Abstract
MicroRNAs (miRNAs) are short noncoding RNAs that regulate gene expression and are important for pre-B and follicular B lymphopoiesis as demonstrated, respectively, by mb-1-Cre– and cd19-Cre–mediated deletion of Dicer, the RNase III enzyme critical for generating mature miRNAs. To explore the role of miRNAs in B-cell terminal differentiation, we use Aicda-Cre to specifically delete Dicer in activated B cells where activation-induced cytidine deaminase is highly expressed. We demonstrate that mutant mice fail to produce high-affinity class-switched antibodies and generate memory B and long-lived plasma cells on immunization with a T cell–dependent antigen. More importantly, germinal center (GC) B-cell formation is drastically compromised in the absence of Dicer, as a result of defects in cell proliferation and survival. Dicer-deficient GC B cells express higher levels of cell cycle inhibitor genes and proapoptotic protein Bim. Ablation of Bim could partially rescue the defect in GC B-cell formation in Dicer-deficient mice. Taken together, our data suggest that Dicer and probably miRNAs are critical for GC B-cell formation during B-cell terminal differentiation.
Introduction
MicroRNAs (miRNAs) are short (∼ 22 nucleotides), endogenous, and noncoding RNAs that have recently emerged as key regulators of gene expression at the posttranscriptional level. They are first transcribed by RNA polymerase II as primary transcripts1,2 that are later processed by the RNase III-type endonucleases, Drosha and Dicer, into mature miRNAs that bind the 3′-untranslated region of target mRNAs and lead to the degradation of mRNA or inhibition of protein translation.3-5 Ablation of either Drosha or Dicer would abort the generation of most mature miRNAs and thus allows one to study the collective requirement of miRNAs in cell differentiation and function.
B cells develop in the bone marrow from committed hematopoietic stem cells into pro-B, pre-B, and immature B cells and later seed the peripheral lymphoid tissues as mature B cells before differentiating into antibody-secreting plasma cells (PCs) and memory B cells on antigen stimulation.6 Germline deletion of Dicer leads to embryonic lethality,7 but tissue-specific deletion of Dicer in B cells demonstrates an essential requirement of miRNAs in early B lymphopoiesis. When Dicer is deleted in the very early stage of murine B-cell development using mb1-Cre–mediated excision of loxP-flanked Dicer1 (Dicer1fl/fl) alleles, B-cell differentiation in the bone marrow is almost completely arrested at the pro-B to pre-B cell transition stage; and as a consequence, immature and mature B cells are hardly detected in the mutant mice. Detailed study shows that Bim, a proapoptotic molecule crucial for life-death decision in developing lymphocyte, is up-regulated in Dicer-deficient pro-B cells and that deletion of Bim or overexpression of Bcl2 can partially rescue B-cell development in the absence of Dicer.8 A recent study further shows that Dicer is important for the generation of follicular B cells from immature B cells as cd19-Cre–mediated deletion of Dicer in developing B lymphocytes results in an over-representation of transitional and marginal zone B cells and an impairment of follicular B-cell generation in the periphery, and this leads to a skewed antibody repertoire.9 Although these studies have given substantial insights into the role of Dicer and probably miRNAs in early B and follicular B-cell development, it remains to be determined whether they play a role in the terminal stages of B-cell differentiation when B lymphocytes encounter specific antigens.
On activation, antigen-specific B cells differentiate into long-lived memory B cells and PCs, which together provide an individual with life-long protective humoral immunity.10-12 It is thought that memory B cells and long-lived PCs are generated with the help of T cells and dendritic cells within a specialized microenvironment known as germinal centers (GCs) that are formed in the secondary lymphoid tissues after B cells' encounter with foreign antigens.13-15 Within GCs, antigen-specific B cells undergo extensive clonal expansion and modify their immunoglobulin (Ig) variable and constant region genes through somatic hypermutation and class-switch recombination, respectively.16-18 The latter 2 processes are mediated by a critical enzyme known as activation-induced cytidine deaminase (AID), which is highly expressed in GC B cells.19 It is also in GC that B cells are subject to extensive selection based on their ability to bind foreign antigens such that cells with high-affinity are selected and allowed to survive while their low affinity peers undergo apoptosis.20-22 The deregulation of GC response may cause autoimmune diseases or lead to B-cell malignancies, such as diffuse large B-cell lymphoma, Burkitt lymphoma, and multiple myeloma.
Here, we have examined the role of Dicer in the differentiation of antigen-activated B cells by taking advantage of the selectively induced and high expression level of AID in GC B cells after B cells encounter with a T cell–dependent antigen.23 We have generated Dicer1fl/flAicdaCre/+ mice in which the expression of the Cre recombinase is under the physiologic control of AID expression and Dicer is ablated only in activated B cells expressing AID but not in naive B cells. We demonstrate that Dicer is required for the production of antigen-specific high-affinity class-switched antibodies during a T cell–dependent immune response. Mutant mice have drastically impaired formation of GC B cells, which could account for their failure to generate memory B cells and long-lived PCs. Dicer-deficient GC B cells exhibit severe defect in cell proliferation and survival, which may be the result of the derepression of cell cycle inhibitor genes and proapoptotic molecule Bim. We further show that the defect in GC B-cell formation in Dicer1fl/flAicdaCre/+ mice can be partially rescued by deleting Bim. Thus, our findings establish a critical role for Dicer and probably miRNAs in GC B-cell formation during B-cell terminal differentiation.
Methods
Mice and immunization
Dicer1fl/fl24 and Bim−/−25 mice were obtained from The Jackson Laboratory. AicdaCre/+ mice were generated in the laboratory of Nancy Jenkins by knocking in Cre cDNA between the stop codon and 3′-untranslated region of Aicda (described in detail elsewhere). Dicer1fl/fl mice were bred with AicdaCre/+ mice to generate Dicer1fl/flAicdaCre/+ mice, and these mice were further crossed with Bim−/− mice to generate Dicer1fl/flAicdaCre/+Bim−/− mice. Eight- to 12-week-old mice were immunized with 100 μg NP38-chicken γ-globulin (CGG; Biosearch Technologies) in Imject alum (Pierce Biotechnology) by intraperitoneal injection. Mice were bred and maintained in our animal facilities and used according to guidelines issued by the National Advisory Committee on Laboratory Animal Research.
