (Figure 3A). The quantity of EBV-DNA in PBMCs was significantly correlated with that in plasma in both T-cell and NK-cell infections, although EBV-DNA was not detected in the plasma from 15 patients. We also compared viral load among clinical groups (Figure 3B-C). Interestingly, the quantity of EBV-DNA in PBMCs was significantly higher in patients with severe mosquito bite allergy and hydroa vacciniforme, but these patients did not have any systemic symptoms.
Viral load in the peripheral blood at the time of diagnosis. EBV-DNA was quantified by real-time PCR. (A) Correlation of viral load between PBMCs and plasma. The correlation was separately estimated in patients with T-cell infection and those with NK-cell infection. (B) Quantity of EBV-DNA in PBMCs among categories of clinical groups. *P < .05. (C) Quantity of EBV-DNA in plasma among categories of clinical groups. SMBA indicates severe mosquito bite allergy; and HV, hydroa vacciniforme.
Viral load in the peripheral blood at the time of diagnosis. EBV-DNA was quantified by real-time PCR. (A) Correlation of viral load between PBMCs and plasma. The correlation was separately estimated in patients with T-cell infection and those with NK-cell infection. (B) Quantity of EBV-DNA in PBMCs among categories of clinical groups. *P < .05. (C) Quantity of EBV-DNA in plasma among categories of clinical groups. SMBA indicates severe mosquito bite allergy; and HV, hydroa vacciniforme.
Efficacy of therapeutic interventions
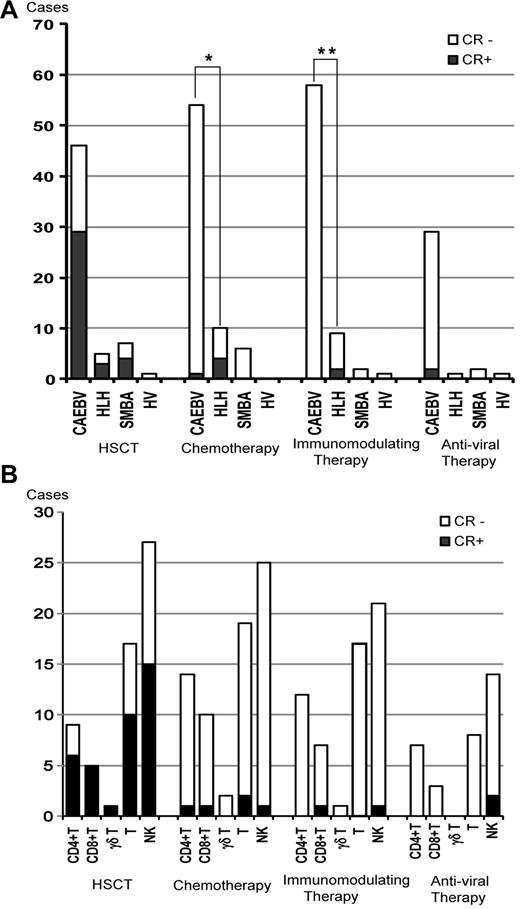
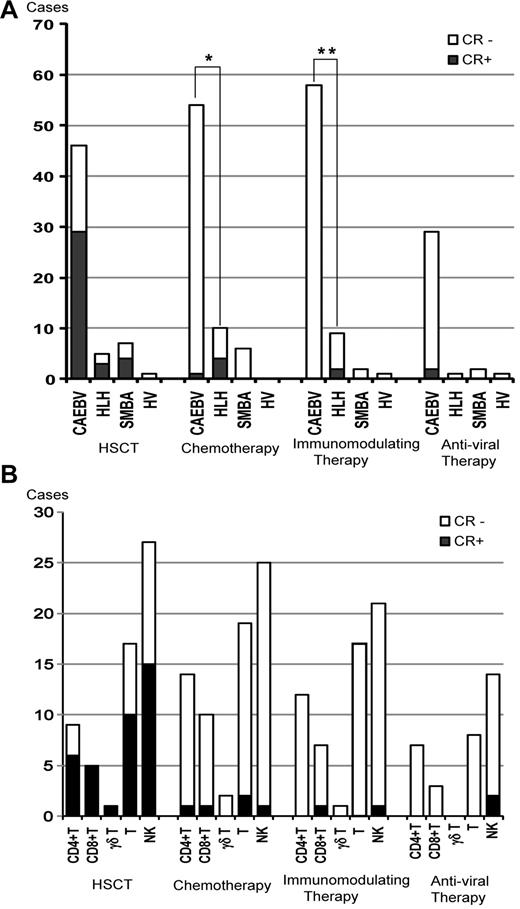
Each patient received a variety of therapies. HSCT was administered to 59 patients, which induced sustained CR in 63% of patients with CAEBV, 60% of HLH patients, and 57% of severe mosquito bite allergy patients (Figure 4A). Seventy patients received chemotherapy such as etoposide/cyclosporine A/dexamethasone, cyclophosphamide/doxorubicin/vincristine/prednisolone (CHOP), CHOP plus etoposide, and high-dose cytosine arabinoside therapy. Chemotherapy was effective in some patients, but the effect was usually transient and failed to induce sustained CR in most cases. Chemotherapy induced sustained CR in only 5 patients, 4 of whom had HLH (Figure 4A). Immunomodulating therapies such as prednisolone, cyclosporine A, high-dose IV immunoglobulin, and methyl prednisolone pulse therapy were administered to 58 patients. The immunomodulating therapies induced sustained CR in 2 patients with HLH (Figure 4A). In patients with HLH, both chemotherapy and immunomodulating therapy induced sustained CR more frequently compared with those with CAEBV (P = .002 and P = .02, respectively). Antiviral therapies such as acyclovir, adenine arabinoside, and ganciclovir were administered to 32 patients. In 2 patients (patients 11 and 45 in supplemental