Abstract
Pediatric arterial ischemic stroke (AIS) is increasingly diagnosed and carries significant risks of recurrence, morbidity, and mortality. Anticoagulant therapy (ACT) is commonly prescribed in childhood AIS. Hemorrhagic complication rates in pediatric stroke are unknown, and adult safety data are of limited applicability. We analyzed a prospectively enrolled cohort of children (aged 1 month-18 years) with acute AIS selected using standardized criteria for protocol-based ACT over14-year period. We assessed ACT-associated intracranial hemorrhage (ICH), including frequency, clinical and radiologic characteristics, predictors, and outcome. Among 215 children with AIS, 123 received ACT within 7 days after diagnosis. During anticoagulation, 14 (11%) children developed new or increased ICH, all within 26 days from diagnosis. ICH was symptomatic in 5 (4%), asymptomatic in 9 (7%), and mild (European Cooperative Acute Stroke Study grades HI1 or HI2) in all but 1 child (ECASS PH-2). Long-term neurologic outcomes after ACT-associated ICH in survivors were abnormal in 73% (8/11). Comparably, 12 of 75 (16%) children treated without anticoagulation developed new or increased ICH on follow-up imaging (P = .3507). We conclude that ACT is relatively safe in children with AIS, with a 4% risk of symptomatic ICH. Based on the safety of ACT in our study, clinical trials of ACT in childhood AIS are warranted.
Introduction
Arterial ischemic stroke (AIS) affects 2 to 6 per 100 000 children annually.1 Mortality risk because of AIS in children is 6% to 8%,2 and ∼ 75% suffer long-term neurologic deficits.3,4 Recurrence rates for AIS and transient ischemic attack (TIA) in non-neonate children are 16% to 50%.4-6 Mechanisms and risk factors for AIS differ between children and adults. In children, prothrombotic disorders, cardiogenic embolism, and arteriopathy including dissection predominate. Such mechanisms may favor fibrin-based thrombosis and targeted therapy, compared with platelet-based processes common in adults, in whom aspirin (ASA) is the initial mainstay of treatment. Common risk factors for hemorrhagic transformation in adults with anticoagulant therapy (ACT), including chronic hypertension and advanced atherosclerosis, are rare in childhood. Several studies have reported potentially beneficial effects of ACT at therapeutic or prophylactic doses in treating children with AIS.2,7-11 Consensus-based pediatric treatment guidelines12-14 suggest that both anticoagulation and ASA are reasonable initial considerations in childhood AIS. However, the evidence base for this is minimal (2C or class II),12,13 including lack of adequate safety data for ACT. This results in controversy in clinical management while impeding the development of clinical trials.
The aim of the present study is to evaluate the safety of protocol-based ACT in a longitudinal, prospectively enrolled sample of children diagnosed with AIS.
Methods
We analyzed a prospectively enrolled cohort of children (1 month-18 years) diagnosed with imaging-confirmed acute AIS and treated with ACT at a tertiary care hospital (The Hospital for Sick Children, Toronto, ON) during 14 years, from January 1, 1992, to December 31, 2005. We also evaluated the risk for intracranial hemorrhage (ICH) in the cohort of children who were not treated with ACT. The study was approved by the Institutional Research Ethics Board of The Hospital for Sick Children, Toronto.
Clinical data
Clinical data were collected from the databases of the Canadian Pediatric Ischemic Stroke Registry–Toronto site based initially on standardized health record review. In addition, stroke-specific history and neurologic examination using an expanded version of the Pediatric Stroke Outcome Measure (PSOM) were conducted at follow-up visits in the Children's Stroke Clinic.4 Stroke risk factors were categorized as follows: vasculopathy (including dissection, moya-moya disease, transient cerebral arteriopathy, vasculitis, postvaricella arteriopathy, and nonspecific arteriopathy)15 ; head and neck infections; other head and neck pathologies; and cardiac disorders (congenital or acquired). Clinical presentations of AIS (eg, hemiparesis and seizures) were documented.
Anticoagulation therapy
All children with AIS who received ACT for a minimum of 24 hours were included. Treatment regimens were based on published institutional antithrombotic (from 1992) and stroke (from 1995) regimens.16 ACT was individualized at the discretion of the caring team in consultation with our institutional hematology (1992-1995), thrombosis (from 1995), neurology (1992-1998) or in-patient stroke (from 1998) services.