Flow cytometry and cell sorting
Cells were harvested from bone marrow, spleen, lymph nodes, peritoneal cavities, and Peyer patches (PPs) of mice and treated with Fc block and stained with biotin- or fluorescent-labeled mAbs as described.26 Biotin conjugates were visualized with streptavidin-conjugated peridinin chlorophyll protein-cyanine 5.5. Fluorescein-labeled peanut agglutinin (PNA) and 4,6-diamidino-2-phenylindole were purchased from Sigma-Aldrich. PE- or allophycocyanin (APC)–labeled NIP-BSA (Biosearch Technologies) was prepared using APC or R-PE labeling kit-SH (Dojindo). Dead cells were excluded electronically using 4,6-diamidino-2-phenylindole. Exclusion staining (Dump) of cells included anti-IgD, anti-CD138, anti-CD3, anti-Th1.2, anti–Gr-1, anti-F4/80, anti-Ter119, anti-DX5, anti-NK1.1, anti-AA4.1, and anti-CD5 mAbs. The following mAbs were from BD Biosciences PharMingen or as specified: FITC anti-Fas, FITC anti-CD19, FITC anti-IgG1, FITC anti-IgA; PE anti-Fas, biotinylated (bio-) anti-CD38, bio-anti–IgM, bio-anti–IgD, bio-anti–CD138, bio-anti–Thy1.2, bio-anti–CD3, bio-anti–Gr-1, bio-anti–F4/80, bio-anti–CD5, bio-anti–Ter119, bio-anti–DX5, bio-anti–NK1.1, and bio-anti–AA4.1; APC anti-CD19, APC anti-CD38 (BioLegend), APC annexin V (BioLegend), APC-Cy7 anti–B220, and APC-Cy7 anti–CD19. For intracellular staining of Bim, splenic GC B cells were stained with FITC-conjugated hamster anti–mouse Bim monoclonal antibody (Santa Cruz Biotechnology). Data were collected using LSR II flow cytometer (BD Biosciences PharMingen) and analyzed with FlowJo Version 7.2.2 (TreeStar). For cell isolation, CD19+CD38−Fas+ activated B cells and CD19+CD38+Fas− nonactivated B cells from PPs of unimmunized mice or CD19+CD38−Fas+PNA+CD90−CD11b−PI− GC B cells from spleens of immunized mice were sorted with FACSAria (BD Biosciences PharMingen).
Histology
Spleens and PPs were harvested, embedded in Tissue-Tek, and “snap-frozen” in dry ice and ethanol and stored at −80°C. Cryostat sections (8 μm in thickness) were prepared, air-dried, and fixed in ice-cold acetone for 15 minutes. Sections were blocked with 5% rat serum and stained with PNA-FITC and the following antibodies: FITC anti–GL-7, PE anti-IgD, and bio-anti–CD35 (BD Biosciences PharMingen). Biotinylated antibodies were detected with streptavidin-APC. Sections were further mounted with CYTOSEAL 60 (Electron Microscopy Sciences) and analyzed with an Olympus FV1000 microscope (Olympus) using a 20× objective, and the images were acquired with Olympus Fluoview Version 2.1 software.
ELISA and ELISPOT assay
Sera 4-hydroxy-3-nitrophenylacytyl (NP)-specific antibodies in immunized mice were measured via ELISA. Briefly, 386-well flat-bottom plates (NUNC) were coated with 5 μg/mL of NP17-BSA or NP2-BSA and blocked with 1% BSA in PBS. Serially diluted sera were added. After washing, the plates were incubated with diluted biotinylated anti-IgM, anti-IgG1, anti-IgG2b, or anti-IgG3 antibodies followed by streptavidin-conjugated HRP. Bound secondary antibodies were assessed by incubation with 3,3′,5,5′ tetramethyl benzidine thiobarbituric acid substrate. To analyze the frequency of antibody-forming cells, an ELISPOT assay was performed as described previously using MultiScreen filter plates (Millipore).27
Cell proliferation and apoptosis assays
Mice were injected intraperitoneally with 2 mg BrdU (Sigma-Aldrich) 4 hours before death. Cell proliferation was examined by BrdU incorporation using BrdU Detection kit (BD Biosciences PharMingen) according to the manufacturer's instructions. For detecting apoptosis, splenocytes were incubated at 37°C with benzyloxycarbony-Val-Ala-Asp-fluoromethyl-ketone-FITC (z-VAD-fmk–FITC; Casp-Glow; Bio Vision) for 45 minutes. Cells were washed according to the manufacturer's protocol and subsequently labeled with the appropriate antibodies for surface staining. For the detection of cell death in vitro, sorted GC B cells (CD19+Fas+PNA+CD38−) were cultured in DMEM supplemented with 10% heat-inactivated FCS for 5 to 6 hours and stained with annexin V.
Statistics
Two-tailed Student t test was used for statistical analyses.
Results
Severe impairment of T cell–dependent antibody responses in immunized Dicer1fl/flAicdaCre/+ mice
To examine the role of Dicer in B-cell terminal differentiation, we have used Aicda-Cre allele to delete Dicer1fl/fl alleles at the stage where B cells are activated by specific antigens and before their differentiation into memory B and antibody-producing cells.
First, we tested Cre-mediated Dicer1 deletion in our system by PCR analyses using activated (CD19+CD38−Fas+) and nonactivated (CD19+CD38+Fas−) B cells from PPs of Dicer1fl/+AicdaCre/+ mice. Our results showed that the floxed-Dicer1 allele was efficiently deleted in activated B cells coexpressing the endogenous Aicda and inserted Cre genes (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Next, we generated Dicer1fl/flAicdaCre/+ mice and found that before immunization Dicer1fl/flAicdaCre/+ mice had normal B-cell subsets in the bone marrow, spleen, lymph nodes, and peritoneal cavities compared with Dicer1+/+AicdaCre/+ wild-type (WT) mice (supplemental Figure 2; supplemental Table 1). However, unchallenged Dicer1fl/flAicdaCre/+ mice displayed lower basal serum levels of IgG1, IgG2b, IgG3, and IgA, whereas their IgM level was normal compared with WT mice (supplemental Figure 2).
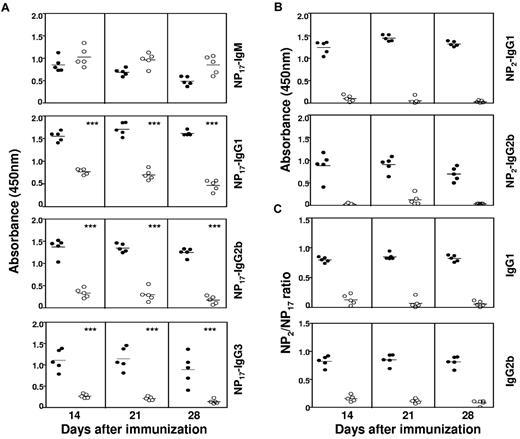
We proceeded to immunize these mice with the T cell–dependent antigen, NP coupled to CGG, and absorbed onto alum. Dicer1fl/flAicdaCre/+ mice produced normal, if not slightly increased, amounts of NP-specific IgM antibodies compared with WT mice at 14, 21, and 28 days after immunization (Figure 1Ai). However, the titers of NP-specific IgG1 antibodies in Dicer1fl/flAicdaCre/+ mice were drastically reduced compared with WT mice for all 3 time points examined when NP17-BSA was used as the capture antigen (Figure 1Aii). Similar reductions in NP-specific IgG2b and IgG3 antibody titers were also evident in Dicer1fl/flAicdaCre/+ mice (Figure 1Aiii-iv), suggesting that antigen-activated B cells lacking Dicer are defective in generating antigen-specific Ig class–switched antibodies. In addition, when NP2-BSA was used to capture high-affinity antibodies, NP-specific IgG1 and IgG2b antibodies were hardly detected in Dicer1fl/flAicdaCre/+ mice (Figure 1B). When the ratios of NP2/NP17-binding antibodies were calculated, it was readily apparent that Dicer1fl/flAicdaCre/+ mice had striking reduction in the NP2/NP17 ratios for IgG1 and IgG2b antibodies compared with WT mice (Figure 1C), implying that the loss of Dicer in antigen-activated B cells drastically compromised the generation of high-affinity class-switched antibodies. Taken together, these data indicated that Dicer in antigen-activated B cells is essential for the generation of high-affinity class-switched antibodies during a primary T cell–dependent immune response.
