Abstract
Few randomized trials have compared therapies in mantle cell lymphoma (MCL), and the role of aggressive induction is unclear. The National Comprehensive Cancer Network (NCCN) Non-Hodgkin Lymphoma (NHL) Database, a prospective cohort study collecting clinical, treatment, and outcome data at 7 NCCN centers, provides a unique opportunity to compare the effectiveness of initial therapies in MCL. Patients younger than 65 diagnosed between 2000 and 2008 were included if they received RHCVAD (rituximab fractionated cyclophosphamide, vincristine, adriamycin, and dexamethasone), RCHOP+HDT/ASCR (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone + high-dose therapy/autologous stem cell rescue), RHCVAD+HDT/ASCR, or RCHOP. Clinical parameters were similar for patients treated with RHCVAD (n = 83, 50%), RCHOP+HDT/ASCR (n = 34, 20%), RCHOP (n = 29, 17%), or RHCVAD+HDT/ASCR (n = 21, 13%). Overall, 70 (42%) of the 167 patients progressed and 25 (15%) expired with a median follow-up of 33 months. There was no difference in progression-free survival (PFS) between aggressive regimens (P > .57), which all demonstrated superior PFS compared with RCHOP (P < .004). There was no difference in overall survival (OS) between the RHCVAD and RCHOP+HDT/ASCR (P = .98). RCHOP was inferior to RHCVAD and RCHOP+HDT/ASCR, which had similar PFS and OS. Despite aggressive regimens, the median PFS was 3 to 4 years. Future trials should focus on novel agents rather than comparing current approaches.
Introduction
Mantle cell lymphoma (MCL) is an uncommon subtype of non-Hodgkin lymphoma (NHL), comprising ∼ 5% of all cases. Although recent data demonstrate that the median survival of MCL has improved over the past decade to ∼ 5 years,1 the disease is not curable with standard therapy and the prognosis remains unfavorable. Few randomized clinical trials have been conducted in MCL, and the optimal initial therapy has not been identified.
Although chemotherapy regimens, such as RCHOP (rituximab, cyclophosphamide, adriamycin, vincristine, and prednisone), have high response rates in previously untreated patients with MCL, the durability of remissions is poor.2,3 More aggressive initial therapy is typically favored in younger patients.4 R-HyperCVAD5 (RHCVAD; rituximab fractionated cyclophosphamide, vincristine, adriamycin, and dexamethasone) and standard or dose-intensified chemotherapy followed by high-dose therapy and autologous stem cell rescue (HDT/ASCR) result in improved progression free-survival (PFS).6–8 These approaches, however, have not been compared directly in randomized trials.
The National Comprehensive Cancer Network (NCCN) Non-Hodgkin Lymphoma (NHL) Database prospectively collects demographic, treatment, and outcome data on patients with NHL treated at 7 National Cancer Institute (NCI)–designated cancer centers. The database provides a unique opportunity to compare the effectiveness of initial treatment approaches in MCL.
Methods
Patient cohort
Patients with previously untreated MCL diagnosed between August 2000 and December 2008 were included in the analysis. The NHL component of the NCCN Oncology Outcomes Database served as the primary source of data. All data used in this analysis, including patient, disease, and treatment characteristics were manually abstracted from medical records by trained data managers at each participating site.9 Eligibility for the NCCN NHL database are restricted to newly diagnosed patients older than 18 years of age who are cancer-free for 5 years before diagnosis and have an NHL diagnosis and histology that are confirmed by a hematopathologist at the treating NCCN center. Participating institutions include: (1) City of Hope Comprehensive Cancer Center, (2) Dana-Farber/Brigham and Women's Cancer Center, (3) Fox Chase Cancer Center, (4) The University of Texas MD Anderson Cancer Center, (5) Roswell Park Cancer Institute, (6) University of Michigan Comprehensive Cancer Center, and (7) Robert H. Lurie Comprehensive Cancer Center of Northwestern. Data collection and storage policies have undergone institutional review board review and approval at each participating institution. Two participating centers required individual patient informed consent for participation in accordance with the Declaration of Helsinki. The remaining participating institutions have deemed this project to be minimal risk research and have granted waivers for informed consent because of demonstration of adequate privacy safeguards.
