To the editor:
Analysis of shed-membrane microparticles (MPs) remains a challenging issue.1,2 György et al recently reported in Blood that measuring MP by flow cytometry was flawed because of the overlapping biophysical properties of immune complexes, causing false-positive signals during analysis of synovial fluid from patients with rheumatoid arthritis.3 They suggested that similar problems might occur during analysis of circulating MP from patients with acute coronary syndrome, where the presence of circulating immune complexes has been reported,4 therefore raising a question on the validity of previous reports.3
In view of György et al's findings, we investigated the potential interaction of immune complexes with flow cytometry analysis of circulating MPs in patients with coronary artery diseases, using MP sensitivity to a brief exposure to Triton detergent.3 Ten patients with acute coronary syndromes (ACS) were included according to their clinical characteristics. Platelet-free plasma samples prepared as previously described5 were analyzed on a Millipore GUAVA EasyCyte 8HT flow cytometer. Fluorescent Megamix beads calibrated from 0.5 to 3μm (Biocytex) were used to define an analysis window (gate) consistent with the size of MPs (< 1 μm). AnnexinV–FITC (Roche), anti–CD41-PC7 (Beckman-Coulter), anti–CD235a-APC (BD-Bioscience), anti–CD144-PE (eBioscience) antibodies, their respective isotypes and Flowcount beads (Beckman-Coulter) were used to measure levels of annexinV+ MPs, platelet (PMPs), erythrocyte-derived (RBC-MPs), and endothelial MPs (EMPs), respectively.5
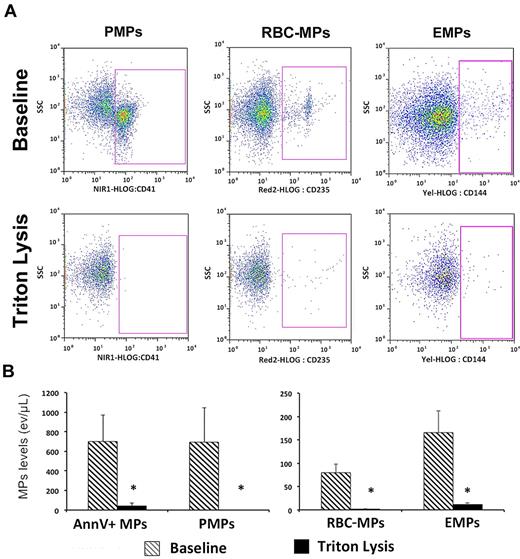
Each plasma sample was evaluated immediately after addition of Triton 0.05% or vehicle, as described by György.3 Preliminary analysis with in vitro generated EMPs and circulating MPs from healthy subjects demonstrated that Triton (0.01 to 0.05%) dose-dependently decreased annexinV+ MP concentration to reach up to 99% inhibition, without affecting the biophysical properties of fluorescent Flowcount beads (not shown). Brief exposure to Triton significantly decreased all plasma MP subpopulations in ACS patients (Figure 1). A comparable effect of Triton on plasma MP analysis was also observed in n = 10 subjects with cardiovascular risk factors but no coronary disease, and in n = 10 patients with symptomatic stable coronary artery disease (data not shown).
Effect of Triton on MP flow cytometry analysis. (A) Representative experiment using plasma from a patient with ACS: PMP, RBC-MPs, and EMP levels were assessed using specific markers, either in absence (top panels; baseline) or presence (bottom panels) of Triton. Labeling with their respective isotype is not shown. MP number dramatically decreases after Triton lysis. (B) Plasma MP levels in ACS patients (n = 10) before and after Triton lysis. Data are expressed as mean ± SEM. AnnV+ indicates annexin V+ MPs (*P < .01; Wilcoxon test).
Effect of Triton on MP flow cytometry analysis. (A) Representative experiment using plasma from a patient with ACS: PMP, RBC-MPs, and EMP levels were assessed using specific markers, either in absence (top panels; baseline) or presence (bottom panels) of Triton. Labeling with their respective isotype is not shown. MP number dramatically decreases after Triton lysis. (B) Plasma MP levels in ACS patients (n = 10) before and after Triton lysis. Data are expressed as mean ± SEM. AnnV+ indicates annexin V+ MPs (*P < .01; Wilcoxon test).
These results demonstrate the absence of significant artifacts resulting from the interference of protein complexes with circulating MP detection in a population of ACS patients where circulating immune complexes have been reported. The discrepancy of the present findings with the work from György et al might result of a lower concentration of immune complexes in our population plasma when compared with inflammatory synovial fluid, or in differences in biophysical properties between these 2 biologic samples. Nevertheless, the results of György et al together with the present data point out the need for systematic use of Triton lysis as an additional control when establishing MP labeling using flow cytometry.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Chantal M. Boulanger, PhD, Paris - Centre de recherche Cardiovasculaire à l'HEGP, Inserm - U970, 56, rue Leblanc, 75737 Paris cedex 15, France; e-mail: chantal.boulanger@inserm.fr.