Tale 1), sustained CR was achieved during oral acyclovir therapy and weekly IV administration of adenine arabinoside (Figure 4A). However, because antiviral therapies had been administered for a long time, it was not clear whether CR was induced by them or if it was spontaneously achieved. The effects of each therapy among cell types are shown in Figure 4B. There was no statistical difference in the CR rate of each therapy among cell types.
Efficacy of therapeutic interventions. (A) Number of patients treated with each therapy and patients who maintained CR are shown among categories of clinical groups. SMBA indictes severe mosquito bite allergy; and HV, hydroa vacciniforme. *P = .002; **P = .02. (B) Numbers of patients who received each therapy and those who maintained sustained CR are shown among categories of EBV-infected cells.
Efficacy of therapeutic interventions. (A) Number of patients treated with each therapy and patients who maintained CR are shown among categories of clinical groups. SMBA indictes severe mosquito bite allergy; and HV, hydroa vacciniforme. *P = .002; **P = .02. (B) Numbers of patients who received each therapy and those who maintained sustained CR are shown among categories of EBV-infected cells.
Factors associated with mortality
The factors associated with mortality were analyzed (Table 3), and univariate analysis showed that sex (female), onset age (≥ 8 years), liver dysfunction, splenomegaly, anemia, and thrombocytopenia were significantly associated with mortality. Conversely, HSCT was inversely correlated with mortality rate (odds ratio, 0.67), and this was statistically significant only in patients with T-cell infection. Multivariate analysis using factors for which P < .10 revealed that onset age and liver dysfunction were independently significant factors that increased mortality (Table 3); again, HSCT was an independent factor that decreased mortality rate.
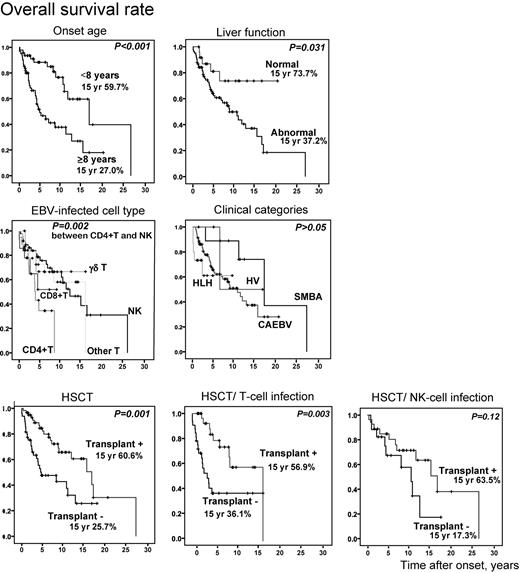
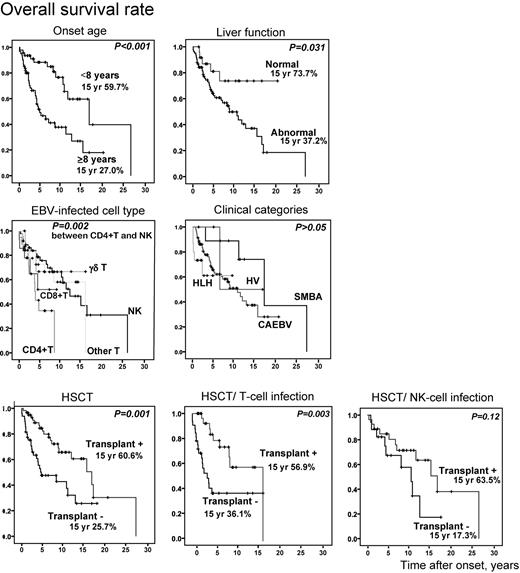
We compared overall survival rates between each subgroup to confirm association of the above factors with mortality (Figure 5). Overall survival rate in patients whose onset was more than 8 years was significantly low (P < .001). Patients with liver dysfunction at the time of diagnosis had lower survival rate (P = .031). When patients were divided into 5 groups based on EBV-infected cells, patients with CD4+ T-cell infection had a significantly lower survival rate compared with those with NK-cell infection (P = .002). However, there was no statistical difference in survival rate among clinical groups, although the numbers in some groups were small. Patients who received HSCT survived longer (P = .001) and, again, this was statistically significant only in patients with T-cell infection (P = .003).