A total of 362 patients with MCL were identified, 242 (67%) of whom were younger than 65 years of age. Patients were further excluded if they: (1) participated in a clinical trial during first-line therapy (n = 33, 14%), (2) did not receive rituximab as part of first-line therapy (n = 32, 13%), (3) did not receive CHOP or HyperCVAD as initial induction therapy (n = 7, 3%), or (4) received sequential CHOP and HyperCVAD where the switch in therapy was because of physician preference or transfer in care (n = 3, 1%). After exclusions, 167 patients were included in the final sample population.
First-line induction and consolidation definitions
The induction component of first-line therapy was defined as the initial chemoimmunotherapy regimen received within 180 days of diagnosis. Consolidation was defined as HDT/ASCR initiated within 180 days of induction therapy.
Identification of survival events
A PFS event was defined as (1) a patient death, (2) a relapse of disease, or (3) an indicator of disease progression. An indicator of disease progression was defined as a progression therapy response or discontinuation of therapy noted in the medical record, or the initiation of second-line therapy. PFS event dates were set to either the date of death, documented date of disease relapse or progression response, or the date of second-line therapy initiation. Overall survival (OS) was determined as a patient death from any cause. Patients without a PFS event or death were censored at their last follow-up visit to the cancer center. Patient deaths were identified from the medical record and institutional tumor registry, and validated against the Social Security Death Index and National Death Index databases.
Definition of data elements
Patients were staged using the Ann Arbor staging system. International Prognostic Index (IPI) scores were computed as outlined by the International Non-Hodgkin Lymphoma Prognostic Factors Project.10 Comorbidity was assessed using the Charlson Comorbidity Index.11
Cycles of therapy were abstracted from the patient medical record. For the HCVAD regimen, parts A (cyclophosphamide, vincristine, doxorubicin, and dexamethasone) and B (high-dose methotrexate and cytarabine) were considered separate cycles of therapy. Receipt of complete induction was defined as having received ≥ 6 cycles of induction therapy. Patients who progressed while on therapy (n = 6) were excluded from all analyses involving cycles of therapy.
A complication of therapy was defined as having at least one associated hospital admission. Complications treated in the outpatient setting, or that arose while admitted for therapy, were excluded. Hospital bed days include inpatient days associated with admissions for both therapy and complications of therapy.
Statistical analysis
The Fisher exact test was used to assess the difference between proportions. Significant differences for interval data were assessed using the Mann-Whitney U test or the Kruskal-Wallis test. Log-rank tests were used to evaluate differences in survival. Time-to-event analyses were measured from diagnosis date. All log-rank statistics are reported as 2-group tests between the individual therapy groupings. Multivariable Cox proportional hazard models were used to compare outcomes between therapy groups in models adjusted for potential confounding factors. All statistical tests were 2-sided with α set at 0.05. All statistical analyses were conducted using SAS Version 9.1 (SAS Institute Inc).
Results
Cohort characteristics
A total of 167 patients were included in the analysis. Demographics are provided in Table 1. The median age was 56 years (range, 29-64); 79% were men. Almost all patients were white (90%). No statistically significant differences in comorbidity, stage at diagnosis, B symptoms, or IPI risk group were observed between the 4 therapy groups, or between the RHCVAD and RCHOP+HDT/ASCR therapy groups (Table 1). IPI risk group was significantly associated with overall survival (log-rank, P = .01). Differences in PFS by IPI risk group did not reach statistical significance (log-rank, P = .07).
