Abstract
The physiologic role played by plasmacytoid dendritic cells (pDCs) in the induction of innate responses and inflammation in response to pathogen signaling is not well understood. Here, we describe a new mouse model lacking pDCs and establish that pDCs are essential for the in vivo induction of NK-cell activity in response to Toll-like receptor 9 (TLR9) triggering. Furthermore, we provide the first evidence that pDCs are critical for the systemic production of a wide variety of chemokines in response to TLR9 activation. Consequently, we observed a profound alteration in monocyte, macrophage, neutrophil, and NK-cell recruitment at the site of inflammation in the absence of pDCs in response to CpG-Dotap and stimulation by microbial pathogens, such as Leishmania major, Escherichia coli, and Mycobacterium bovis. This study, which is based on the development of a constitutively pDC-deficient mouse model, highlights the pivotal role played by pDCs in the induction of innate immune responses and inflammation after TLR9 triggering.
Introduction
Plasmacytoid DCs (pDCs) are characterized by their ability to contribute to antiviral innate immunity by producing type I IFNs on stimulation.1 These cells display a CD11clow B220+ Ly6C+ CD45RA+ phenotype and also express markers, such as CD317 (BST-2)2 and SiglecH.3 Their Toll-like receptor (TLR) expression pattern is limited to TLR7 and TLR9, which recognize viral single-stranded RNA and unmethylated DNA, respectively. The constitutive expression of IFN regulatory factor 7 enables pDCs to rapidly produce high levels of type I IFNs after TLR stimulation. After activation via TLR7 or TLR9 signaling, pDCs produce cytokines, such as IL-12, IL-6, and TNF-α, and chemokines, including CCL3, CCL4, CCL5, CXCL9, and CXCL10, in addition to type I IFNs.4
NK cells exhibit potent cytotoxic activity against infected or tumor cells and are efficient producers of several cytokines and chemokines.5 NK-cell activation is controlled by the recognition of ligands expressed on the surface of target cells. However, NK cells require additional signals for activation, including type I IFNs and IL-12.6 Because of their ability to produce these cytokines, pDCs may play an important role in stimulating and inducing NK-cell responses. Indeed, pDCs can promote murine cytomegalovirus clearance by NK cells through TLR9 interaction.7 Furthermore, NK cells express the chemokine receptors CCR5 and CXCR3, which interact with the chemokines produced by activated pDCs,8 suggesting that pDCs may also influence their recruitment. In addition to NK cells, immature conventional DCs (cDCs), monocytes, macrophages, polymorphonuclear basophiles (PMBs) and eosinophils (PMEs) also respond to type I IFNs9 and to CCL3, CCL4, and CCL5,8 suggesting that pDCs participate in the activation and recruitment of inflammatory cells.
To directly assess the physiologic role of pDCs in innate immunity, it is crucial to analyze these responses in vivo in the absence of pDCs. pDCs could be immunodepleted in vivo with antibodies against molecules expressed by pDCs, such as anti-GR1 (Ly6C/G)1 and anti-CD317 mAbs.2 However, pDC depletion following this strategy is incomplete and transient and affects other cells that express these markers. Conditional pDC-depleted models have been described in which the diphtheria toxin receptor is expressed under the control of promoters specifically expressed by pDCs.10,11 Although of great interest, the limitations of these models are the transient depletion of pDCs and the cell death induced by the toxin treatment that could affect the innate immunity by modifying the inflammatory environment. Recently, a new mouse model that constitutively lacks pDCs has been developed.12 This model was used to evaluate the impact of pDC deficiency on acute and chronic viral infection. In the present study, we developed an alternative mouse model based on the constitutive depletion of pDCs. A hypomorphic mutation in the Ikaros locus (IKL/L) leads to the expression of low levels of functional Ikaros protein in hematopoietic cells and is associated with the absence of pDCs in periphery.13 However, these IKL/L mice also exhibit abnormalities in B-cell development and function14 and develop a lethal T-cell leukemia beginning at 6 to 8 weeks of age. To circumvent these deleterious effects, we crossed IKL/L mice with syngeneic recombination activating gene 2 (Rag2)–deficient mice to obtain a homozygous strain carrying both the hypomorphic mutation in the Ikaros locus, as well as a knockout of the Rag2 gene (IKL/L Rag−/− mice).
Using the IKL/L Rag−/− mouse model, we analyzed the innate response induced after TLR9 stimulation. We show that pDCs constitute a unique source of type I IFNs and are required for the induction of NK-cell responses to TLR9 activation. Furthermore, our results demonstrate, for the first time, that pDCs are absolutely essential for systemic chemokine production after TLR9 stimulation. The lack of pDCs was associated with a strong defect in the recruitment of NK cells, polymorphonuclear neutrophils (PMNs), monocytes, and macrophages to the site of inflammation. Similar results were obtained in response to stimulation by microbial pathogens. In contrast, these responses were not affected in IKL/L Rag−/− mice after stimulation through TLR3, a TLR expressed on cDCs but not on pDCs, demonstrating that pDCs are involved only after direct activation. Thus, pDCs constitute a crucial factor in the initiation of TLR9-dependent inflammation and NK-cell responses.
Methods
Mice
C57BL/6 mice and 129sv mice were purchased from Charles River Laboratories and used at 6 to 10 weeks of age. OT-I15 in the Rag2-deficient background and OT-II16 T-cell receptor-transgenic mice, specific for the Kb-restricted OVA257-264 and the I-Ab-OVA323-339 epitopes, respectively, were bred at the animal facilities of the Pasteur Institute. IKL/L mice13 were crossed into a Rag2-deficient background.17 Rag−/−γc−/− and β2m−/− mice were kindly provided by James Di Santo and Matthew Albert, respectively (Institut Pasteur, France). All animal work was approved by departmental veterinarian services (Paris).