ACT included monotherapy or combination therapy (either sequential or concurrent) with initial unfractionated heparin (UFH), warfarin (Coumadin), or low-molecular-weight heparin (LMWH; Enoxaparin, Rhone Poulenc Rorer). Concomitant ASA or clopidogrel use was noted. ACT dose intensity, duration, side effects, and safety were recorded. ACT doses were adjusted based on published algorithms.12,16 Intravenous UFH was initiated without loading dose and titrated to activated partial thromboplastin time of 60 to 85 seconds or anti–factor Xa (anti-Xa) level of 0.35 to 0.7 U/mL. LMWH was injected subcutaneously twice daily at a 1 mg/kg/dose (1.5-mg/kg/dose for infants < 2 months of age), and titrated to anti-Xa levels of 0.5 to 1.0 U/mL at 4 hours after injection. Warfarin initial dose was 0.2 mg/kg/d titrated to international normalized ratio 2.0 to 3.0. Patients were considered to be still receiving ACT for the following intervals after receiving the last dose: up to 24 hours for UFH, 48 hours for LMWH, 5 days for warfarin, and 7 days for ASA. Children who received multiple ACT medications over time were represented in multiple categories. Brain computed tomography (CT) or magnetic resonance imaging scans were performed at 3 and 7 to 10 days post-ACT initiation and with any clinical suspicion of ICH.
ACT safety
The primary safety outcomes of the study were ACT-associated death, ICH, or serious systemic bleeding. ICH episodes were categorized clinically as asymptomatic or symptomatic and by radiologic severity (see “Radiologic data”).17 Symptomatic ICH included new or increased neurologic deficit (focal or diffuse), headache, or seizures. Laboratory parameters at the time of ICH included complete blood count, coagulation tests, and anticoagulant therapeutic drug monitoring.
Radiologic data
Radiologic data included number and location of infarcts (vascular territory, brain structures, and laterality) and presence of ICH, including hemorrhagic conversion of infarcts at AIS diagnosis and during ACT. For every child with ACT-associated ICH, all brain imaging studies were re-evaluated by the study pediatric neuroradiologist (S.L.) and a pediatric neurologist (A.K. or G.d.V.) blinded to patient clinical data including ACT. ICH scoring was classified according to the European Cooperative Acute Stroke Study (ECASS) definitions: H0, no hemorrhage detected; HI1, hemorrhagic infarct 1: small petechiae, < 50% of infarct area (includes laminar necrosis); HI2, hemorrhagic infarct 2: confluent petechiae, > 50% of infarct area; PH1, parenchymal hemorrhage 1: blood ≤ 30% of infarct area, no or slight space effect; and PH2, parenchymal hemorrhage 2: blood ≥ 30% of infarct area, substantial space effect.18 Changes in ECASS scores over time were documented.
Neurologic outcome
Children were evaluated in the Children's Stroke Clinic, The Hospital for Sick Children, 3 and 12 months after diagnosis and annually thereafter with the PSOM.4 The PSOM is a standardized, validated Likert-scaled neurologic assessment with 5 spheres: sensori-motor right, sensori-motor left, language production, language comprehension, and cognitive/behavioral. Summary scores for each sphere reflect no deficit (0), mild (0.5), moderate (slowed function, 1), or severe (missing function, 2). Clinical outcome was dichotomized as favorable (no or mild deficits) or unfavorable (total PSOM score ≥ 1 with impact on function). Outcomes were compared in children receiving ACT either with or without ICH.
Statistical analysis
We used descriptive statistics and association testing for potential predictors for ICH. A priori variables include treatment, multiple infarcts and cardiac risk factor. Group comparisons and association of ICH and neurologic outcome also were conducted. Univariate analyses included Fisher exact, χ2 for nominal data and Wilcoxon Mann-Whitney for nonparametric continuous data. Associations with P values ≤ .05 (2-tailed) were considered significant, and values between .05 and .10 were considered trends.