First-line therapy characterization
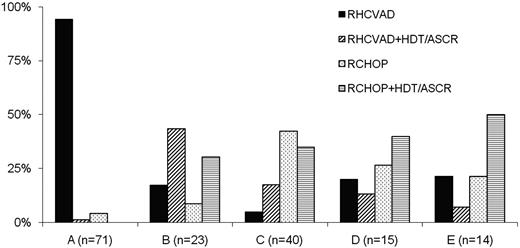
Patients received RHCVAD (n = 83, 50%), RCHOP+HDT/ASCR (n = 34, 20%), RCHOP (n = 29, 17%), or RHCVAD+HDT/ASCR (n = 21, 13%) as first-line therapy. Therapy selection varied considerably between institutions (Figure 1). Of the 63 patients receiving RCHOP induction, 3 patients (5%) progressed while on therapy, compared with 3 patients (3%) among 104 who received RHCVAD induction (P = .67). Some patients received rituximab maintenance (n = 30, 18%) including 2 (7%) in the RCHOP, 11 (13%) in the RHCVAD, 9 (26%) in the RCHOP+HDT/ASCR, and 8 (38%) in the RHCVAD+HDT/ASCR group (P = .01). The difference in R maintenance between RHCVAD and RCHOP+HDT/ASCR was not significantly different (P = .11). Median time to HDT/ASCR after RCHOP (10 weeks) and RHCVAD (8 weeks) induction was not significantly different (P = .61). The most common conditioning regimens used for patients treated with RCHOP+HDT/ASCR were CBV (cyclophosphamide, BCNU, VP-16; n = 20, 58%), BEAM/R-BEAM/Z-BEAM [(BCNU, etopside, cytarabine, melphalan)/(rituximab, BCNU, etoposide, cytarabine, melphalan)/(zecalin, BCNU, etoposide, cytarabine, melphalan)] (n = 6, 18%), Bu/Cy (n = 3, 9%), or FTBI/VP/Cy (fractionated total body irradiation, VP-16, cyclophosphamide; (n = 3, 9%). The most common conditioning regimens used for patients treated with RHCVAD+HDT/ASCR were CBV (n = 9, 43%), BEAM/R-BEAM/Z-BEAM (n = 6, 29%), or FTBI/VP/Cy (n = 2, 9%). Some patients received additional drug therapy after initial induction therapy in the RCHOP (n = 3, 10%), RCHOP+HDT/ASCR (n = 8, 23%), RHCVAD (n = 4, 5%), or RHCVAD+HDT/ASCR (n = 1, 5%) groups without evidence of progression. In most cases, patients received one or 2 cycles of either RICE (rituximab, ifosfamide, carboplatin, etoposide), RDHAP (rituximab, dexamethasone, cytarabine, cisplatin), RHCVAD, or RCHOP.
Institutional variability in the selection of first-line therapy for MCL patients (n = 167). Two institutions that contributed 4 patients to the study, 2 from each institution, are not included in the plot. All 4 patients received RHCVAD.
Institutional variability in the selection of first-line therapy for MCL patients (n = 167). Two institutions that contributed 4 patients to the study, 2 from each institution, are not included in the plot. All 4 patients received RHCVAD.
In the RHCVAD group, 79% of patients who did not progress during induction received ≥ 6 cycles of therapy with 10% having received ≥ 8cycles (1 patient received 10 cycles, Table 2). In the RCHOP+HDT/ASCR group, 94% of patients received ≥ 6 cycles of induction compared with 84% in the RCHOP group (P = .39). Fewer patients in the RHCVAD+HDT/ASCR therapy group received ≥ 6 cycles of induction (n = 7, 33%) than in any of the other 3 therapy groups (P < .001 for all pairwise comparisons).
Overall, 147 (89%) patients had reliable admissions data. Patients receiving RHCVAD required more inpatient bed days (median = 29) compared with patients who received RCHOP+HDT/ASCR (median = 23, P = .05, Table 2). Patients receiving RHCVAD+HDT/ASCR had a median of 53 inpatient bed days which was significantly greater than either the RHCVAD (P < .001) or RCHOP+HDT/ASCR (P < .001) therapy groups.
Therapy tolerability
Therapy tolerability was indirectly assessed using 2 indicators: (1) having received incomplete induction therapy, or (2) having at least one complication of therapy resulting in hospitalization (Table 3). The most common complications included febrile neutropenia (n = 42, 29%) and infection (n = 12, 8%). Rates of febrile neutropenia were highest in the RHCVAD+HDT/ASCR therapy group (n = 7, 44%), followed by the RHCVAD (n = 27, 38%), RCHOP (n = 4, 14%), and RCHOP+HDT/ASCR (n = 4, 13%) therapy groups
More patients in the RHCVAD (53%, P < .001) or RHCVAD+HDT/ASCR (75%, P < .001) therapy groups had at least one therapy complication requiring hospital admission than patients receiving RCHOP+HDT/ASCR (16%). The difference in complications requiring admission between the RHCVAD and RHCVAD+HDT/ASCR therapy groups did not reach statistical significance (P = .16), nor did the complication rates between the RCHOP (21%) and RCHOP+HDT/ASCR therapy groups (P = .74).