Defective T cell–dependent antibody response in Dicer1fl/flAicdaCre/+ mice. (A-B) Serum anti–NP IgM (1:1000 dilution), IgG1 (1:9000 dilution), IgG2b (1:3000 dilution), and IgG3 (1:1000 dilution) antibodies from NP38-CGG immunized Dicer1+/+AicdaCre/+ (●) and Dicer1fl/flAicdaCre/+ (○) mice were measured by ELISA using NP17-BSA (A) or NP2-BSA (B) as coating antigens. Mice were immunized on day 0, and sera were collected at the indicated time points after immunization. (C) Ratios of anti-NP IgG1 and IgG2b titers detected with NP2-BSA compared with NP17-BSA as coating antigens in ELISA. Each symbol represents 1 mouse. Data shown are representative of 3 repeated experiments and each experimental group contained at least 5 mice. ***P < .001 (Student t test).
Defective T cell–dependent antibody response in Dicer1fl/flAicdaCre/+ mice. (A-B) Serum anti–NP IgM (1:1000 dilution), IgG1 (1:9000 dilution), IgG2b (1:3000 dilution), and IgG3 (1:1000 dilution) antibodies from NP38-CGG immunized Dicer1+/+AicdaCre/+ (●) and Dicer1fl/flAicdaCre/+ (○) mice were measured by ELISA using NP17-BSA (A) or NP2-BSA (B) as coating antigens. Mice were immunized on day 0, and sera were collected at the indicated time points after immunization. (C) Ratios of anti-NP IgG1 and IgG2b titers detected with NP2-BSA compared with NP17-BSA as coating antigens in ELISA. Each symbol represents 1 mouse. Data shown are representative of 3 repeated experiments and each experimental group contained at least 5 mice. ***P < .001 (Student t test).
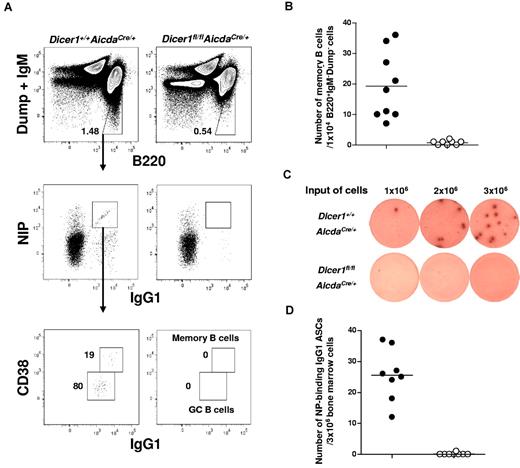
Absence of antigen-specific memory B and long-lived PCs in Dicer1fl/flAicdaCre/+ mice
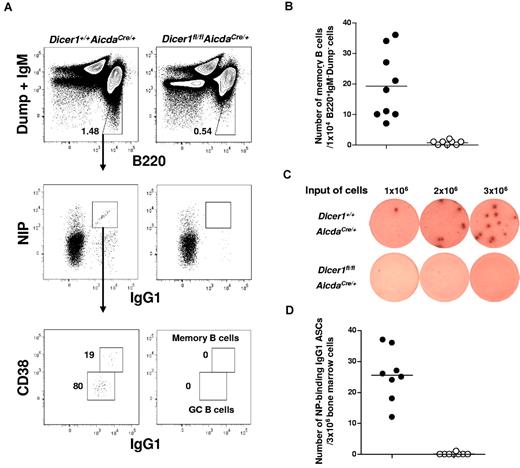
During T cell–dependent immune responses, memory B cells and long-lived PCs are generated to establish life-long protective humoral immunity in an organism.10,11,13 After immunization with NP-CGG, mice produce predominantly NP-specific antibody of the IgG1 isotype.28,29 We first analyzed the frequency of NP-specific CD38+IgG1high (hi) memory B cells in the spleens of Dicer1fl/flAicdaCre/+ mice at day 56 after primary immunization when the antibody response would have already matured. NP-specific B cells can be detected via flow cytometry by staining cells with fluorochrome-conjugated NIP, an analog of NP.30 As shown in Figure 2A, antigen-specific NIP+IgG1+ B cells and NIP+CD38+IgG1hi memory B cells were detected in WT but not in Dicer1fl/flAicdaCre/+ mice. Quantification of these antigen-specific memory B cells showed that they were virtually absent in Dicer1fl/flAicdaCre/+ mice (Figure 2B).
Long-lived plasma cells and memory B cells are absent in immunized Dicer1fl/flAicdaCre/+ mice. (A) Flow cytometric analysis of NP-specific IgG1 memory B cells in the spleen of mice 56 days after immunization with NP38-CGG. Splenic cells were stained for B220, IgG1, and CD38 expression and NIP binding after exclusion of dead cells (4,6-diamidino-2-phenylindole), non-B cells (Dump), and IgM+ cells. The numbers adjacent to the outlined areas indicate the percentage of gated cells (upper panel) and the absolute number of cells per 1 × 104 IgM−Dump−B220+ cells (bottom panel). (B) NIP-binding CD38+IgG1hi memory B cells in the spleens of Dicer1+/+AicdaCre/+ (●) and Dicer1fl/flAicdaCre/+ mice (○) mice 56 days after immunization. (C) ELISPOT assays of anti–NP IgG1 ASCs cells in the bone marrow of day 56 postimmunized Dicer1+/+AicdaCre/+ and Dicer1fl/flAicdaCre/+ mice using BSA-NP17 as coating antigens. (D) Anti–NP IgG1 ASCs in the bone marrow of Dicer1+/+AicdaCre/+ (●) and Dicer1fl/flAicdaCre/+ (○) mice 56 days after immunization were quantified by ELISPOT assay. Each symbol represents 1 mouse.
Long-lived plasma cells and memory B cells are absent in immunized Dicer1fl/flAicdaCre/+ mice. (A) Flow cytometric analysis of NP-specific IgG1 memory B cells in the spleen of mice 56 days after immunization with NP38-CGG. Splenic cells were stained for B220, IgG1, and CD38 expression and NIP binding after exclusion of dead cells (4,6-diamidino-2-phenylindole), non-B cells (Dump), and IgM+ cells. The numbers adjacent to the outlined areas indicate the percentage of gated cells (upper panel) and the absolute number of cells per 1 × 104 IgM−Dump−B220+ cells (bottom panel). (B) NIP-binding CD38+IgG1hi memory B cells in the spleens of Dicer1+/+AicdaCre/+ (●) and Dicer1fl/flAicdaCre/+ mice (○) mice 56 days after immunization. (C) ELISPOT assays of anti–NP IgG1 ASCs cells in the bone marrow of day 56 postimmunized Dicer1+/+AicdaCre/+ and Dicer1fl/flAicdaCre/+ mice using BSA-NP17 as coating antigens. (D) Anti–NP IgG1 ASCs in the bone marrow of Dicer1+/+AicdaCre/+ (●) and Dicer1fl/flAicdaCre/+ (○) mice 56 days after immunization were quantified by ELISPOT assay. Each symbol represents 1 mouse.