Results
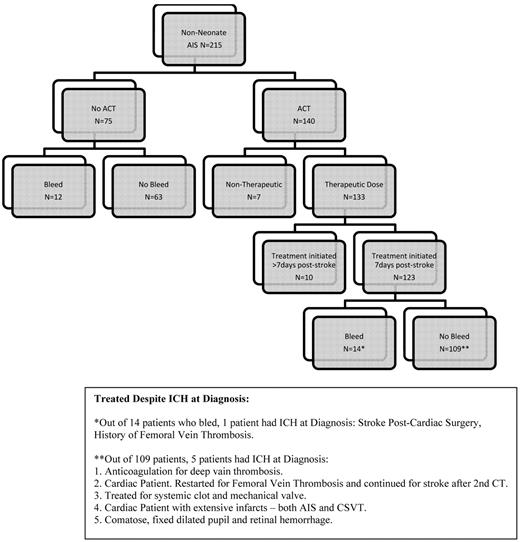
Two-hundred and fifteen children were diagnosed with AIS during the study period (Figure 1). We excluded 17 children treated with ACT because ACT was initiated > 7 days after AIS diagnosis (n = 10, none had ACT-associated ICH), or because ACT was limited to prophylactic dosing provided for preventing systemic thrombosis (n = 7). Table 1 presents patient characteristics of 198 children (123 treated with ACT initiated within 7 days after AIS and 75 receiving only ASA [n = 25] or no ACT/ASA [n = 50]). In the 198 children, mean patient age was 6.2 years (range, 1 month-17.7 years) and 115 (58%) were male. Common presentations included hemiparesis (77%) and seizures (29%). Risk factors for AIS frequently included cardiac disorders (34%) and vasculopathy (44%; 3 in ACT group had arterial dissection). Infarcts at presentation were multiple in 82 (41%) and basal ganglia structure was affected in 96 children (48%). Fourteen children (6 ACT group and 8 no ACT group) had ICH at stroke diagnosis.
ICH post-AIS diagnosis occurred in 12/75 (16%) patients in the no ACT group and 14/123 (11%) in the ACT group. No significant predictors of ICH were found: treatment with ACT (P = .3507), multiple infarcts (P = .7430), and cardiac disease (P = .7227). In addition, no difference was found in ICH rates between the first 7 years and last 7 years of enrolled patients (P = .4837).
Anticoagulant therapy
ACT was initiated within 72 hours from AIS diagnosis in 102 (83%) of the 123 children treated with ACT. Among 123 children receiving initial ACT within 7 days after AIS, 51 received a single ACT medication, 64 had 2 sequential ACT medications, and 8 had 3 sequential ACT medications during the treatment period. Seventy-two (58%) children switched ACT medication during treatment. ICH rates were similar for each ACT regimen or combination. Median duration of ACT was 74 days (range, 1 day-6.4 years). Six children received ACT despite evidence of ICH at diagnosis, either because the ICH was minor (ECASS grade HI1) or the treating physician graded the risk of recurrent thromboembolism as major.
Intracranial hemorrhage
Fourteen (11%) children had ACT-associated ICH. Nine children had asymptomatic ICH, detected within 6 to 26 days (mean, 12.4 days) from AIS diagnosis. Five children had symptomatic ICH, and symptoms began 2 to 15 days post-AIS diagnosis and 2 to 13 days post-ACT initiation. ICH symptoms included irritability, seizures, headache, facial droop, aphasia, or decreasing level of consciousness (Table 2). The incidence of ICH on ACT in the first 4 weeks was 4% in the first week, 6.4% in the second week, 2.3% in the third week, and 1.3% in the fourth week. Table 2 summarizes additional clinical and radiologic data, respectively, on the 14 children with ACT-associated ICH (the first 9 children are the asymptomatic group, and the last 5 are the symptomatic group). Non-ICH systemic bleeding did not occur in any patient. All the patients without evidence of ICH had brain imaging immediately after AIS diagnosis to rule out ICH or AIS progression as well as additional follow-up imaging from 7 days after diagnosis (52 patients imaged 7-30 days after diagnosis of AIS; 30 patients between day 30 and day 90; 84 patients > 90 days).
Anticoagulant therapy at time of ICH
Among 9 children with asymptomatic ICH, 7 children were receiving ACT monotherapy with LMWH (n = 4), UFH (n = 2), or warfarin (n = 1). Two had ICH on combination therapy with UFH and ASA (n = 1) or LMWH and warfarin (n = 1) during medication transition. Two children had hematologic risks for ICH. One child developed thrombocytopenia (platelets count, 32 000) during catastrophic antiphospholipid antibody syndrome and had mildly elevated UFH level (anti-Xa = 0.81; therapeutic range, 0.35-0.7).19 The second child had an international normalized ratio of 3.0 (normal range, 0.8-1.2) while on LMWH.
Among the 5 children with symptomatic ICH, 3 children were receiving ACT monotherapy with LMWH (n = 1) and UFH (n = 2). Two had ICH on combination therapy with UFH and ASA (n = 1) or LMWH and warfarin (n = 1) during medication transition.