Survival analysis
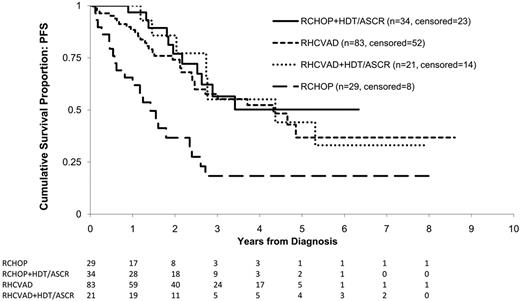
Patients alive at the time of censoring were followed for a median of 33 months. Overall, 70 (42%) of the 167 patients had a progression event during the study period including 31 (37%) in the RHCVAD, 7 (33%) in the RHCVAD+HDT/ASCR, 11 (32%) in the RCHOP+HDT/ASCR, and 21 (72%) in the RCHOP therapy group. There were no statistically significant differences in PFS between the RHCVAD, RHCVAD+HDT/ASCR, or RCHOP+HDT/ASCR (> 0.50 for all pairwise comparisons, Figure 2). Compared with the RCHOP therapy group, the other 3 therapy groups demonstrated superior PFS (RHCVAD [P < .001], RHCVAD+HDT/ASCR [P = .004], and RCHOP+HDT/ASCR [P < .001]). Salvage therapy was received by 87% of patients who progressed or relapsed (n = 61). Of those treated, 13 patients were enrolled on clinical trials, 24 received rituximab plus cytotoxic therapy, 16 received bortezomib (single agent or combination therapy), and 13 received HDT/ASCR (11 allogeneic and 2 autologous).
KM estimates of PFS from diagnosis by therapy group. Log-rank statistics were used to compare the 4 therapy groups. No significant difference was observed between the RHCVAD and RCHOP+HDT/ASCR therapy groups (P = .50), or between the RHCVAD (P = .57), RCHOP+HDT/ASCR (P = .96), and RHCVAD+HDT/ASCR therapy groups. Patients in the RHCVAD (P < .001), RHCVAD+HDT/ASCR (P = .004), and RCHOP+HDT/ASCR (P < .001) therapy groups had significantly superior PFS compared with patients in the RCHOP therapy group. Median follow-up: 33 months; 3-year PFS: RHCVAD = 58% (95% CI: 44%, 69%), RCHOP+HDT/ASCR = 56% (95% CI: 33%, 74%), RHCVAD+HDT/ASCR = 55% (95% CI: 22%, 79%), RCHOP = 18% (95% CI: 6%, 36%).
KM estimates of PFS from diagnosis by therapy group. Log-rank statistics were used to compare the 4 therapy groups. No significant difference was observed between the RHCVAD and RCHOP+HDT/ASCR therapy groups (P = .50), or between the RHCVAD (P = .57), RCHOP+HDT/ASCR (P = .96), and RHCVAD+HDT/ASCR therapy groups. Patients in the RHCVAD (P < .001), RHCVAD+HDT/ASCR (P = .004), and RCHOP+HDT/ASCR (P < .001) therapy groups had significantly superior PFS compared with patients in the RCHOP therapy group. Median follow-up: 33 months; 3-year PFS: RHCVAD = 58% (95% CI: 44%, 69%), RCHOP+HDT/ASCR = 56% (95% CI: 33%, 74%), RHCVAD+HDT/ASCR = 55% (95% CI: 22%, 79%), RCHOP = 18% (95% CI: 6%, 36%).