Medium, proteins, TLR-L, and pathogens
Complete medium consisted of RPMI 1640 (Invitrogen) supplemented with 10% FCS (Valeant Pharmaceuticals) and antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin; Invitrogen). Ovalbumin (OVA) was obtained from Calbiochem. Polyinosinic acid/polycytidic acid (PIC, TLR3 ligand) was purchased from Invivogen and was administered intravenously at 25 μg per mouse. Unmethylated cytosine-guanine (CpG, TLR9 ligand) motif 2216 (5′-GGGACGATCGTG-3′; Proligo) was administrated intravenously at 25 μg per mouse unless indicated otherwise. For the CpG-Dotap complex, 25 μg of CpG was diluted in 50 μL of PBS and mixed with 30 μg of Dotap (Roche Diagnostics) diluted in 100 μL of PBS. Pathogens and vaccines were as follows: heat-inactivated Leishmania major promastigotes produced as described,18 heat-inactivated Escherichia coli strain DH5α, and Bacille Calmette-Guerin (BCG) vaccine (1331 strain; Statens Serum Institute).
Flow cytometry
Cell suspensions were prepared as previously described19 and labeled with mAbs purchased from BD Biosciences, except for anti-CD317, which was purchased from AbCys. Events were acquired on a Cyan flow cytometer (Dako Denmark) and analyzed using FlowJo Version 9.4 software (Tree-Star). For intracellular staining, cell surface labeling with mAbs was performed in the presence of brefeldine A (1 μg/mL, BD Biosciences) for IFN-γ detection, and cells were fixed, permeabilized, and incubated with anti–IFN-γ (BD Biosciences) or anti–Granzyme B (GrzB; Invitrogen) mAb, or the control isotype.
Multispectral imaging
A high-throughput multispectral fluorometric technique was used to quantitate the number of GrzB+ granules in NKp46+ NK1-1+ DX5+ cells. Cells were stained with mAbs, and digital imaging was performed on a multispectral imaging flow cytometer (ImageStreamX; Amnis Corporation). At least 104 NKp46+ cells were imaged for each sample and analyzed using the manufacturer's software (IDEAS Version 4.0; Amnis Corporation).
Preparation of splenic DCs and NK cells
For cDC purification, spleen cells were incubated with MACS–anti-CD11c and isolated on an AutoMACS (Miltenyi Biotec). Cell suspensions were composed of 90% to 95% CD11c+ cells. For pDC purification, spleen cells were stained with biotinylated anti-CD317 mAb (mPDCA1; Miltenyi Biotec) and selected by AutoMACS using MACS–anti-biotin. pDCs were more than or equal to 95% pure. For NK-cell enrichment, splenocytes were incubated with MACS–anti-DX5 and selected by AutoMACS. DX5+ cells represent more than 60% of the enriched population.
Cytokine ELISA
The IL-12-p40 concentration in the culture supernatant was determined using an ELISA, as previously described.19
Antigen presentation assays
Antigen presentation by cDCs was assessed by their ability to induce proliferation of OVA-specific OT-I (class I presentation) or OT-II (class II presentation) lymph node (LN) cells as previously described.20
Cytokine and chemokine concentrations in sera
Cytokine and chemokine concentrations in sera were assessed with Luminex xMAP technology using the Milliplex mouse cytokine/chemokine immunoassay from Millipore. IFN-α and IFN-β concentrations were determined using the Procarta cytokine assay (Affymetrix).
In vivo killing assay
Splenocytes from C57BL/6 or β2m−/− mice were depleted of pDCs and NK cells using MACS–anti-CD317 and MACS–anti-DX5 on an AutoMACS. β2m−/− and C57BL/6 cells were subsequently stained with CFSE (Sigma-Aldrich) at a concentration of 2.5μM and 0.25μM, respectively. C57BL/6, IK+/+ Rag−/−, or IKL/L Rag−/− mice previously stimulated with TLR agonists were injected with 5 × 106 cells of each population. Rag−/−γc−/− mice were used as a control. At 4 hours after injection, the proportion of target cells in the spleen was determined using flow cytometry. Specific lysis of β2m−/− cells was determined as follows: 100 − 100 × (% CFSEhigh cells/CFSElow cells)/(% CFSEhigh cells in Rag−/−γc−/− mouse/% CFSElow cells in Rag−/−γc−/− mouse).
Statistical analysis
The statistical significance of differences between groups of mice was determined by the Student t test.