Probability of survival rates from time of disease onset. Overall survival rates from onset (n = 108) were calculated from Kaplan-Meier estimates between each subgroup (onset age ≥ 8 years or < 8 years, with or without liver dysfunction, EBV-infected cell types, clinical categories, and with or without HSCT). HSCT patients were divided into groups based on T-cell infection (n = 64) and NK-cell infection (n = 44) and independently analyzed. SMBA indicates severe mosquito bite allergy; and HV, hydroa vacciniforme.
Probability of survival rates from time of disease onset. Overall survival rates from onset (n = 108) were calculated from Kaplan-Meier estimates between each subgroup (onset age ≥ 8 years or < 8 years, with or without liver dysfunction, EBV-infected cell types, clinical categories, and with or without HSCT). HSCT patients were divided into groups based on T-cell infection (n = 64) and NK-cell infection (n = 44) and independently analyzed. SMBA indicates severe mosquito bite allergy; and HV, hydroa vacciniforme.
Characteristics of patients after HSCT
Of 59 patients who underwent HSCT, 39 patients (66%) survived 1-144 months after transplantation (median, 35.5 months). Conversely, 20 patients (34%) died 1 day to 48 months after transplantation (median, 1.8 months). Detailed characteristics of each patient are shown in supplemental Table 3. Main causes of death were multiple organ failure (n = 5), intracranial hemorrhage (n = 5), sepsis (n = 2), and other (n = 8). Of the 20 deaths, 15 were considered to be treatment related. We compared various factors between patients who lived and those who died after HSCT (Table 4). Univariate analysis showed that age at HSCT was higher and patients with active disease status at the time of HSCT died more frequently after HSCT (Table 4). Time from disease onset to HSCT and intensity of the conditioning regimen (either myeloablative or reduced) were marginally associated with death (P = .059 and P = .086, respectively). To determine independent risk factors, we performed multivariate analysis using factors for which P < .10, and found that none was an independent risk factor for death (data not shown).