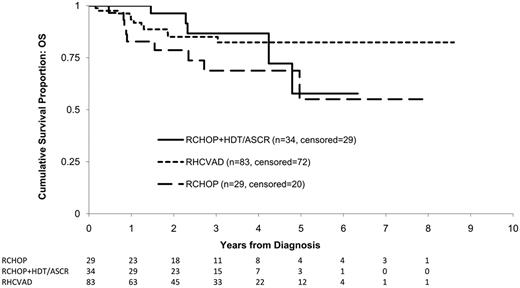
Overall, 25 patients (15%) died within the study period including 11 (13%) in the RHCVAD, 5 (15%) in the RCHOP+HDT/ASCR, and 9 (31%) in the RCHOP group (Figure 3). No patients died within the RHCVAD+HDT/ASCR therapy group. There was no significant difference in OS between the RHCVAD and RCHOP+HDT/ASCR therapy groups (0.98, Figure 3). The ob-served differences in OS between the RCHOP and the RHCVAD (P = .07) or RCHOP+HDT/ASCR (P = .20) therapy groups did not reach statistical significance. Data were not yet mature enough to include patients treated with RHCVAD+HDT/ASCR in analyses of OS. The most common cause of death was progressive disease (n = 15, 60%), followed by infection (n = 3, 12%), and GVHD (n = 2, 25%). One patient receiving RHCVAD therapy died of renal and liver failure because of excessive toxicity.
KM estimates of OS from diagnosis by therapy group. Log-rank statistics were used to compare the 3 therapy groups. No significant difference was observed between the RHCVAD and RCHOP+HDT/ASCR (P = .98) or between the RCHOP+HDT/ASCR and RCHOP (P = .20) therapy groups. Patients in the RHCVAD therapy group had marginally greater OS than those in the RCHOP therapy group (P = .07). Data were not yet mature enough to examine survival for patients receiving RHCVAD+HDT/ASCR. Median follow-up: 33 months; 3-year OS: RCHOP+HDT/ASCR = 87% (95% CI: 64%, 95%), RHCVAD = 85% (95% CI: 74%, 92%), RCHOP = 69% (95% CI: 46%, 83%).
KM estimates of OS from diagnosis by therapy group. Log-rank statistics were used to compare the 3 therapy groups. No significant difference was observed between the RHCVAD and RCHOP+HDT/ASCR (P = .98) or between the RCHOP+HDT/ASCR and RCHOP (P = .20) therapy groups. Patients in the RHCVAD therapy group had marginally greater OS than those in the RCHOP therapy group (P = .07). Data were not yet mature enough to examine survival for patients receiving RHCVAD+HDT/ASCR. Median follow-up: 33 months; 3-year OS: RCHOP+HDT/ASCR = 87% (95% CI: 64%, 95%), RHCVAD = 85% (95% CI: 74%, 92%), RCHOP = 69% (95% CI: 46%, 83%).
PFS and OS were also evaluated using multivariable Cox proportional hazard models to adjust for potential confounding in patient prognosis between therapy groups. All models were adjusted for use of rituximab maintenance, IPI, and comorbidity. No significant difference in PFS (hazard ratio [HR]: 0.8; 95% confidence interval [CI; 0.4, 1.6], P = .48) or OS (HR: 1.3; 95% CI [0.5, 4.0], P = .58) was observed between the RHCVAD (reference) and RCHOP+HDT/ASCR therapy groups. In addition, no significant difference in PFS was observed between the RHCVAD+HDT/ASCR therapy group and the RHCVAD (HR: 1.0; 95% CI [0.4, 2.4], P = .92) or RCHOP+HDT/ASCR (HR: 0.8; 95% CI [0.3, 2.1], P = .67) therapy groups. RCHOP demonstrated poorer PFS survival compare with RHCVAD (HR: 2.7; 95% CI [1.6, 4.8], P < .001), RCHOP+HDT/ASCR (HR: 3.5; 95% CI [1.7, 7.4], P < .001), and RHCVAD+HDT/ASCR (HR: 2.8; 95% CI [1.2, 6.8], P = .02). In addition, patients receiving RCHOP had poorer OS compared with the RHVCAD (HR: 2.5; 95% CI [1.0, 6.2], P = .04). The difference between RCHOP and RCHOP+HDT/ASCR was not statistically significant (HR: 1.9; 95% CI [0.6, 5.7], P = .27). Pooling patients in the 3 intensive therapy groups, both OS (HR: 0.4; 95% CI [0.2, 0.8], P = .02) and PFS (HR: 0.3; 95% CI [0.2, 0.6], P < .001) were significantly improved versus patients receiving RCHOP alone. Rituximab maintenance was associated with a statistically significant improvement in PFS (HR: 0.3; 95% CI [0.1, 0.9, P = .02]). The association between rituximab maintenance and OS did not reach statistical significance (HR: 0.6; 95% CI [0.1, 2.6, P = .50]).