For children treated with single or combination ACT medications the risk for ICH for each regimen was 8% (4/50 children) for UFH alone, 6% (5/81 patients) for LMWH alone, 3% (1/39 patients) for warfarin alone, 14% (2/14 children) for UFH plus ASA, and 9% (2/22 children) for LMWH plus warfarin.
Radiologic Findings of ICH after ACT
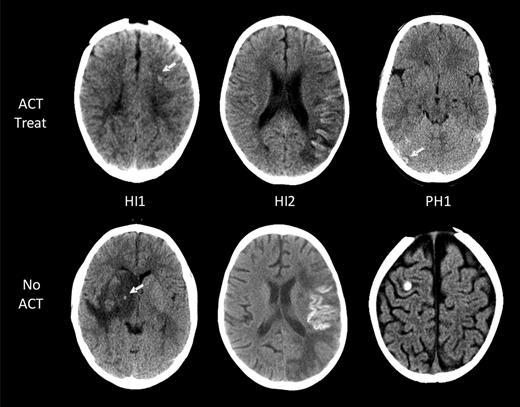
Eleven of the 14 children with ICH had images available for reanalysis and ECASS grading of ICH severity (Table 2).18 The ACT-associated ICH grades in the 7 asymptomatic patients were as follows: HI1 (no cortical laminar necrosis) in 3 patients, HI2 in 3, and PH2 in 1 (Figure 2). All 4 symptomatic ICH patients had HI1 (including cortical laminar necrosis) bleed severity. Among them, 1 patient had evidence of laminar necrosis only, 2 patients had evidence of both laminar necrosis and petechial hemorrhage, and the fourth patient had petechial hemorrhage.
ICH in children with AIS. Intracranial hemorrhages are classified according to the ECASS grading system. Representative examples of the 3 grades encountered (HI1, HI2, and PH1) are shown for both children on ACT (top row) and those not on ACT (bottom row). No larger hematomas or ECASS PH2 were observed in the ACT-treated group. White arrows point at areas of ICH.
ICH in children with AIS. Intracranial hemorrhages are classified according to the ECASS grading system. Representative examples of the 3 grades encountered (HI1, HI2, and PH1) are shown for both children on ACT (top row) and those not on ACT (bottom row). No larger hematomas or ECASS PH2 were observed in the ACT-treated group. White arrows point at areas of ICH.
Potential risk factors for ACT-associated ICH
Potential risk factors for ACT-associated bleeding were evaluated in all 123 patients. No significant predictors of ACT-associated ICH were found for multiple infarcts (4/14 [29%] vs 46/109 [42%]; P = .3283) or cardiac disease (7/14 [50%] vs 38/109 [42%]; P = .2683).
Neurologic outcome in the ACT group
Three of the 14 children with ICH after ACT died because of underlying cardiac disorder (n = 2) or fatal leukemia-induced catastrophic antiphospholipid antibody syndrome (n = 1).19 None died from ICH. Moderate-to-severe neurologic deficits were documented in 8/11 (73%) surviving children. Outcomes in the 7 survivors with asymptomatic ACT-related ICH were as follows: severe deficit (n = 4), moderate deficit (n = 1), mild deficit (n = 1), and normal (n = 1). Outcomes in the 4 survivors with symptomatic ACT-related ICH were as follows: severe deficit (n = 2), moderate deficit (n = 1), and mild deficit (n = 1). There is no significant difference in the neurologic outcomes between patients with asymptomatic (poor outcome, 7/9 [78%]) and symptomatic (poor outcome, 4/5 [80%]) ACT-related ICH (P = .9227).
Follow-up data were available for 95 of the 109 ACT-treated children who did not develop ICH. Fifty (53%) had poor outcome, including 9 patients who died. Neurologic outcomes in the 86 survivors were as follows: severe deficit (n = 24), moderate (n = 17), mild deficit (n = 24), and normal (n = 21). Children with ACT-associated ICH had a trend toward abnormal outcome compared with those who did not (P = .068). It is possible that prior ICH affects long-term neurologic outcome. The data from the 6 patients treated with ACT despite ICH at diagnosis suggests that there is also variability in the outcome: normal (n = 1), mild (n = 1), severe (n = 2), deceased (n = 1), and data unavailable for 1 patient.
Discussion
We studied the risk of ICH in a prospectively enrolled cohort of 215 children with AIS over 14 years. To address the question of ACT safety, we focused on symptomatic and asymptomatic ACT-related ICH in a cohort of 123 children treated with protocol-based ACT for acute ischemic stroke. Symptomatic ICH developed in 4%, and overall the ICH risk was 11%. ICH was mild with ECASS grades HI1 or HI2 in 10 of 11 affected children and occurred in all within the first month of ACT. The risk for new or increased ICH in children managed without ACT was similar at 16%. Although no ICH episodes were fatal, long-term follow-up demonstrated a moderate or severe neurologic deficit in two thirds of children with ACT-associated ICH.