Results
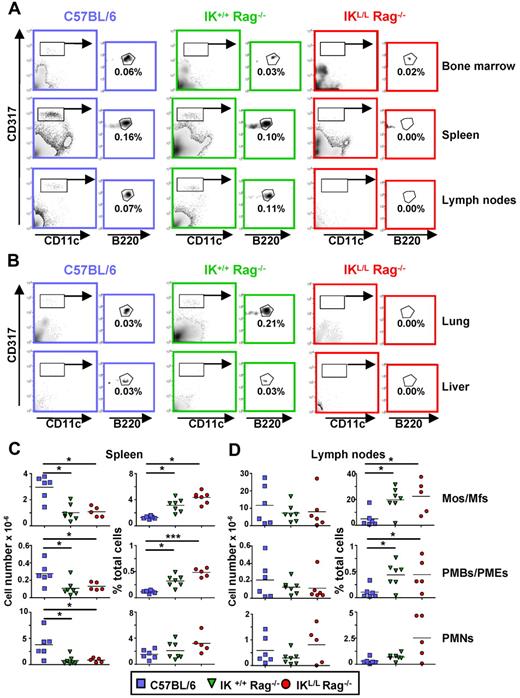
IKL/L Rag−/− mice lack pDCs, B cells, and T cells
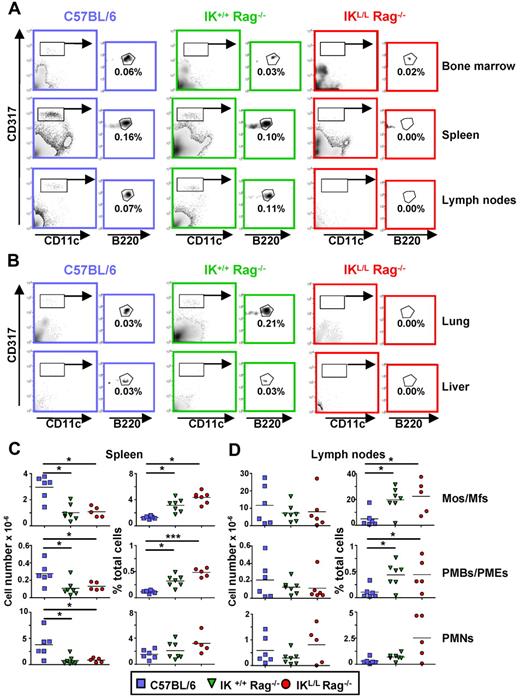
To generate a mouse model lacking pDCs, we crossed IKL/L mice13 with syngeneic Rag2-deficient mice to obtain a new strain of mice homozygous for the IKL/L gene and knocked out for the Rag2 gene (IKL/L Rag−/− mice). These mice are long lived (at least 18 months) and did not develop intrinsic diseases. The IKL/L Rag−/− mice exhibited a selective defect in CD11cint CD317+ cells, a phenotype characteristic of the pDC population, in the peripheral organs except BM (Figure 1A-B). These results demonstrate that, in IKL/L Rag−/− mice, pDCs are absent from the periphery but are present in the BM. These BM pDCs were SiglecH, CD11c, and Ly6C positive (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). They expressed higher levels of CD8, MHC class II, and CD24 compared with BM pDCs from IK+/+ Rag−/− and C57BL/6 mice and did not express costimulatory molecules, such as CD40 and CD80. Thus, these cells were phenotypically identical to the pDCs observed in BM from the original IKL/L mice, strongly suggesting that they belong to the pDC lineage but are blocked in their differentiation.13 As expected, Rag2-deficient mice lacked both T and B cells (supplemental Figure 2). Monocyte/macrophage, PMB/PME, and PMN populations were quantified in the spleen and lymph nodes. There was no statistically significant difference in the percentage or total number of these cell subsets between IK+/+ Rag−/− and IKL/L Rag−/− mice, demonstrating that the Ikaros mutation did not quantitatively affect these populations (Figure 1C-D). In contrast, higher numbers of these cell subsets were observed in the spleen, but not the LN, of C57BL/6 mice compared with the Rag2-deficient strains, indicating that B and T lymphocytes may influence the homing of hematopoietic cells. The higher percentages of these populations observed in the 2 strains of Rag2-deficient mice were mainly the result of the absence of lymphocytes. Similar cell profiles were observed in elderly mice (up to 15 months of age), demonstrating the stability of the phenotype (data not shown). Overall, these results indicate that a low level of expression of the Ikaros gene does not affect the development of hematopoietic populations in IKL/L Rag−/− mice.
Cell populations present in IKL/L Rag−/− mice. (A-B) Total cells from the BM, spleen, and lymph nodes (A) or 40% Percoll gradient-enriched cells from the lung and liver (B) of C57BL/6, IK+/+ Rag−/−, or IKL/L Rag−/− mice were stained and analyzed by flow cytometry. pDCs were gated on CD317+ CD11clow B220+. Numbers indicate the percentage of cells among total live cells from the indicated organ. Data shown are representative of at least 3 experiments. (C-D) Spleen (C) and lymph node (D) cells from the indicated mouse strains were stained and analyzed by flow cytometry. Total cell numbers (left column) and percentages (right column) of monocytes/macrophages (Mos/Mfs; F4/80+ CD11c− CD11bhigh Ly6C− GR1−), PMBs/PMEs (SSChigh F4/80+ CD11c− CD11bint Ly6C+ GR1low), and PMNs (F4/80low CD11c− CD11b+ Ly6C+ GR1high) among total live cells are shown. Each dot represents results obtained from individual mice, and bars represent the mean. Data shown are from 3 experiments. *P < .05. ***P < .001.
Cell populations present in IKL/L Rag−/− mice. (A-B) Total cells from the BM, spleen, and lymph nodes (A) or 40% Percoll gradient-enriched cells from the lung and liver (B) of C57BL/6, IK+/+ Rag−/−, or IKL/L Rag−/− mice were stained and analyzed by flow cytometry. pDCs were gated on CD317+ CD11clow B220+. Numbers indicate the percentage of cells among total live cells from the indicated organ. Data shown are representative of at least 3 experiments. (C-D) Spleen (C) and lymph node (D) cells from the indicated mouse strains were stained and analyzed by flow cytometry. Total cell numbers (left column) and percentages (right column) of monocytes/macrophages (Mos/Mfs; F4/80+ CD11c− CD11bhigh Ly6C− GR1−), PMBs/PMEs (SSChigh F4/80+ CD11c− CD11bint Ly6C+ GR1low), and PMNs (F4/80low CD11c− CD11b+ Ly6C+ GR1high) among total live cells are shown. Each dot represents results obtained from individual mice, and bars represent the mean. Data shown are from 3 experiments. *P < .05. ***P < .001.