When long-lived PCs in bone marrow were examined by ELISPOT assays, it was apparent that NP-specific IgG1 antibody-secreting cells (ASCs) were also absent in Dicer1fl/flAicdaCre/+ mice. In contrast, a sizeable number of these cells were found in the bone marrow of WT mice (Figure 2C-D). The complete absence of memory B cells in the spleens and long-lived PCs in the bone marrow of Dicer1fl/flAicdaCre/+ mice suggest that the expression of Dicer and probably miRNAs in antigen-activated B cells is required for the formation of memory B cells and long-lived PCs during T cell–dependent immune response.
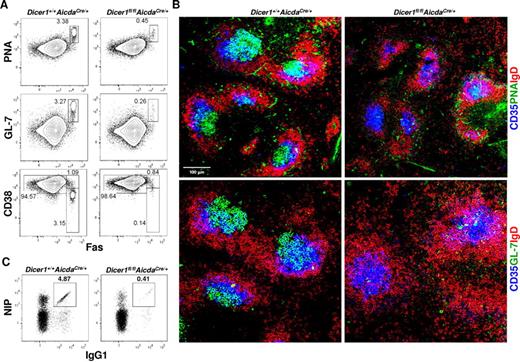
Defective GC B-cell formation in Dicer1fl/flAicdaCre/+ mice
The impaired production of class-switched and high-affinity antigen-specific antibodies and the absence of memory B cells and long-lived PCs in Dicer1fl/flAicdaCre/+ mice prompted us to determine the precise stage in which Dicer deficiency impacted on the antigen-driven phase of B-cell differentiation. It is known that, in T cell–dependent immune response, GCs are critical sites for antigen-specific B cells to expand and modify their Ig genes through somatic hypermutation and class switching such that cells with higher affinities are selected and further differentiate into memory B cells and long-lived PCs.11,13-15 Hence, we examined GC response in immunized Dicer1fl/flAicdaCre/+ mice.
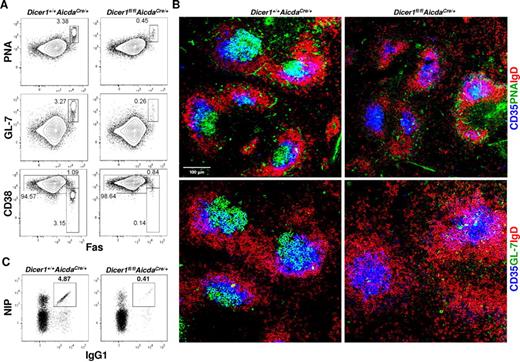
As shown in Figure 3A, significant population of GC B cells (defined as CD19+PNA+Fas+GL-7+CD38−) was detected in the spleens of immunized WT mice at day 10 after immunization. By contrast, this population of GC B cells was drastically decreased by 6- to 10-fold in immunized Dicer1fl/flAicdaCre/+ mice. The profound reduction in GC B cells in immunized Dicer1fl/flAicdaCre/+ mice was also evident when GCs were visualized by confocal microscopy. GC B cells, which were PNA+ and IgD−, were readily detected in the central area of splenic follicles and found to be associated with CD35+ follicular dendritic cells (FDCs) in spleens of WT mice at day 10 after immunization (Figure 3B). In contrast, very few, if any, PNA+IgD− B cells were detected in the spleen follicles of immunized Dicer1fl/flAicdaCre/+ mice, although CD35+ FDCs were found to be present. Similar results were also obtained when GL-7 was used to stain for GC B cells (Figure 3B).
Dicer-deficiency in antigen-activated B cells leads to severe impairment in GC B-cell formation. (A) Flow cytometric analysis of splenic GC B cells from Dicer1+/+AicdaCre/+ and Dicer1fl/flAicdaCre/+ mice 10 days after immunization with NP38-CGG. CD19+ splenic B cells were stained for the expression of PNA, GL-7, CD38, and Fas. (B) Confocal immunofluorescence microscopy of spleen cryosections from Dicer1+/+AicdaCre/+ and Dicer1fl/flAicdaCre/+ mice 10 days after immunization performed as described in “Histology.” The sections were stained with anti-IgD (red), anti-CD35 (blue), anti–GL-7 (green) antibodies or PNA (green) to visualize B-cell follicles (IgD+), FDCs (CD35+), and GC B cells (GL-7+ or PNA+). (C) Flow cytometric analysis of NP-specific IgG1 cells in the spleens of mice 10 days after immunization using the same gating as in Figure 2A. Numbers adjacent to outlined area indicate the percentage of cells in the gate. Data are representative of more than 10 (A,C) or 8 (B) experiments.
Dicer-deficiency in antigen-activated B cells leads to severe impairment in GC B-cell formation. (A) Flow cytometric analysis of splenic GC B cells from Dicer1+/+AicdaCre/+ and Dicer1fl/flAicdaCre/+ mice 10 days after immunization with NP38-CGG. CD19+ splenic B cells were stained for the expression of PNA, GL-7, CD38, and Fas. (B) Confocal immunofluorescence microscopy of spleen cryosections from Dicer1+/+AicdaCre/+ and Dicer1fl/flAicdaCre/+ mice 10 days after immunization performed as described in “Histology.” The sections were stained with anti-IgD (red), anti-CD35 (blue), anti–GL-7 (green) antibodies or PNA (green) to visualize B-cell follicles (IgD+), FDCs (CD35+), and GC B cells (GL-7+ or PNA+). (C) Flow cytometric analysis of NP-specific IgG1 cells in the spleens of mice 10 days after immunization using the same gating as in Figure 2A. Numbers adjacent to outlined area indicate the percentage of cells in the gate. Data are representative of more than 10 (A,C) or 8 (B) experiments.
We next determined the expression levels of miRNAs in those rudimentary GC B cells (CD19+Fas+PNA+CD38−) from immunized Dicer1fl/flAicdaCre/+ mice. We randomly chose miR-140, miR-25, miR-191, and miR-93, which have been shown to be highly expressed in GC B cells by a recent deep-sequencing study31 and found that these miRNAs were significantly reduced (70%-90%) in mutant GC B cells compared with WT cells (supplemental Figure 3). Taken together, these data suggest that Dicer and probably Dicer-dependent miRNAs are critical for the formation of GC B cells during T cell–dependent immune response.
When class-switched antigen-specific NIP+IgG1+ B cells were further examined, there was a more than 10-fold reduction in the population of antigen-specific IgG1 B cells in spleens of immunized Dicer1fl/flAicdaCre/+ mice compared with WT mice at day 10 after immunization (Figure 3C; supplemental Figure 4), indicating that few class-switched antigen-specific IgG1 B cells were formed at this time point during the immune response. It was possible that the significant reduction of antigen-specific IgG1 B cells in the spleens of immunized Dicer1fl/flAicdaCre/+ mice at day 10 after immunization could be the result of delayed differentiation of these cells in the absence of Dicer, and they might accumulate at later time points. However, the population of NIP+IgG1+ B cells was even more severely decreased in Dicer1fl/flAicdaCre/+ mice compared with WT mice at day 28 and day 56 after immunization (supplemental Figure 4), thus excluding the possibility of delayed appearance of these antigen-specific IgG1 B cells in the mutant mice.