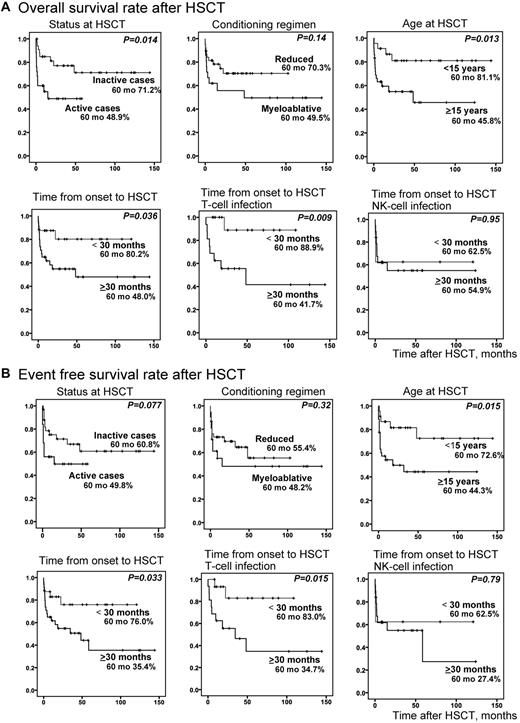
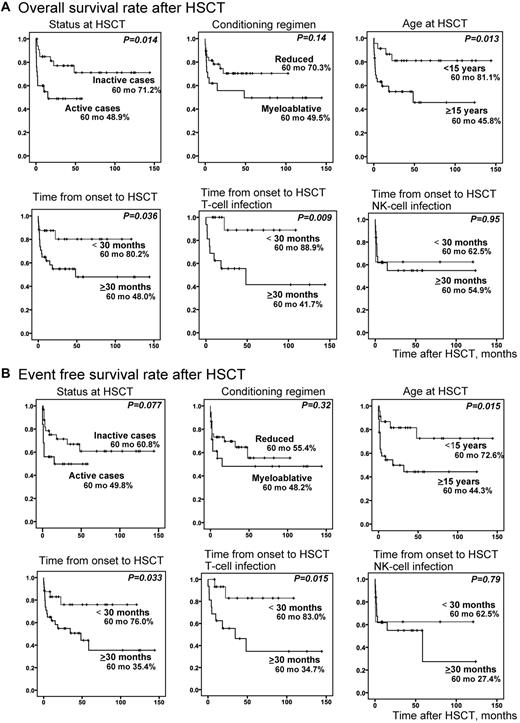
We compared overall survival rates (Figure 6A) and event-free survival rates (Figure 6B) of transplanted patients between each subgroup. Although disease status at HSCT was not an independent risk factor by multivariate analysis, overall survival rate was significantly higher in patients with inactive disease at the time of HSCT (P = .014); however, its significance diminished for the event-free survival rate. Patients who received HSCT at an age less than 15 years had significantly higher overall (P = .013) and event-free survival rates (P = .015). Patients whose time from onset to HSCT was less than 30 months also had significantly higher overall (P = .036) and event-free survival rates (P = .033). Interestingly, these were statistically significant only in patients with T-cell infection.
Probability of survival rates after HSCT. Survival rates after HSCT were calculated from Kaplan-Meier estimates between each subgroup (inactive or active cases at HSCT, reduced or myeloablative conditioning, age ≥ 15 years or < 15 years at HSCT, and time from onset to HSCT ≥ 30 months or < 30 months). Stratified ages were analyzed in advance, and ≥ 15 years was chosen as the age factor. Similarly stratified times from onset to HSCT were analyzed in advance, and ≥ 30 months was chosen as the time factor. (A) Overall survival rate after HSCT (n = 59). (B) Event-free survival rate after HSCT (n = 59). For time from onset to HSCT, patients were divided into T-cell infection (n = 32) and NK-cell infection (n = 27) groups and independently analyzed.
Probability of survival rates after HSCT. Survival rates after HSCT were calculated from Kaplan-Meier estimates between each subgroup (inactive or active cases at HSCT, reduced or myeloablative conditioning, age ≥ 15 years or < 15 years at HSCT, and time from onset to HSCT ≥ 30 months or < 30 months). Stratified ages were analyzed in advance, and ≥ 15 years was chosen as the age factor. Similarly stratified times from onset to HSCT were analyzed in advance, and ≥ 30 months was chosen as the time factor. (A) Overall survival rate after HSCT (n = 59). (B) Event-free survival rate after HSCT (n = 59). For time from onset to HSCT, patients were divided into T-cell infection (n = 32) and NK-cell infection (n = 27) groups and independently analyzed.
Discussion
Determining the phenotype of EBV-infected cells is mandatory for our further understanding of the pathogenesis of EBV+ T/NK-LPDs and related biologic behaviors. In the present study, we used unfixed peripheral blood to determine the phenotypes of EBV-infected cells. One caveat of this study is that we may have missed EBV-associated T/NK-LPDs if EBV-infected cells failed to migrate into the peripheral blood.33 Furthermore, EBV-infected cells in the peripheral blood might be different from those existing in tissues, although there was no discordant result between tissue biopsy and peripheral blood.