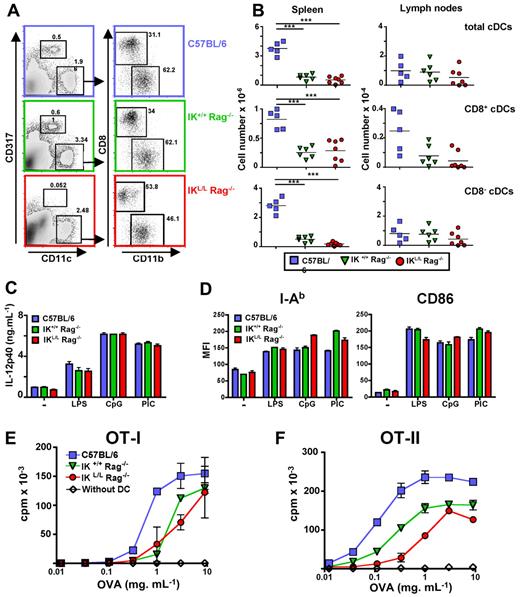
cDCs from IKL/L Rag−/− mice are functional
Because cDCs play a key role in the induction of immunity, we next performed a phenotypic and functional analysis of cDCs in IKL/L Rag−/− mice. Although the ratio between CD11b+ and CD8+ cDCs was slightly modified in IKL/L Rag−/− mice compared with the other strains, no statistically significant difference was observed in the absolute number of cDC subsets in the spleen and LN of IK+/+ Rag−/− and IKL/L Rag−/− mice (Figure 2A-B). As shown for other hematopoietic cell populations, the total number of cDCs was significantly lower in the spleen of Rag2-deficient mice compared with C57BL/6 mice (Figure 2B), but not in the LN (Figure 2B) or the blood (data not shown). To assess the possible effect of the Ikaros mutation on cDC functions, we analyzed cytokine production and maturation of purified cDCs in response to TLR-L. Stimulation with TLR-Ls induced the production of similar quantities of IL-12p40 by splenic CD11c+ DCs purified from C57BL/6, IK+/+ Rag−/−, and IKL/L Rag−/− mice (Figure 2C). These cells up-regulated the expression of MHC class II and CD86 molecules to a similar degree after stimulation (Figure 2D). Although the cDCs from Rag2-deficient strains showed weaker Ag-presenting ability for both class I and II responses compared with cDCs from C57BL/6 mice, cDCs from IKL/L Rag−/− mice were able to induce both OT-I and OT-II LN cell proliferation (Figure 2E,F). These data demonstrate that cDCs from IKL/L Rag−/− mice were functional, even if these cells appeared to be less effective to present Ag for MHC class II-restricted T-cell responses.
cDCs from IKL/L Rag−/− mice are functional. Spleen cells from C57BL/6, IK+/+ Rag−/−, or IKL/L Rag−/− mice were stained and analyzed using flow cytometry. (A) CD11chigh CD317− cells were gated and analyzed for the expression of CD8 and CD11b markers. Numbers indicate the percentage of events within the indicated gate. (B) Cumulative data of numbers of CD11chigh cells (first row), CD11chigh CD8+ cDCs (second row), and CD11chigh CD8− cDCs (third row) from the spleen (first column) or LN (second column). Each dot corresponds to results obtained from individual mice, and bars represent the mean. ***P < .001. (C-D) Purified CD11chigh spleen cells from C57BL/6, IK+/+ Rag−/−, and IKL/L Rag−/− mice were cultured in medium alone or in the presence of LPS (2 μg/mL), CpG (10 μg/mL), or PIC (10 μg/mL) for 18 hours. The concentration of IL-12p40 in the supernatants was determined by ELISA (C), and cells were labeled and analyzed by flow cytometry (D). I-Ab and CD86 expression on CD11chigh-gated cells from the indicated mouse strains is expressed as the mean fluorescence intensity (MFI). One representative experiment of 4 is shown. (E-F) A total of 104 purified CD11chigh spleen cells from the indicated mouse strains were incubated for 2 hours in the presence of various concentrations of OVA. Cells were washed twice and cocultured with 2 × 104 LN cells from OT-I (E) or OT-II (F) transgenic mice for 36 hours. T-cell proliferation was measured by 3H-labeled thymidine incorporation during the final 6 hours of culture. Proliferation of OT-I and OT-II LN cells in the absence of DCs is shown (white diamonds). Results are expressed as the mean cpm ± SD for duplicate wells. One representative experiment of 3 is shown.