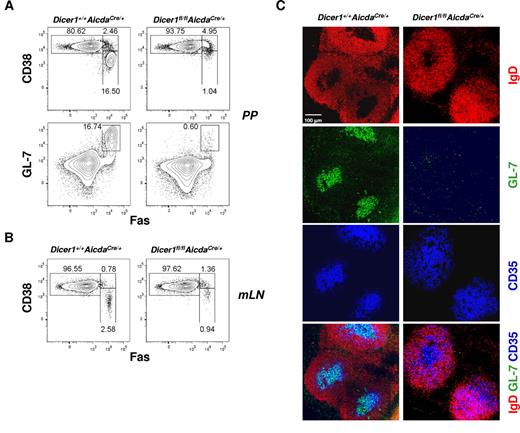
GC B cells are also formed in the gut-associated lymphoid tissues, such as the PPs and mesenteric lymph nodes of nonimmunized mice, because of the chronic stimulation of immune cells in these sites by gut-derived microbes.32 As shown in Figure 4A, the CD19+CD38−Fas+ or CD19+GL-7+Fas+ GC B cell population was decreased by 15- to 20-fold in the PPs of Dicer1fl/flAicdaCre/+ mice compared with WT mice. Microscopically, we could detect only very few GL-7+IgD− cells in PPs follicles of Dicer1fl/flAicdaCre/+ mice, whereas a considerable population of GL-7+IgD− cells was found to reside in the PP follicles and associate with FDCs in WT mice (Figure 4C). The reduction of GC B-cell population was also observed in the mesenteric lymph nodes of Dicer1fl/flAicdaCre/+ mice compared with WT counterparts (Figure 4B). Thus, our results suggest that Dicer also plays an important role in GC B-cell formation during chronic GC responses in gut-associated lymphoid tissues similar to its role during acute GC response when mice are challenged with T cell–dependent antigens.
Absence of chronic GCs in the gut-associated lymphoid tissues of Dicer1fl/flAicdaCre/+ mice. Flow cytometric analysis of GC B cells in the Peyer patches (PP; A), or mesenteric lymph nodes (mLN; B), of unchallenged Dicer1+/+AicdaCre/+ and Dicer1fl/flAicdaCre/+ mice. GC B cells were stained as described in Figure 3A. Numbers adjacent to outlined area indicated percentage of cells in the individual gate. (C) Confocal microscopy of PP cryosections from unchallenged Dicer1+/+AicdaCre/+ and Dicer1fl/flAicdaCre/+ mice. The sections were stained with the same cocktail of antibodies as described in Figure 3B to visualize B-cell follicles, FDCs, and GC B cells. Data are representative of more than 6 experiments (A-B) or 3 experiments (C).
Absence of chronic GCs in the gut-associated lymphoid tissues of Dicer1fl/flAicdaCre/+ mice. Flow cytometric analysis of GC B cells in the Peyer patches (PP; A), or mesenteric lymph nodes (mLN; B), of unchallenged Dicer1+/+AicdaCre/+ and Dicer1fl/flAicdaCre/+ mice. GC B cells were stained as described in Figure 3A. Numbers adjacent to outlined area indicated percentage of cells in the individual gate. (C) Confocal microscopy of PP cryosections from unchallenged Dicer1+/+AicdaCre/+ and Dicer1fl/flAicdaCre/+ mice. The sections were stained with the same cocktail of antibodies as described in Figure 3B to visualize B-cell follicles, FDCs, and GC B cells. Data are representative of more than 6 experiments (A-B) or 3 experiments (C).
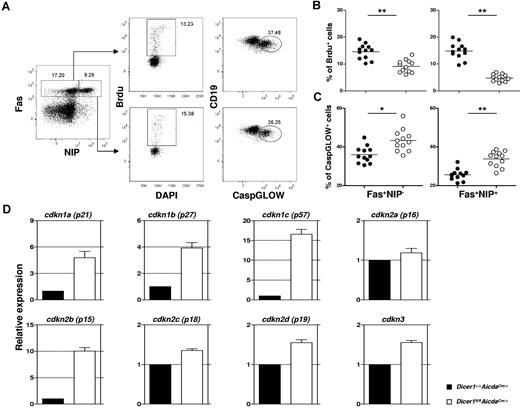
Antigen-activated B cells lacking Dicer exhibit defects in proliferation and survival
We further investigated the reason for the defective formation of GC B cells in immunized Dicer1fl/flAicdaCre/+ mice. During GC response, antigen-activated B cells undergo rapid proliferation followed by somatic hypermutation to diversify their Ig variable genes and class-switching to other Ig isotypes.16-18 Another characteristic of GC B cells is their propensity to undergo cell death, and only those GC B cells with high affinity for foreign antigens could survive, whereas their low-affinity peers were deleted by apoptosis.12,20,33 It is possible that the near-absence of GC B cells in antigen-challenged Dicer1fl/flAicdaCre/+ mice is the result of reduced proliferation or greater apoptosis of GC B cells or both.
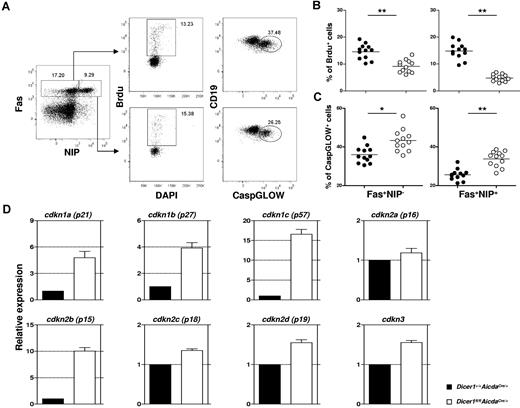
We assessed the proliferation of GC B cells by measuring their incorporation of the thymidine analog BrdU. Mice at day 10 after immunization were pulsed with BrdU for 4 hours before analysis. As shown in Figure 5A and quantified in Figure 5B, significant population of GC B cells (CD19+Fas+NIP+ and CD19+Fas+NIP−) in spleens of Dicer1+/+AicdaCre/+ mice incorporated BrdU, suggesting that they were proliferating. By contrast, there were 3- to 5-fold less BrdU-incorporated GC B cells in similarly treated Dicer1fl/flAicdaCre/+ mice (Figure 5B), indicating that the proliferative capacity of Dicer-deficient GC B cells is profoundly impaired.
Combined defects in cell proliferation and survival and aberrant expression of cell cycle inhibitor genes in Dicer-deficient GC B cells. (A) Flow cytometric analysis of BrdU incorporation and CaspGLOW stainings in splenic GC B cells after immunization with NP38-CGG. A representative FACS profile and gating strategy for the detection of BrdU+ and CaspGLOW+ cells among CD19+Fas+ Dump−NIP− and CD19+Fas+Dump−NIP+ cells from Dicer1+/+AicdaCre/+ mice at day 10 after immunization are shown. Mice were pulsed with BrdU (2 mg) 4 hours before analysis. Numbers adjacent to the outlined area indicate the percentage of cells in the individual gate. Data are representative of more than 10 experiments. (B-C) The frequencies of splenic B cells that are BrdU+ (B) or CaspGLOW+ (C) among splenic CD19+Fas+Dump−NIP− and CD19+Fas+Dump−NIP+ cells from Dicer1+/+AicdaCre/+ (●) and Dicer1fl/flAicdaCre/+ (○) mice 10 days after immunization are quantified. Each symbol represents 1 mouse. (D) Real-time RT-PCR analyses of various CDK inhibitor gene expression in Dicer1+/+AicdaCre/+ (filled) and Dicer1fl/flAicdaCre/+ (empty) GC B cells (CD19+Fas+PNA+CD38−). Bars represent relative expression of genes normalized to the expression of hprt (mean values of 3 independently sorted GC B-cell samples). **P < .01 (Student t test).