In the present study, EBV-infected cells in EBV+ T/NK-LPDs were immunophenotypically divided into CD4+ T cells, CD8+ T cells, γδ T cells, and NK cells, the variable proportions of which were observed in each of the clinical categories. Kasahara et al reported that CAEBV and EBV-associated HLH were largely caused by CD4+ T or NK cells and CD8+ T cells, respectively.22 We demonstrated that CAEBV was caused by not only CD4+ T and NK cells but also by CD8+ T and γδ T cells. We also demonstrated that EBV-infected cells in nearly half of hydroa vacciniforme or hydroa vacciniforme–like lymphoma patients were γδ T cells, which is in agreements with our previous observations.36 Interestingly, all of these cells express molecules characteristic of cytotoxic cells. In fact, EBER+ lymphocytes in EBV+ T/NK-LPDs usually express cytotoxic molecules including perforin, granzyme B, and TIA-1, as shown in this study and in previous studies.7,44 The mechanism underlying EBV infection of T and NK cells, which do not express CD21, remains unresolved. It has been shown that NK cells activated by EBV-infected B cells acquire CD21 by synaptic transfer, and these ectopic receptors allow EBV binding to NK-cell hosts.45 It is plausible that killer cells in close contact with EBV-infected B cells may acquire EBV infection directly and then proliferate with clonality.
In the present study, we evaluated prognostic factors among patients with EBV+ T/NK-LPDs. Multivariate analysis showed that age at onset of disease (≥ 8 years) and liver dysfunction were independent risk factors for mortality, and that patients receiving transplantations had a better prognosis. We found previously that older onset age (≥ 8 years) was associated with mortality in patients with CAEBV.29 Furthermore, a recent report demonstrated that adult patients with CAEBV had progressive and more aggressive courses than those of childhood onset cases.46 Interestingly, patients with CD4+ T-cell infection had shorter survival rates than those with NK infection, whereas clinical categories were not correlated with survival rates. Onset age of patients with CD4+ T-cell infection was high (median, 14.5 years). These results suggest that adult patients with CD4+ T-cell infection may have more aggressive features and are likely to develop multiple organ failure. Although the reason is unclear, we should be cautious about rapid progression in patients with CD4+ T-cell infection.
We surveyed administered therapies based on physician questionnaire responses. A potential limitation of this study design was the use of retrospective questionnaires; therefore, we should be cautious about the evaluation of treatment efficacy. Nevertheless, it seems that only HSCT induced CR in patients with EBV-associated T/NK-LPDs except for HLH. Some EBV-associated HLH patients responded well to chemotherapy and immunomodulating therapies,47 but patients with CAEBV were generally refractory to chemotherapy. Similar findings were reported in patients with CAEBV in the United States.20 Furthermore, Kaplan-Meier estimates indicated that shorter time from onset to HSCT (< 30 months) and inactive disease at HSCT resulted in long survival times, suggesting that earlier HSCT in patients in good condition is preferred. Patients with CAEBV have a higher risk of transplantation-related complications.41,48 Recently, Kawa et al reported excellent outcome of HSCT with reduced-intensity conditioning.40 Although the superiority of reduced-intensity conditioning over myeloablative conditioning did not reach statistical significance in that study, it appears that a reduced-intensity regimen is sufficient to prevent transplantation-related deaths.40,49
The concept of EBV+ T/NK-LPD was initially proposed by Kawa et al, and then examined by other researchers.27,44 This umbrella term encompasses specific clinical diseases of the CAEBV T/NK–cell type, EBV-associated HLH, severe mosquito bite allergy, and hydroa vacciniforme, the distinction of which are differentiated based on clinical manifestations. However, if the clinical data are absent regarding the prodromal phase of expansion of EBV+ T/NK–cells with variable clonality, we cannot discriminate systemic diseases such as ANKL and extranasal ENKL from EBV+ NK-LPDs, because EBV+ proliferating cells are indistinguishable in morphology and phenotype. Recently, this issue was highlighted by Takahashi et al.50 Interestingly, 4 patients of the present series developed ANKL in their clinical course, 2 of whom had only skin symptoms categorized as severe mosquito bite allergy at the time of the diagnosis. In addition, 6 patients who were clinically categorized as CAEBV NK-cell type (4 cases) and T-cell type (2 cases) developed ENKL; the major clinical difference from de novo ENKL was its early onset (median age, 8.5 years). Three patients had hypersensitivity to mosquito bites. There were no differences in pathologic features between these patients and de novo ENKL patients.50 Furthermore, new development of chromosomal aberrations was seen in 6 patients during follow-up. In this study, most of the patients with EBV+ T/NK-LPDs had clonality of EBV-infected cells. These results indicate that patients with clonally expanding EBV-infected T or NK cells in EBV+ T/NK-LPD eventually develop overt leukemia and lymphoma, the clinicopathologic findings of which are in keeping with those well documented in extranasal ENKL, ANKL, and PTCL, with additional mutations in cancer genes or tumor-suppressor genes.