cDCs from IKL/L Rag−/− mice are functional. Spleen cells from C57BL/6, IK+/+ Rag−/−, or IKL/L Rag−/− mice were stained and analyzed using flow cytometry. (A) CD11chigh CD317− cells were gated and analyzed for the expression of CD8 and CD11b markers. Numbers indicate the percentage of events within the indicated gate. (B) Cumulative data of numbers of CD11chigh cells (first row), CD11chigh CD8+ cDCs (second row), and CD11chigh CD8− cDCs (third row) from the spleen (first column) or LN (second column). Each dot corresponds to results obtained from individual mice, and bars represent the mean. ***P < .001. (C-D) Purified CD11chigh spleen cells from C57BL/6, IK+/+ Rag−/−, and IKL/L Rag−/− mice were cultured in medium alone or in the presence of LPS (2 μg/mL), CpG (10 μg/mL), or PIC (10 μg/mL) for 18 hours. The concentration of IL-12p40 in the supernatants was determined by ELISA (C), and cells were labeled and analyzed by flow cytometry (D). I-Ab and CD86 expression on CD11chigh-gated cells from the indicated mouse strains is expressed as the mean fluorescence intensity (MFI). One representative experiment of 4 is shown. (E-F) A total of 104 purified CD11chigh spleen cells from the indicated mouse strains were incubated for 2 hours in the presence of various concentrations of OVA. Cells were washed twice and cocultured with 2 × 104 LN cells from OT-I (E) or OT-II (F) transgenic mice for 36 hours. T-cell proliferation was measured by 3H-labeled thymidine incorporation during the final 6 hours of culture. Proliferation of OT-I and OT-II LN cells in the absence of DCs is shown (white diamonds). Results are expressed as the mean cpm ± SD for duplicate wells. One representative experiment of 3 is shown.
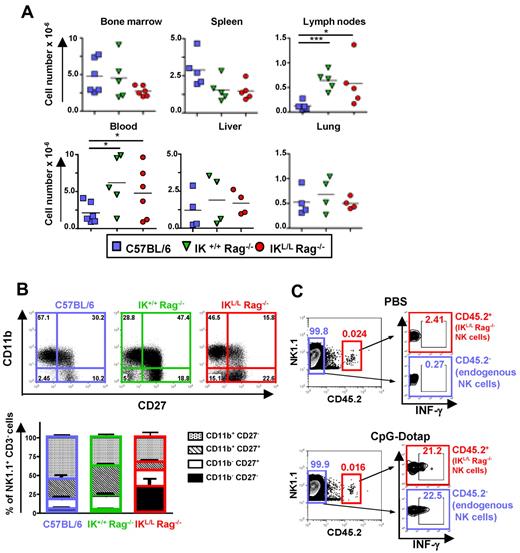
NK cells from IKL/L Rag−/− mice are functional
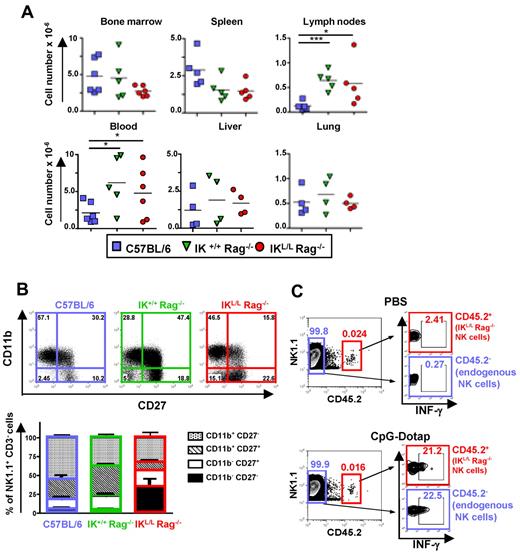
We next determined whether the Ikaros mutation influences the phenotype and function of IKL/L Rag−/− NK cells. Although NK cell numbers were higher in the blood and LN of Rag2-deficient strains compared with C57BL/6 mice, no statistically significant difference was observed between IK+/+ Rag−/− and IKL/L Rag−/− mice in all of the organs analyzed (Figure 3A). However, IKL/L Rag−/− mice exhibited more immature NK cells (CD11b− CD27−) in the spleen compared with the 2 other strains (Figure 3B). Mature NK cells (CD11b+ CD27−) were more abundant in C57BL/6 mice, whereas semimature NK cells (CD11b+ CD27+) were more numerous in IK+/+ Rag−/− mice. Similar differences were also observed in the lung, liver, and blood (supplemental Figure 3). These results suggest that NK-cell maturation may be affected by both the Ikaros mutation and the presence of T and B cells. To assess the effect of the Ikaros mutation on NK-cell function, we transferred NK cells from IKL/L Rag−/− mice (CD45.2+) into CD45.1+ C57BL/6 mice and analyzed the IFN-γ response of both endogenous (CD45.1+) and transferred (CD45.2+) NK cells to TLR9 activation in vivo. We found that both NK-cell populations responded equally to CpG-Dotap, demonstrating that the Ikaros mutation does not affect NK-cell function (Figure 3C).
Representation and phenotypic characterization of NK cells. (A) Numbers of NK1.1+ CD3− cells from the indicated organs from C57BL/6, IK+/+ Rag−/−, or IKL/L Rag−/− mice. Each dot represents results from individual mice, and bars represent the mean. *P < .05. ***P < .001. (B) CD11b and CD27 expression on NK1.1+ CD3− cells from the spleens of the indicated mouse strains as characterized by flow cytometry. Results are expressed as a dot plot from 1 representative experiment (top panel), and numbers represent the percentage of events in the corresponding quadrant. The cumulative results from 5 mice are shown (bottom panels). (C) C57BL/6 CD45.1+ mice were injected intravenously with 105 DX5+ CD45.2+-enriched cells from IKL/L Rag−/− mice. The following day, mice were injected with PBS (top panel) or CpG-Dotap (bottom panel); and after 4 hours, IFN-γ production by injected (CD45.2+) or endogenous (CD45.2−) NK cells from the host spleen was determined by intracellular staining. The numbers in quadrants represent the percentage of cells in the selected gates. One representative experiment of 2 is shown.