Combined defects in cell proliferation and survival and aberrant expression of cell cycle inhibitor genes in Dicer-deficient GC B cells. (A) Flow cytometric analysis of BrdU incorporation and CaspGLOW stainings in splenic GC B cells after immunization with NP38-CGG. A representative FACS profile and gating strategy for the detection of BrdU+ and CaspGLOW+ cells among CD19+Fas+ Dump−NIP− and CD19+Fas+Dump−NIP+ cells from Dicer1+/+AicdaCre/+ mice at day 10 after immunization are shown. Mice were pulsed with BrdU (2 mg) 4 hours before analysis. Numbers adjacent to the outlined area indicate the percentage of cells in the individual gate. Data are representative of more than 10 experiments. (B-C) The frequencies of splenic B cells that are BrdU+ (B) or CaspGLOW+ (C) among splenic CD19+Fas+Dump−NIP− and CD19+Fas+Dump−NIP+ cells from Dicer1+/+AicdaCre/+ (●) and Dicer1fl/flAicdaCre/+ (○) mice 10 days after immunization are quantified. Each symbol represents 1 mouse. (D) Real-time RT-PCR analyses of various CDK inhibitor gene expression in Dicer1+/+AicdaCre/+ (filled) and Dicer1fl/flAicdaCre/+ (empty) GC B cells (CD19+Fas+PNA+CD38−). Bars represent relative expression of genes normalized to the expression of hprt (mean values of 3 independently sorted GC B-cell samples). **P < .01 (Student t test).
Concurrently, we examined the apoptosis of GC B cells at day 10 after immunization by detecting caspase activation in these cells using a fluorescent irreversible inhibitor of caspase (CaspGLOW) that binds activated caspases. As seen in Figure 5A and quantified in Figure 5C, Dicer1fl/flAicdaCre/+ GC B cells exhibited a 30% to 50% increase in the fraction of cells stained as CaspGLOW+, indicating the occurrence of enhanced apoptosis in these mutant cells compared with WT GC B cells. We also found that GC B cells purified from immunized Dicer1fl/flAicdaCre/+ mice exhibited approximately 30% more cell death after 6 hours of in vitro culture compared with their WT counterparts, suggesting that Dicer-deficient GC B cells are more prone to cell death (supplemental Figure 5A-B). Taken together, these results suggest that the absence of Dicer affects both the proliferation and survival of GC B cells and that the combined defects in proliferation and survival probably lead to the drastic reduction of the GC B cells in Dicer1fl/flAicdaCre/+ mice.
Derepression of cell cycle inhibitor genes in Dicer-deficient GC B cells
To better understand the proliferative defect of Dicer-deficient GC B cells, we proceeded to investigate how GC B-cell proliferation was regulated by Dicer-dependent miRNAs. We focused on genes involved in the negative regulation of cell proliferation, which might be derepressed on ablation of Dicer-dependent miRNAs. The mammalian cell cycle is regulated mainly by a family of serine/threonine protein kinases consisting of regulatory cyclin subunits and catalytic cyclin-dependent kinases (CDKs).34 The CDK activity is negatively controlled by several inhibitors, including CDKN1A, CDKN1B, CDKN1C, CDKN2A, CDKN2B, CDKN2C, CDKN2D, and CDKN3. Splenic GC B cells (CD19+Fas+PNA+CD38−) were sorted from both WT and Dicer1fl/flAicdaCre/+ mice at day 10 after immunization, and the expression of CDK inhibitor genes was examined by real-time RT-PCR. As shown in Figure 5D, Dicer1fl/flAicdaCre/+ GC B cells have approximately 16- and 10-fold higher expression levels of Cdkn1c (p57Kip2) and Cdkn2b (p15INK4b) genes, respectively, compared with WT GC B cells. The expression levels of Cdkn1a (p21Cip1) and Cdkn1b (p27Kip) genes were also increased by approximately 5- and 4-fold, whereas the levels of Cdkn2a (p16INK4a), Cdkn2c (p18INK4c), Cdkn2d (p19INK4d), and Cdkn3 (KAP) were only increased by 1.2- to 1.6-fold in Dicer1fl/flAicdaCre/+ GC B cells.
To gain insights into how these CDK inhibitor genes are regulated by miRNAs in GC B cells, we searched for the predicted targeting of these CDK inhibitor genes by miRNAs highly expressed in GC B cells as determined in a previous deep-sequencing study,31 using MicroCosm Targets software (http://www.ebi.ac.uk/enright-srv/microcosm/htdocs/targets/v5/). We selected 37 of 600 mature mouse miRNAs as highly expressed miRNAs, which were detected at more than 1000 sequence tags per million in GC B cells.31 Interestingly, all 8 CDK inhibitor genes examined in our study were predicted to be targeted at least once by 24 miRNAs, which account for approximately 60% of the 37 miRNAs abundantly expressed in GC B cells (supplemental Table 2). Moreover, 3 of 8 CDK inhibitor genes (cdkn1a, cdkn1c, and cdkn2b), which were the most highly up-regulated and were increased by 5-, 16-, and 10-fold, respectively (Figure 5D), were predicted to be targeted by more than 5 miRNAs from the list of 37 miRNAs (supplemental Table 2). Therefore, the release of inhibition of a spectrum of CDK inhibitor genes by miRNAs in Dicer-deficient GC B cells could be one of the underlying mechanisms for the defective proliferation and dramatic reduction of GC B cells in Dicer1fl/flAicdaCre/+ mice.
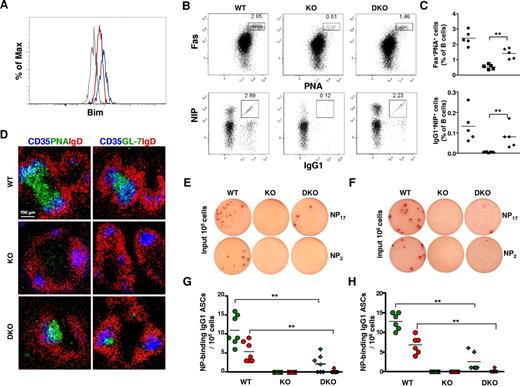
GC B cells in Dicer1fl/flAicdaCre/+ mice have elevated Bim expression and can be partially rescued by Bim deficiency
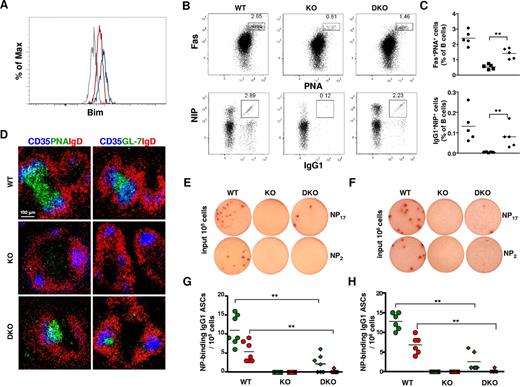
It has been shown that developmentally arrested mutant pro-B cells in Dicer1fl/flmb-1Cre/+ mice had increased levels of the proapoptotic molecule Bim, and they exhibited enhanced cell death.8 We next examined whether the expression level of Bim was also deregulated in Dicer1fl/flAicdaCre/+ GC B cells by intracellular flow cytometry analyses using a Bim-specific antibody. As shown in Figure 6A, the level of Bim protein in splenic Dicer1fl/flAicdaCre/+ GC B cells was increased compared with that found in WT GC B cells, suggesting that, similar to the situation found in Dicer-deficient pro-B cells, GC B cells lacking Dicer also have increased Bim expression, which might account for their enhanced apoptosis.