In 2008, an international meeting was organized at the National Institute of Health to better define the pathogenesis, classification, and treatment of EBV-associated LPDs in nonimmunocompromised hosts.39 At that meeting, acute and chronic EBV syndromes of T cells and NK cells were clarified to have a broad spectrum, in which hydroa vacciniforme, hydroa vacciniforme–like lymphoma, severe mosquito bite allergy, and systemic EBV+ T-LPD of childhood were listed as EBV+ T/NK-LPDs under an umbrella term of CAEBV of T/NK–cell type.39 In the present study, EBV+ T/NK-LPD is characterized by the systemic distribution of EBV+ clones beyond the clinical categorization currently proposed as CAEBV, HLH, severe mosquito bite allergy, and hydroa vacciniforme. Furthermore, we also shed light on the clinicopathologic distinctiveness of patients with NK-cell infection, which has not been well addressed in the past even though these patients comprise approximately 40% of EBV+ T/NK-LPD cases. This phenotype was more closely associated with hypersensitivity to mosquito bite and a relatively indolent clinical course, the biologic significance of which should be clarified in the future.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank S. Kumagai, F. Ando, and H. Yamada for the excellent technical support and the following collaborating institutions and their staff for providing the specimens and patient data: Aichi Cancer Center, Dokkyo University Hospital, Ehime Prefectural Central Hospital, Ehime University Hospital, Fujita Health University Hospital, Gifu Prefectural General Medical Center, Gifu University Hospital, Gifu Municipal Hospital, Gunma Children's Medical Center, Hamamatsu University School of Medicine, Hyogo Prefectural Kobe Children's Hospital, Ibaraki Children's Hospital, Ichinomiya Municipal Hospital, Ikeda Municipal Hospital, Japanese Red Cross Kitami Hospital, Japanese Red Cross Nagoya Daiichi Hospital, Juntendo University Hospital, Kansai Medical University Hospital, Kitasato University School of Medicine, Kochi Medical School Hospital, Kumamoto University Hospital, Kyoto Prefectural University of Medicine, Kyoto University Hospital, Matsushita Memorial Hospital, Meitetsu Hospital, Nagasaki University Hospital, Nagoya Medical Center, Niigata Cancer Center Hospital, Niigata University Hospital, Nippon Medical School, NTT Medical Center Tokyo, Ogaki Municipal Hospital, Oita University Hospital, Okayama University Hospital, Okazaki City Hospital, Osaka City General Hospital, Osaka City University Hospital, Osaka University Hospital, Ohta General Hospital, Rinku General Medical Center, Sakai Hospital Kinki University Faculty of Medicine, Saitama Children's Medical Center, Shimane University Hospital, Shinshu University Hospital, Shizuoka Cancer Center, Shizuoka Children's Hospital, Shizuoka General Hospital, Showa University Fujigaoka Hospital, Social Insurance Kinan Hospital, Steel Memorial Hirohata Hospital, Teine Keijinkai Hospital, Tokyo Medical and Dental University, Tohoku University Hospital, Tosei General Hospital, Toyama University Hospital, Toyohashi Medical Center, Toyohashi Municipal Hospital, Toyokawa City Hospital, Tsukuba University Hospital, The Institute of Medical Sciences, The University of Tokyo, The University of Tokyo Hospital, Yamagata University, Yamaguchi University Hospital, University of Miyazaki Hospital, Yokohama City University Hospital, Yokohama Minami Kyousai Hospital, and Wakayama Medical University Hospital.
This study was supported in part by a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan (21591384) and a Health and Labor Science Research Grant on intractable diseases from the Ministry of Health, Labor and Welfare of Japan (H22-Nanchi-080 to H.K.).
Authorship
Contribution: H.K. designed the study, followed the patients, analyzed the data, and wrote the manuscript; Y.I. contributed to the study design, followed the patients, and helped to edit the manuscript; S. Kawabe, K.G., and S.E. performed the experiments; Y.T., S. Kojima, and T.N. followed the patients, collected the clinical data, and helped to edit the manuscript; A.K., A.S., and K.K. followed the patients and collected the clinical data; K.O. performed the experiments and helped to edit the manuscript; and S.N. contributed to the study design, performed the experiments, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hiroshi Kimura, MD, PhD, Department of Virology, Nagoya University Graduate School of Medicine, 65 Tsurumai-cho, Showa-ku, Nagoya 466-8550, Japan; e-mail: hkimura@med.nagoya-u.ac.jp.