Representation and phenotypic characterization of NK cells. (A) Numbers of NK1.1+ CD3− cells from the indicated organs from C57BL/6, IK+/+ Rag−/−, or IKL/L Rag−/− mice. Each dot represents results from individual mice, and bars represent the mean. *P < .05. ***P < .001. (B) CD11b and CD27 expression on NK1.1+ CD3− cells from the spleens of the indicated mouse strains as characterized by flow cytometry. Results are expressed as a dot plot from 1 representative experiment (top panel), and numbers represent the percentage of events in the corresponding quadrant. The cumulative results from 5 mice are shown (bottom panels). (C) C57BL/6 CD45.1+ mice were injected intravenously with 105 DX5+ CD45.2+-enriched cells from IKL/L Rag−/− mice. The following day, mice were injected with PBS (top panel) or CpG-Dotap (bottom panel); and after 4 hours, IFN-γ production by injected (CD45.2+) or endogenous (CD45.2−) NK cells from the host spleen was determined by intracellular staining. The numbers in quadrants represent the percentage of cells in the selected gates. One representative experiment of 2 is shown.
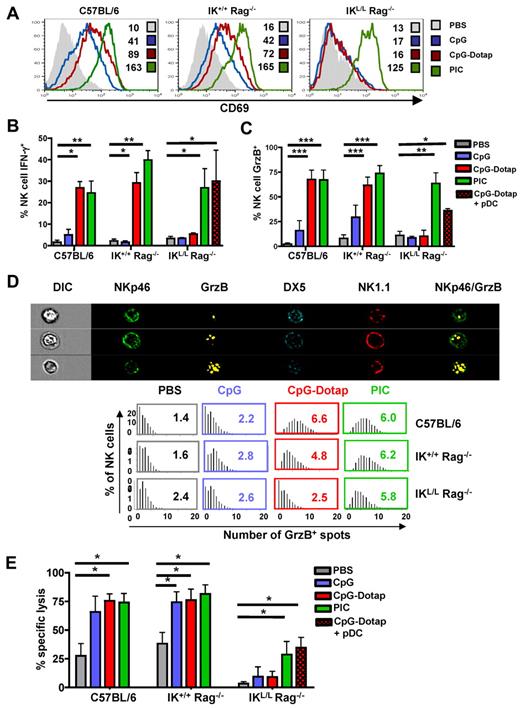
Impaired NK activity in the absence of pDCs in response to TLR9 activation
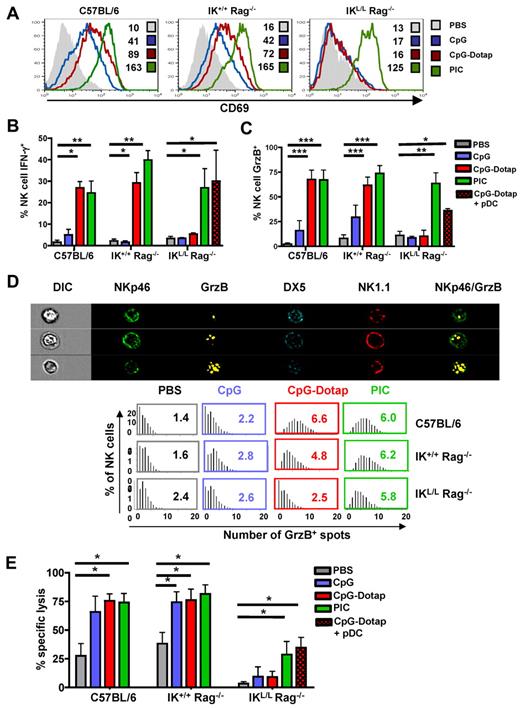
Multiple studies have suggested a role for pDCs in the induction of NK-cell activity.21-23 To analyze the role of pDCs in the induction of NK-cell responses, we measured splenic NK-cell activation, IFN-γ and GrzB production, and cytotoxic activity after the in vivo administration of either CpG alone or CpG complexed to Dotap (CpG-Dotap). In contrast to C57BL/6 and IK+/+ Rag−/− NK cells, NK cells from IKL/L Rag−/− mice did not exhibit increased expression of the activation marker CD69 18 hours after CpG or CpG-Dotap treatment (Figure 4A). Whereas CpG alone did not stimulate the production of IFN-γ in the 3 strains of mice, a strong IFN-γ response was observed in C57BL/6 and IK+/+ Rag−/− NK cells after administration of CpG-Dotap. In contrast, no response to CpG-Dotap was observed in IKL/L Rag−/− mice (Figure 4B). A similar defect was observed for GrzB production after CpG or CpG-Dotap injection of IKL/L Rag−/− mice (Figure 4C). A significant GrzB response was observed in C57BL/6 and IK+/+ Rag−/− mice in response to CpG, demonstrating that pDCs are also involved in NK activation in response to the free form of CpG. These results were confirmed by the quantification of GrzB+ granules in NK cells after in vivo activation. Indeed, the number of GrzB+ granules did not increase in IKL/L Rag−/− NK cells after CpG-Dotap stimulation, in contrast with IK+/+ Rag−/− and C57BL/6 NK cells (Figure 4D). Thus, the lack of pDCs in IKL/L Rag−/− mice strongly affected both the percentage of GrzB+ NK cells and the level of GrzB expressed by individual NK cells. Finally, NK cells from CpG-Dotap-stimulated IKL/L Rag−/− mice were unable to kill MHC class I knockout splenocytes in vivo, whereas NK cells from CpG- or CpG-Dotap–stimulated IK+/+ Rag−/− and C57BL/6 mice very efficiently killed these target cells. Overall, these data demonstrate that pDCs are strictly required for NK-cell responses after TLR9 stimulation. Importantly, the injection of pDCs into IKL/L Rag−/− mice before CpG-Dotap stimulation restored NK-cell functions, such as IFN-γ and GrzB production and cytotoxic activity (Figure 4B-E), which demonstrates that IKL/L Rag−/− mice were able to mount efficient NK-cell responses and that the defect of NK-cell activity in response to TLR9 activation was essentially the result of the absence of pDCs.