Our relatively low rate of ACT-associated ICH is consistent with several previous studies in childhood AIS. In a recent study, 37 children with childhood AIS and non-moyamoya arteriopathy received ACT in prophylactic (57%) or therapeutic (43%) doses. None developed ICH or major bleeding over a period of 1329 patient-months.20 Dix et al reported 29 newborns and children treated with LMWH for ischemic stroke.8 None developed ICH, although extracranial bleeds (1 major and 8 minor) were observed primarily in neonates.8 Burak et al reported 2 minor bleeding episodes in 8 children with AIS receiving LMWH.7 Strater et al published a nonrandomized study of 135 children with AIS (idiopathic, n = 79; cardiac, n = 15; vascular, n = 30; and infectious, n = 11) of whom 86 received prophylactic dose LMWH (4-hour anti-Xa activity, 0.2-0.4).10 No drug-related adverse events or major bleeding were reported. Conversely, a recent study of 63 children with AIS reported 30% of them to develop hemorrhagic transformation in the first month of therapy. However, only 2 (3%) of the 19 children had symptomatic ICH. Five of the 24 children treated with ACT developed ICH (21%), and 12 of the 34 children treated with antiplatelet therapy. The development of ICH was not significantly associated with ACT versus antiplatelet therapy but was associated with larger infarct volume. A trend toward increased risk of ICH was found in children with cardiac conditions and meningitis.21
In our study, ICH was mild in almost all patients: ECASS grades were HI1 or HI2 in all but 1 child at diagnosis. We included children with cortical laminar necrosis in our definition of “ICH” and assigned them an ECASS grade of HI1 (n = 1). In routine CT or MRI imaging of pediatric patients, it is challenging to differentiate whether cortical laminar necrosis is because of petechial cortical bleeding or cortical neuronal death with mineralization.22 Therefore, our conservative approach to assign an ECASS HI1 ICH score to all cortical laminar necrosis cases implies that our estimation of risk is maximal, and overall ICH risk in children receiving ACT for AIS may be even lower. In a study performed in 15 children with cortical laminar necrosis, susceptibility-weighted MR imaging identified bleeding in only 20%.22 This is supported by our observation that most children with ICH were asymptomatic and were diagnosed solely by scheduled follow-up imaging. The implication is that in children with AIS treated with ACT routine imaging is necessary to detect subclinical ICH. Despite the mild severity of most ICH episodes on imaging, the neurologic outcome tended to be worse in children with ACT-related ICH compared with children with no ICH.
In our study, ACT-associated ICH occurred early, within 26 days of AIS diagnosis. Although the majority of children continued therapy beyond the first month from AIS diagnosis, and up to 6.4 years after, none has experienced ICH beyond the first month. This finding may identify a short vulnerable period for development of ICH in children receiving ACT for stroke. Physiologic vascular and tissue stabilization probably develops over several weeks, decreasing ICH risks. Similarly, in adults, heparin-related ICH typically occurs early after stroke onset, usually within 72 hours.23 Also as in adults, the most common type of treatment-related ICH in our study was hemorrhagic conversion of a bland infarct, which is expected to occur in the days and early weeks post-stroke onset.
The underlying risk factors for ICH differ between pediatric and adult stroke. In adults, hypertension, atherosclerosis, and concomitant thrombolytic therapy17,24 are major risk factors for hemorrhagic conversion. These factors are not represented in most pediatric stroke series. Conversely, children with AIS have either normal brain arteries (most children with cardiac-related stroke) or arteriopathy limited to focal involvement of intracranial arteries.
No risk factors for ICH were identified in our study, possibly because of limited sample size. However, nearly half of children with ACT-associated ICH in our study had underlying cardiac disorders. In adults, cardio-embolic AIS is associated with an increased risk of hemorrhagic conversion.25 Because many cardiac strokes are associated with cardiac surgery, additional surgical factors may further increases the risk of hemorrhagic conversion. Because multivariate analysis was not feasible, due to a relatively small cohort compared with adult series, whether presentation with seizures and pre-existing ICH are independent of cardiac disorders could not be definitively determined.