Bim ablation partially rescues GC- and NP-specific IgG1+ B cells in Dicer1fl/flAicdaCre/+ mice. (A) Flow cytometric analysis of Bim protein levels in splenic GC and non-GC B cells from Dicer1+/+AicdaCre/+ and Dicer1fl/flAicdaCre/+ mice 10 days after immunization with NP38-CGG. Dump−CD19+Fas+NIP+ cells from Dicer1+/+AicdaCre/+ (red), Dicer1fl/flAicdaCre/+ (blue), and Dicer1+/+AicdaCre/+Bim−/− (gray) mice were fixed, permeabilized, and stained intracellularly with an anti–Bim antibody. (B) Flow cytometric analysis of GC B cells and NP-specific IgG1 cells in the spleens of Dicer1+/+AicdaCre/+Bim+/+ (WT), Dicer1fl/flAicdaCre/+Bim+/+ (KO), and Dicer1fl/flAicdaCre/+Bim−/− (DKO) mice at day 10 after immunization with NP38-CGG as described in Figure 3. Numbers adjacent to the outlined areas indicate the percentage of cells in the individual gate. (C) Frequency of CD19+Fas+PNA+ (top panel) and NIP+IgG1+ (bottom panel) cells among total B cells in the spleens of WT (●), KO (■), and DKO (♦) mice at day 10 after immunization. Each symbol represents one mouse. (D) Confocal microscopy of spleen sections of WT, KO, and DKO mice 10 days after immunization with NP38-CGG as described in Figure 3B. (E-F) ELISPOT assays of anti–NP IgG1 ASCs cells in spleens (E) and bone marrow (F) of day 12 postimmunized WT, KO, and DKO mice using BSA-NP17 (top panel) or BSA-NP2 (bottom panel) as coating antigens. (G-H) Frequency of anti-NP17 (green symbol) and anti-NP2 (red symbol) IgG1 ASCs in spleens (G) and bone marrow (H) of WT (circles), KO (squares), and DKO (diamonds) mice at day 12 after immunization. Each symbol represents 1 mouse. Data are representative of 3 independent experiments (A,D) or 5 independent experiments (B,E-F).
Bim ablation partially rescues GC- and NP-specific IgG1+ B cells in Dicer1fl/flAicdaCre/+ mice. (A) Flow cytometric analysis of Bim protein levels in splenic GC and non-GC B cells from Dicer1+/+AicdaCre/+ and Dicer1fl/flAicdaCre/+ mice 10 days after immunization with NP38-CGG. Dump−CD19+Fas+NIP+ cells from Dicer1+/+AicdaCre/+ (red), Dicer1fl/flAicdaCre/+ (blue), and Dicer1+/+AicdaCre/+Bim−/− (gray) mice were fixed, permeabilized, and stained intracellularly with an anti–Bim antibody. (B) Flow cytometric analysis of GC B cells and NP-specific IgG1 cells in the spleens of Dicer1+/+AicdaCre/+Bim+/+ (WT), Dicer1fl/flAicdaCre/+Bim+/+ (KO), and Dicer1fl/flAicdaCre/+Bim−/− (DKO) mice at day 10 after immunization with NP38-CGG as described in Figure 3. Numbers adjacent to the outlined areas indicate the percentage of cells in the individual gate. (C) Frequency of CD19+Fas+PNA+ (top panel) and NIP+IgG1+ (bottom panel) cells among total B cells in the spleens of WT (●), KO (■), and DKO (♦) mice at day 10 after immunization. Each symbol represents one mouse. (D) Confocal microscopy of spleen sections of WT, KO, and DKO mice 10 days after immunization with NP38-CGG as described in Figure 3B. (E-F) ELISPOT assays of anti–NP IgG1 ASCs cells in spleens (E) and bone marrow (F) of day 12 postimmunized WT, KO, and DKO mice using BSA-NP17 (top panel) or BSA-NP2 (bottom panel) as coating antigens. (G-H) Frequency of anti-NP17 (green symbol) and anti-NP2 (red symbol) IgG1 ASCs in spleens (G) and bone marrow (H) of WT (circles), KO (squares), and DKO (diamonds) mice at day 12 after immunization. Each symbol represents 1 mouse. Data are representative of 3 independent experiments (A,D) or 5 independent experiments (B,E-F).
To further determine the functional relevance of elevated Bim expression in Dicer-deficient GC B cells, we introduced Bim deficiency into immunized Dicer1fl/flAicdaCre/+ mice. In striking contrast to the drastic reduction of GC B cells in Dicer1fl/flAicdaCre/+ mice at day 10 after immunization, the population of Fas+PNA+ GC B cells was significantly increased in the spleens of Dicer1fl/flAicdaCre/+Bim−/− mice (Figure 6Bi,C). When GCs in the spleen of immunized Dicer1fl/flAicdaCre/+Bim−/− mice were examined via confocal microscopy, a sizeable number of PNA+IgD− or GL-7+IgD− GC B cells were found to be present in the B-cell follicles and in close contact with CD35+ FDCs (Figure 6D). The population of antigen-specific B cells was also largely rescued by Bim deficiency as the percentage of NIP+IgG1+ GC B cells was significantly increased in the spleen of immunized Dicer1fl/flAicdaCre/+Bim−/− mice compared with Dicer1fl/flAicdaCre/+ mice (Figure 6Bii,C). Thus, these data indicate that Dicer deficiency in GC B cells leads to increased expression of Bim, which contributes to their enhanced apoptosis and compromises their subsequent differentiation.
We next determined whether the rescued NIP+IgG1+ GC B cells in Dicer1fl/flAicdaCre/+Bim−/− mice would generate antigen-specific ASCs using ELISPOT assays. NP-specific IgG1 ASCs were detected in spleens of Dicer1+/+AicdaCre/+Bim+/+ mice but not in Dicer1fl/flAicdaCre/+Bim+/+ mice at day 12 after immunization (Figure 6E). Interestingly and in contrast to more than 50% rescue of NIP+IgG1+ GC B cells in spleens of Dicer1fl/flAicdaCre/+Bim−/− mice (Figure 6C), the frequency of anti–NP17 IgG1 ASCs in the spleens of these mice was only approximately 20% to 25% of that found in WT mice; and more importantly, the high-affinity anti–NP2 IgG1 ASCs were hardly detected in Dicer1fl/flAicdaCre/+Bim−/− mice (Figure 6E,G). A similar result was obtained when NP-specific ASCs were examined in the bone marrow of these mice (Figure 6F,H). These results indicate that rescued Dicer-deficient antigen-specific GC B cells could not efficiently differentiate into ASCs; and in particular, they fail to differentiate into high-affinity ASCs, suggesting that Dicer might also be required for the generation of GC-derived antigen-specific ASCs during a T cell–dependent immune response.