Functional defect in the NK-cell activity of IKL/L Rag−/− mice after TLR9 stimulation. (A) CD69 expression on splenic NK cells (gated on NK1.1+ DX5+) from C57BL/6, IK+/+ Rag−/−, or IKL/L Rag−/− mice 18 hours after intravenous injection of PBS, CpG, CpG-Dotap, or PIC. Numbers indicate mean fluorescence intensity for each histogram. (B-C) C57BL/6, IK+/+ Rag−/−, or IKL/L Rag−/− mice were injected with PBS, CpG, CpG-Dotap, or PIC. Some IKL/L Rag−/− mice were also reconstituted with 1 to 2 × 106 purified pDCs 1 day before CpG-Dotap injection (CpG-Dotap + pDC). After 4 hours (B) or 18 hours (C), spleen cells were labeled, and IFN-γ (B) or GrzB (C) production by NK cells (NK1.1+ DX5+ cells) was determined by intracellular staining. Results are expressed as the mean of the percentage of IFN-γ+ (B) or GrzB+ (C) NK cells ± SD from 5 mice. Data are from 3 experiments. *P < .05. **P < .01. ***P < .001. (D) Top panel: the morphology and enumeration of GrzB+ spots in NKp46+ DX5+ NK1.1+ cells from C57BL/6 mice 18 hours after injection of CpG-Dotap by multispectral imaging. Representative images show NK cells (NKp46+ DX5+ NK1.1+) with 1 (first row), 3 (second row), and 10 (third row) GrzB+ spots. Bottom panel: the indicated mouse strains were injected with PBS alone, CpG, CpG-Dotap, or PIC. After 18 hours, the numbers of GrzB+ spots in NK cells were quantified. Bars represent the distribution of GrzB+ spot counts on NKp46+ DX5+ NK1.1+ cells, and numbers indicate the mean of GrzB+ spots per NK cell. One representative experiment of 4 is shown. (E) C57BL/6, IK+/+ Rag−/−, and IKL/L Rag−/− mice were injected with PBS, CpG, CpG-Dotap, or PIC. Some IKL/L Rag−/− mice were also reconstituted with 1 to 2 × 106 purified pDC 1 day before CpG-Dotap injection (CpG-Dotap + pDC). After 18 hours, NK-cell cytotoxic activity was determined with a 4-hour in vivo killing assay using β2m knockout splenocytes as target cells. Data shown are from 4 experiments. *P < .05.
Functional defect in the NK-cell activity of IKL/L Rag−/− mice after TLR9 stimulation. (A) CD69 expression on splenic NK cells (gated on NK1.1+ DX5+) from C57BL/6, IK+/+ Rag−/−, or IKL/L Rag−/− mice 18 hours after intravenous injection of PBS, CpG, CpG-Dotap, or PIC. Numbers indicate mean fluorescence intensity for each histogram. (B-C) C57BL/6, IK+/+ Rag−/−, or IKL/L Rag−/− mice were injected with PBS, CpG, CpG-Dotap, or PIC. Some IKL/L Rag−/− mice were also reconstituted with 1 to 2 × 106 purified pDCs 1 day before CpG-Dotap injection (CpG-Dotap + pDC). After 4 hours (B) or 18 hours (C), spleen cells were labeled, and IFN-γ (B) or GrzB (C) production by NK cells (NK1.1+ DX5+ cells) was determined by intracellular staining. Results are expressed as the mean of the percentage of IFN-γ+ (B) or GrzB+ (C) NK cells ± SD from 5 mice. Data are from 3 experiments. *P < .05. **P < .01. ***P < .001. (D) Top panel: the morphology and enumeration of GrzB+ spots in NKp46+ DX5+ NK1.1+ cells from C57BL/6 mice 18 hours after injection of CpG-Dotap by multispectral imaging. Representative images show NK cells (NKp46+ DX5+ NK1.1+) with 1 (first row), 3 (second row), and 10 (third row) GrzB+ spots. Bottom panel: the indicated mouse strains were injected with PBS alone, CpG, CpG-Dotap, or PIC. After 18 hours, the numbers of GrzB+ spots in NK cells were quantified. Bars represent the distribution of GrzB+ spot counts on NKp46+ DX5+ NK1.1+ cells, and numbers indicate the mean of GrzB+ spots per NK cell. One representative experiment of 4 is shown. (E) C57BL/6, IK+/+ Rag−/−, and IKL/L Rag−/− mice were injected with PBS, CpG, CpG-Dotap, or PIC. Some IKL/L Rag−/− mice were also reconstituted with 1 to 2 × 106 purified pDC 1 day before CpG-Dotap injection (CpG-Dotap + pDC). After 18 hours, NK-cell cytotoxic activity was determined with a 4-hour in vivo killing assay using β2m knockout splenocytes as target cells. Data shown are from 4 experiments. *P < .05.
In contrast to the defective response to CpG-Dotap by IKL/L Rag−/− NK cells, the response to PIC, a TLR3-L that cannot directly activate pDCs, was not affected by the absence of pDCs (Figure 4), demonstrating that IKL/L Rag−/− mice were able to generate an NK-cell response after a TLR9-independent activation pathway.
