To the editor:
In a recent perspective, Ablain and de The challenged the classic model of acute promyelocytic leukemia (APL), whereby differentiation-impairment in lineage-committed progenitors causes self-renewal, and instead proposed that APL arises from deregulation of stem cell self-renewal pathways in leukemia initiating cells, with the curative potential of arsenic (As2O3) relating to abrogation of these pathways.1 However, differentiation syndrome occurs at a similar rate with As2O3 as with all-trans retinoic acid (ATRA), and furthermore, disseminated intravascular coagulation, the potentially fatal complication frequently triggered by cytotoxic treatment, is not typical.2 Hence, clinical experience suggests that important actions of As2O3 include terminating APL proliferation by differentiation. To evaluate this further, the APL cell line NB4 was treated with low concentrations of As2O3 which do not induce early apoptosis3 (Figure 1A; supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). These concentrations rapidly activated CEBPE, a key myeloid late-differentiation driver which is repressed by PML-RARA4 and which directly represses MYC5 (supplemental Figure 2; MYC is an oncogene product that drives myeloid progenitor proliferation6 ). As expected therefore, CEBPE activation was accompanied by MYC repression (Figure 1B). MXD1, a down-stream target of CEBPE7 and MYC-antagonist, was also activated, but subsequent to activation of CEBPE (Figure 1B). CEBPA, a key lineage-specifying transcription factor that drives CEBPE expression,8 is expressed at high levels in NB4 and primary APL cells (supplemental Figure 1B), providing a potential explanation for rapid activation of CEBPE after repressive actions of PML-RARA are inhibited by As2O3. Time-course changes in CEBPE and MYC protein levels reiterated the time-course changes in expression of mRNA (Figure 1C). The cyclin-dependent kinase inhibitor p27/CDKN1B mediates cell cycle exit by differentiation and was up-regulated by As2O3 at late-time points (Figure 1C); p21/CDKN1A mediates cell cycle exit by differentiation or apoptosis, and was also up-regulated (Figure 1C). Phosphorylation and up-regulation of the master regulator of apoptosis p53 was also observed, but as a late event that occurred subsequent to up-regulation of CEBPE and down-regulation of MYC (Figure 1C). Both ATRA and As2O3 were anti-proliferative, with the greater effect from As2O3 (Figure 1D). Others have shown that As2O3 0.1-0.5μM induces morphologic differentiation in primary APL and NB4 cells,3 but not to the same extent as ATRA,3 an observation we recapitulated (Figure 1E).
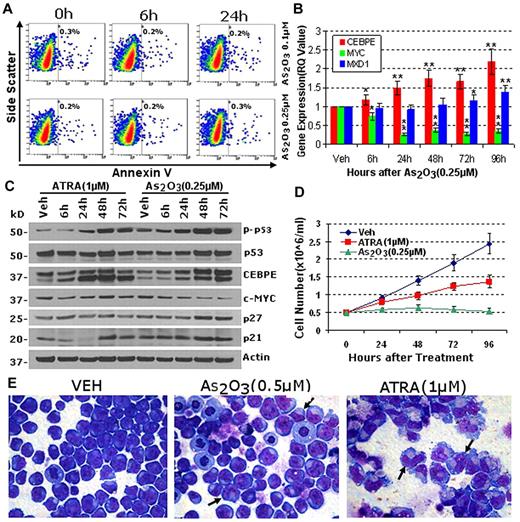
Concentrations of As2O3 that do not cause apoptosis activate key late-differentiation genes (CEBPE, MXD1) that repress MYC. (A) Concentrations of As2O3 that do not cause early apoptosis in NB4 cells were identified. Apoptosis measured by annexin-staining and flow cytometry. (B) As2O3 0.25μM activated the myeloid late-differentiation genes CEBPE and MXD1, and repressed MYC. mRNA levels measured by QRT-PCR. *P < .05, **P < .01 compared with baseline (t test). (C) Time-course changes in protein expression reiterated changes in mRNA expression. Protein expression measured by Western blot after As2O3 0.25μM or ATRA 1μM. Antibodies for MXD1 did not work. (D) As2O3 was more anti-proliferative than ATRA. Cumulative cell counts by automated counter. Error bars = SD, 3 experiments. (E) Both As2O3 and ATRA induced morphologic differentiation, although this was much more extensive with ATRA. Giemsa-stained cytospins 96 hours after addition of As2O3 0.5μm or ATRA 1μm. Black arrows indicate cells with maturation changes.
Concentrations of As2O3 that do not cause apoptosis activate key late-differentiation genes (CEBPE, MXD1) that repress MYC. (A) Concentrations of As2O3 that do not cause early apoptosis in NB4 cells were identified. Apoptosis measured by annexin-staining and flow cytometry. (B) As2O3 0.25μM activated the myeloid late-differentiation genes CEBPE and MXD1, and repressed MYC. mRNA levels measured by QRT-PCR. *P < .05, **P < .01 compared with baseline (t test). (C) Time-course changes in protein expression reiterated changes in mRNA expression. Protein expression measured by Western blot after As2O3 0.25μM or ATRA 1μM. Antibodies for MXD1 did not work. (D) As2O3 was more anti-proliferative than ATRA. Cumulative cell counts by automated counter. Error bars = SD, 3 experiments. (E) Both As2O3 and ATRA induced morphologic differentiation, although this was much more extensive with ATRA. Giemsa-stained cytospins 96 hours after addition of As2O3 0.5μm or ATRA 1μm. Black arrows indicate cells with maturation changes.
In normal hematopoiesis, MYC-mediated proliferation in progenitors is self-limited by progressive maturation that activates key late-differentiation genes such as CEBPE and MXD14–7 (supplemental Figure 2). Thus, aberrant epigenetic repression of CEBPE and other key late-differentiation genes by PML-RARA4 (supplemental Figure 2), and as a consequence of cooperating genetic abnormalities (eg, UTX deletion), can potentially contribute to MYC-related self-renewal (proliferation at the same level of differentiation) in progenitors,4,9,10 is consistent with the MYC up-regulation and amplification that is characteristic of APL,11 and as shown here, is a molecular pathway targeted by As2O3 to potentially explain the anti–self-renewal effects of this drug. The curative potential of As2O3 compared with ATRA could reflect superior pharmacodynamics, that is, more effective degradation of oncogenic PML-RARA.3,12 Hence, in important aspects, As2O3 could be a differentiation-therapy drug for APL, with a mechanism of action that supports the classic model of this disease, even if it does not recapitulate the full-spectrum morphologic differentiation that is produced by the more physiologic interactions of ATRA with the RARA moiety in PML-RARA.
Authorship
The online version of this article contains a data supplement.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yogen Saunthararajah, Taussig Cancer Institute, Cleveland Clinic, 9500 Euclid Ave, MC R40, Cleveland, OH 44195; e-mail: saunthy@ccf.org.
References
National Institutes of Health