Discussion
We have provided definitive evidence in this study for a role of Dicer in B-cell terminal differentiation by specifically deleting Dicer in antigen-activated B cells using an Aicda-Cre allele, which is highly expressed only on B-cell stimulation. We show that Dicer1fl/flAicdaCre/+ mice have a severe impairment of antibody response on immunization with the T cell–dependent antigen NP38-CGG. The mutant mice fail to produce high-affinity class-switched NP-specific antibodies and generate long-lived PCs and memory B cells. We further show that Dicer1fl/flAicdaCre/+ mice have a defect in GC B-cell formation during both antigen-induced acute immune response and gut-associated chronic antigenic challenges. Thus, our data demonstrate an essential B cell–intrinsic role for Dicer in the formation of GC B cells before their subsequent differentiation into memory and plasma cells.
We further demonstrated that Dicer-deficient GC B cells have severe defects in cell proliferation and survival, which could account for the impairment in GC B-cell formation. Consistent with these defects, the rudimentary Dicer-deficient GC B cells that we managed to isolate have up-regulated expression of genes encoding CDK inhibitors and apoptotic protein Bim. However, the severe scarcity and increased susceptibility to apoptosis of Dicer-deficient GC B cells make it difficult for us to obtain enough Dicer-deficient GC B cells for a systemic transcriptome study on how Dicer-dependent miRNAs globally regulate gene expression in GC B cells. B cells stimulated in vitro with anti–CD40 antibody and IL-4 were also not ideal for such study and for examining the role of Dicer in Ig class–switching because of the delayed and incomplete deletion of Dicer in the cultured Dicer1fl/flAicdaCre/+ B cells (supplemental Figure 5C-D). Therefore, we took a bioinformatics approach and focused on the possible regulation of CDK inhibitor genes by Dicer-dependent miRNAs. We found that approximately 60% of the 37 highly expressed miRNAs (with TMP > 1000) in GC B cells as identified recently by a deep-sequencing approach31 were predicted to target at least one of the CDK inhibitor genes, and 3 of the most highly up-regulated CDK inhibitor genes (cdkn1a, cdkn1c, and cdkn2b) were predicted to be targeted by more than 5 miRNAs from the list of 37 miRNAs (Figure 5D; supplemental Table 2). Indeed, some of these miRNAs have been demonstrated in other cell types to target CDK inhibitor genes. For instance, miR-221 and miR-181a have been shown to repress p27Kip1,35-37 miR-25 and miR-221 can suppress p57Kip2,35,38 and miR-93 can inhibit the expression of p21Cip1.35 Given that GC B cells divide every 6 hours and are among the most rapidly proliferating cell types in the body,39 it is tantalizing to hypothesize that, in WT GC B cells, genes encoding cell cycle inhibitors are largely repressed by Dicer-dependent miRNAs either directly or indirectly to ensure the robust expansion of GC B cells and the subsequent differentiation of these GC B cells into long-lived PCs and memory B cells. In the absence of Dicer or Dicer-dependent miRNAs as is in the situation of antigen-stimulated Dicer1fl/flAicdaCre/+ GC B cells, the induced repression of these cell cycle inhibitor genes does not occur, and this results in elevated expression of cell cycle inhibitors, which inhibit cell proliferation and subsequently affect GC B-cell formation.
Similar to the situation in Dicer1fl/flmb1Cre/+ mice where Dicer-deficient pro-B cells can be partially rescued by deleting Bim,8 the ablation of Bim can also partially rescue the defective formation of GC B cells and NP-specific IgG1 B cells in Dicer1fl/flAicdaCre/+ mice. However, the GCs in Dicer1fl/flAicdaCre/+ mice were still smaller than those found in WT mice, which is probably because Bim is a proapoptotic molecule and not involved in cell proliferation and expansion. In support of this argument, Dicer-deficient pro-B cells found in Dicer1fl/fl mb-1Cre/+Bim−/− failed to proliferate in response to IL-7 treatment in vitro, and this correlated with an incomplete rescue of early B-cell development in these mice.8 Interestingly, the rescued antigen-specific GC B cells in Dicer1fl/flAicdaCre/+Bim−/− mice failed to efficiently generate antibody-secreting PCs. This is probably not the result of Bim deficiency per se, as Bim−/− mice have increased the number of NP-specific IgG1-secreting PCs after primary immunization.40 Hence, Dicer-dependent miRNAs might also play a role in the generation of high-affinity PCs either affecting their differentiation, proliferation, or survival. Nevertheless, the small population of rescued Dicer-deficient GC B cells in Bim-deficient genetic background could be invaluable to study Dicer-dependent mechanisms affecting memory B cells and PC differentiation.
Overall, our study has defined an essential role for Dicer in the formation of GC B cells. Together with previous studies of Dicer in early B lymphopoiesis8 and follicular B-cell development,9 it is now clear that Dicer or Dicer-dependent miRNAs are essential for both antigen-independent and antigen-dependent phases of B-cell differentiation. It will be interesting to identify the specific miRNAs important for GC B-cell differentiation, and even for early B and follicular B lymphopoiesis. However, this is a formidable task and would require the systematic ablation of individual miRNA or combinations of various miRNAs that are specifically and abundantly expressed at each stage of B-cell differentiation.
Previous studies have shown that mice deficient in miR-155 had defective GC B-cell formation.41,42 However, the kinetics and formation of GCs were not affected by miR-155 deficiency in B cells, and the anatomic structure of GCs was still normal, although the size of GCs was reduced by 50% to 60%.41 Furthermore, the proliferation and survival of activated B cells were also not affected by miR-155 deficiency.42 These results would suggest that miR-155 might not be critically involved in the GC B-cell formation per se, and other Dicer-dependent miRNAs could be important for GC B cells.
Last but not least, it is known that Dicer has additional functions other than generating miRNAs. Dicer is also involved in the generation of endogenous siRNAs.43 A more recent study showed that Dicer could be converted from ribonuclease into death-promoting deoxyribonuclease by protease and plays an unexpected role in chromosome fragmentation.44 Whether these additional functions of Dicer affect GC B-cell formation is unknown and awaits the generation of Dicer mutant mice that are deficient in some of these functions for analysis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Nancy Jenkins (IMCB, A-STAR) for the gift of AicdaCre/+ mice, Masaki Hikida (Kyoto University) for suggestions on ELISPOT assays, and Annabelle Pang and Yingjie Zhuo for technical assistance.
This work was supported by the Biomedical Research Council of the Singapore Agency for Science Technology and Research.
Authorship
Contribution: S.X. and K.-P.L. designed the research; Q.Z. provided reagents. S.X., K.G., and J.H. performed research; and S.X. and K.-P.L. analyzed data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shengli Xu, Laboratory of Immunology, Bioprocessing Technology Institute, Agency for Science, Technology and Research, 20 Biopolis Way, #06-01 Centros, Singapore 138668; e-mail: xu_shengli@bti.a-star.edu.sg; and Kong-Peng Lam, Laboratory of Immunology, Bioprocessing Technology Institute, Agency for Science, Technology and Research, 20 Biopolis Way, #06-01 Centros, Singapore 138668; e-mail: lam_kong_peng@bti.a-star.edu.sg.