A PFS-sensitivity analysis was performed comparing patients who completed RCHOP without progression (n = 26) and patients who received RCHOP+HDT/ASCR. In this analysis, RCHOP+HDT/ASCR demonstrated significantly superior PFS to RCHOP (P = .001).
Discussion
In our study from the NCCN NHL outcomes database, RHyperCVAD, RHyperCVAD followed by HDT/ASCR, and RCHOP followed by HDT/ASCR had similar disease-specific outcomes. Patients treated with RCHOP alone had inferior progression-free and overall survival. The toxicity of the RHyperCVAD arms was greater in terms of unplanned hospital admissions, in particular, febrile neutropenia. Although this is not a randomized-controlled trial, patients enrolled in the database have similar characteristics to patients reported in clinical trials and other studies in MCL. The majority of patients in all treatment arms had low or low-intermediate risk IPI scores. The Mantle Cell Lymphoma International Prognostic Index (MIPI) may be more relevant than the IPI for patients with MCL.12 Recent data validate its predictive value in patients receiving RHyperCVAD,13 as well as those undergoing HDT/ACSR consolidation in first remission.14 We were unable to calculate MIPI scores given that not all elements were included in the database. In addition, the Ki-67 fraction has emerged as a strong predictor of outcome in MCL but was not examined in all of our NCCN cases.15,16
Recent data from Cornell demonstrated no adverse impact on survival for select patients undergoing initial observation in MCL.17 Our data do not contradict this finding, as all patients in the current analysis received therapy within 180 days of presentation to the NCCN center. Outcome for patients receiving RCHOP alone was poor, and median PFS was comparable with previously published reports.2,3 A previously reported retrospective series suggests that intensive treatment regimens do not yield superior overall survival compared with sequential, standard therapies.18 However, our data show a survival disadvantage for younger patients receiving RCHOP alone, compared with more aggressive regimens.
In terms of patients receiving RHyperCVAD, this study demonstrated inferior PFS (58% at 3 years) and OS (85% at 3 years) compared with published data from the MD Anderson Cancer Center, where the 3-year failure-free survival in patients under 65 was 73%.5 When the regimen was studied by Southwest Oncology Group in the multicenter setting; however, the 2-year failure-free survival was 64% and a significant proportion of patients were unable to receive all the planned therapy predominantly because of hematologic toxicity.19 These differences are likely because of patient selection.
The outcome of patients undergoing RCHOP followed by HDT/ASCR in our study was similar to that reported in the study from Germany by Dreyling et al.7 In this trial, patients were randomized after standard chemotherapy to high-dose chemotherapy with cytoxan/total body irradiation conditioning versus IFN with a 3-year event-free survival (EFS) of 54% in the transplant arm. Improved results have been reported by the Nordic group using dose-intensified RCHOP followed by high-dose cytarabine plus rituximab, with 3-year EFS of 70%, although some patients in this series received preemptive therapy at the time of molecular relapse.8 It is unclear whether the more intensive induction therapy in this regimen resulted in prolonged EFS compared with the German study, where most patients received CHOP or CHOP-like regimens. In our study, the use of RHyperCVAD before transplantation did not yield improved PFS compared with RCHOP followed by transplantation or RHyperCVAD, though the number of patients in each of the transplant arms was small. Our findings are in keeping with a recent analysis of MCL patients treated at a single institution comparing the impact of standard versus intensive induction regimens followed by autologous stem cell transplant (ASCT). The MIPI score was an independent predictor of survival, and there was not a benefit for intensive regimens after adjusting for MIPI.20 Preliminary results from a large European study, however, comparing RCHOP versus RCHOP alternating with RDHAP followed by ASCT were recently presented and suggest a benefit in the RDHAP containing arm. Interestingly, patients who achieved a complete response after RCHOP had equivalent outcomes to the RDHAP containing arm.21 In terms of maintenance rituximab, we found a significant improvement in PFS. Preliminary results from a recent study from the MCL Network in patients older than 60 years of age who were not eligible for transplantation also demonstrated that maintenance rituximab was associated with significant prolongation in remission duration compared with IFN or observation.22
The comparison of RHyperCVAD with or without HDT/ASCR, and RCHOP followed by HDT/ASCR in our study is limited by the fact that this is not a randomized controlled study. Patients in both transplant arms received a variety of mobilization and conditioning regimens. There is a possible selection bias in favor of patients receiving RCHOP followed by HDT/ASCR, as it is difficult to ascertain the treating physician's plan for each patient at diagnosis. It is conceivable that for patients starting on RCHOP with the initial intention of consolidation with high-dose therapy, a decision was later made to stop with RCHOP alone, either because of an inadequate response to therapy, patient comorbidities, or poor chemotherapy tolerance. However, given the very high response rates observed with RCHOP in MCL, this is unlikely a significant bias in the current study.