In the treatment of childhood AIS, current approaches frequently include ACT. Recent observational data from the International Pediatric Stroke Study indicates that among all 640 children with AIS treated since 2003, 43% received in-hospital ACT.26 In children with AIS because of cardiac disease, arterial dissection, or major prothrombotic disorders, initial treatment with ACT is now recommended by multiple published pediatric stroke guidelines.13,14,27 In these scenarios, ACT use is favored over antiplatelet agents because thrombus formation is believed to involve predominantly fibrin thrombus formation by the coagulation system, against which antiplatelet agents are ineffective. These predisposing conditions also frequently are treated with ACT in adults. In children with AIS because of other causes, the use of ACT is controversial. In our study, most children were treated with initial ACT regardless of suspected etiology and then transitioned to antiplatelet agents after the initial week(s) of therapy, if dissection, cardiac disorders, or prothrombotic disorders were excluded. The relatively low rate of serious ICH, observed with ACT in the current study supports the safety of this practice. However, efficacy data remain to be determined, because in observational studies, children selected for ACT may have higher inherent rates of recurrent stroke than those selected for antiplatelet agents (selection bias).
Adult stroke trials comparing aspirin versus ACT have yielded consistent findings that hemorrhagic risks outweigh the benefits of ACT in noncardiac stroke. A Cochrane meta-analysis reported an absolute risk increase of 0.9% in symptomatic intracranial and 0.9% for additional extracranial hemorrhage risk with ACT compared with antiplatelet therapy, offsetting the benefits of ACT in preventing stroke recurrence.28 However, individual prospective studies have reported benefit of LMWH in adult AIS without increased risk of ICH.29,30 A subanalysis of the Warfarin Aspirin Recurrent Stroke Study demonstrated overall improved outcomes with ACT over ASA in the subgroup of stroke patients most similar to children with stroke, namely, nonhypertensive adults with cryptogenic stroke. This included a 30% risk reduction for recurrent stroke (P < .02).31
The main limitations of our study are the small number of safety outcome events (ICH or major extracranial bleeding) and relatively small number of subjects, compared with adult series. This factor may have accounted for our inability to determine statistically significant predictors of ICH. Another limitation was the shift in practice from UFH to LMWH as initial ACT when LMWH became increasingly available several years after study initiation. In addition,our limited number of routine follow-up CT scans, necessitated by concerns about radiation exposure of the pediatric brain, resulted in only one consistent early time point for CT detection of asymptomatic ICH. Finally, clustering of ACT-associated ICH occurring early after diagnosis may result from methodologic biases, namely, loss of subjects for follow-up and incomplete and nonuniform imaging frequency over time after the initial week after diagnosis. Importantly, in this nonrandomized study, although we did not find ACT to increase the risk for ICH, the possibility of selection of patients with inherently lower risk of ICH for initial ACT makes this comparison inconclusive.
In summary, we found that protocol-based ACT for pediatric AIS was safe with a 4% risk of symptomatic ICH, limited to low-grade imaging score (ECASS grades HI1 or HI2) in all but 1 patient. The risk for developing ICH was low compared with the risk in adults. Importantly, this risk was limited to the first 4 weeks of anticoagulant therapy in children with AIS. The favorable safety profile we observed supports the inclusion of ACT in current consensus-based management guidelines and the advancement of therapeutic clinical trials in childhood AIS.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by an Auxilium Foundation grant (G.d.V.)
Authorship
Contribution: T.S. performed the data compilation and literature search, interpreted the data, and wrote the initial manuscript; A.K. collected additional clinical details regarding presentations of bleeds and stroke and assisted in assembling and reviewing the neuroradiology images with S.L.; S.L. analyzed the neuroradiology images and classified using ECASS grades; A.-M.P. collected the clinical data, created the database, and planned and performed statistical data analyses; Y.F. assisted with data analysis and interpretation and preparation of the manuscript; D.M. participated in the database design and outcome assessments for patients; A.C. participated in the study design and development of anticoagulation protocol; G.d.V. and L.R.B. participated in developing the study plan, supervised the study progress and analysis, and cowrote the manuscript with T.S.; G.d.V. provided neurologic outcomes for some patients and final revisions of manuscript; and L.R.B. provided additional anticoagulation treatment details for the patients.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tal Schechter, Division of Hematology/Oncology, Hospital for Sick Children, University of Toronto, Toronto, ON M5G 1X8, Canada; e-mail: tal.schechter-finkelstein@sickkids.ca.
References
Author notes
G.d.V. and L.R.B. contributed equally to the manuscript and are therefore co–senior authors.