This study is also limited by power because of smaller sample sizes among the therapy groups. As such, differences between the intensive therapy groups may be observed that are not statistically significant. However, the Kaplan-Meier PFS estimates between the 3 intensive strategies and the KM OS estimate between the RHCVAD and RCHOP+HDT/ASCR therapy strategies are very similar and appear clinically insignificant. Therefore, we infer that these strategies likely have similar disease-specific outcomes based on these data.
When we evaluated the Charlson comorbidity index, as well as median age, there was no significant difference between the RCHOP alone and the other 3 arms. Maintenance rituximab was received by a minority of patients with a greater use in the patients receiving HDT/ASCR. However, a similar pattern of results was observed in multivariable Cox proportional hazard models (adjusted for rituximab maintenance, IPI, and comorbidity) as in the unadjusted KM curves. In addition, the choice of therapy appeared to be more closely associated with institutional practice, rather than based on patient related or prognostic factors. The institutions included in the study are all large NCI-designated cancer centers with experience in delivering high-dose chemotherapy and stem cell transplantations. Therefore, while institutional practice appears to drive therapy selection, it is unlikely that the relationship between therapy selection and patient outcomes is confounded by the treating institution.
MCL is not a curable disease in most cases, and therefore comparative effectiveness analyses need to consider therapy-related morbidities and quality of life. Although PFS is a robust endpoint for clinical benefit,23 the toxicity of these regimens was also evaluated. Formal quality-of-life assessments are outside the scope of the NCCN NHL database and were not conducted. Therapy tolerability was indirectly assessed from the number of cycles of treatment administered and treatment-related toxicities resulting in unplanned hospitalizations. Tolerability of therapy appeared to be superior in the RCHOP arms. Moreover, although we did not compare the cost effectiveness of the treatment strategies, aggregate hospital bed days were analyzed and were greater in both RHyperCVAD arms compared with RCHOP followed by HDT/ASCR. The overall cost of HDT/ASCR, however, including mobilization of stem cells and transplantation, may offset the shorter hospital stay in the RCHOP followed by HDT/ASCR arm. Future prospective trials, including aggressive approaches in MCL, should include these important secondary endpoints of morbidity and cost.
Although these results demonstrate comparable outcomes for patients treated with RHyperCVAD, with or without HDT/ASCT, and RCHOP followed by HDT/ASCT, neither intensive therapy strategy seems to result in durable remission of disease. The current randomized study of RHyperCVAD versus rituximab plus bendamustine followed by ASCT by the US cooperative groups will provide definitive evidence regarding the role of intensive induction in younger patients with MCL. Subsequent future clinical trials should focus on the incorporation of novel agents, such as bortezomib and lenalidomide, into initial therapeutic regimens, and examine the role of maintenance strategies. In our cohort, only 14% and 25% of all MCL patients in the database, all of whom were treated at major cancer centers, participated in clinical trials in the upfront and relapsed settings, respectively. Patient participation in prospective clinical trials is critical to improve outcomes in MCL.
Presented in abstract form at the 2009 American Society of Hematology annual meeting, December 5-8, New Orleans, LA.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.S.L., M.A.R., M.S.C., A.P.N., D.W.B., L.I.G., M.M., E.M.L., A.D.Z., J.N., and J.W.F. contributed to conception and design, data analysis and interpretation, manuscript writing, and final approval of manuscript; and J.L.V., G.A.A., A.L.C., and A.V. contributed to conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing and final approval of manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ann S. LaCasce, MD, Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02115; e-mail: ann_lacasce@dfci.harvard.edu.