Impaired cytokine and chemokine production in IKL/L Rag−/− mice after CpG stimulation
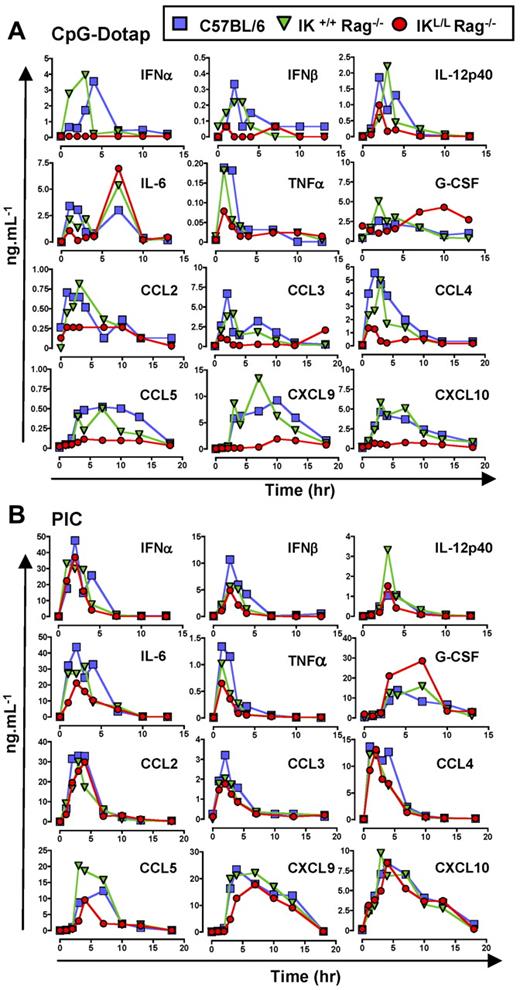
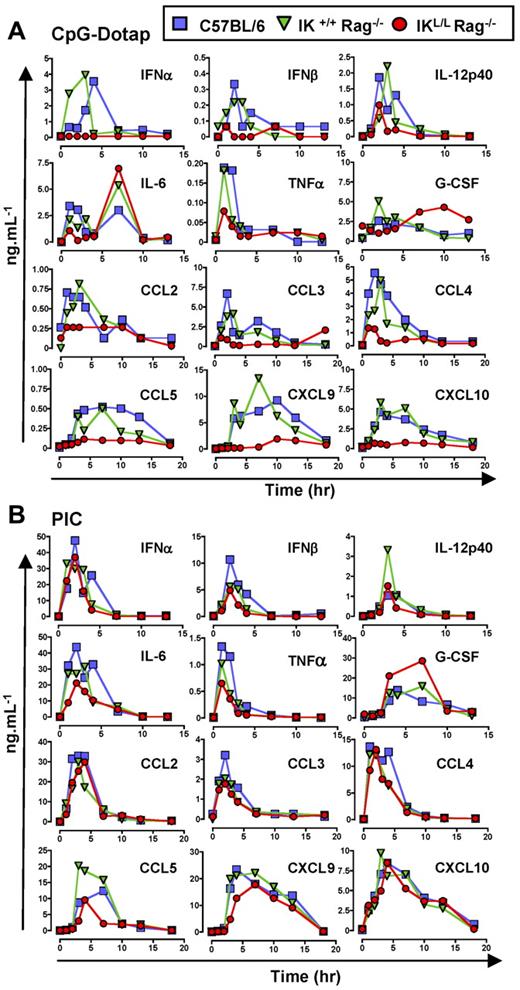
Because pDCs have been reported to be the main producer of type I IFNs after microbial stimulation,24 we first examined IFN-α/β concentrations in the sera of IKL/L Rag−/− mice at different time points after CpG-Dotap or PIC injection. IFN-α/β was not detectable in the sera of these mice; in contrast, C57BL/6 and IK+/+ Rag−/− mice produced these cytokines at early time points after injection of CpG-Dotap (Figure 5A), demonstrating that pDCs were the only source of type I IFNs in response to TLR9 activation. However, similar IFN-α/β responses were observed for these 3 strains of mice after PIC injection (Figure 5B), demonstrating that cells other than pDCs were responsible for type I IFN production in response to TLR3 activation. Injection of IKL/L Rag−/− mice with pDCs before TLR9 activation led to type I IFN production, confirming that the absence of IFN-α/β production observed in IKL/L Rag−/− mice was the result of the lack of pDCs (supplemental Figure 4A).
The in vivo production of cytokines and chemokines after TLR9 stimulation is impaired in the absence of pDCs. C57BL/6, IK+/+ Rag −/−, and IKL/L Rag−/− mice were injected with CpG-Dotap (A) or PIC (B), and sera were collected at different time points after injection. Levels of IFN-α, IFN-β, IL-12p40, IL-6, TNF-α, G-CSF, CCL2, CCL3, CCL4, CCL5, CXCL9, and CXCL10 were determined using xMAP. Data shown are from 2 experiments.
The in vivo production of cytokines and chemokines after TLR9 stimulation is impaired in the absence of pDCs. C57BL/6, IK+/+ Rag −/−, and IKL/L Rag−/− mice were injected with CpG-Dotap (A) or PIC (B), and sera were collected at different time points after injection. Levels of IFN-α, IFN-β, IL-12p40, IL-6, TNF-α, G-CSF, CCL2, CCL3, CCL4, CCL5, CXCL9, and CXCL10 were determined using xMAP. Data shown are from 2 experiments.
To assess the contribution of pDCs in cytokine production, the levels of various cytokines were quantified in the sera of mice at different time points after systemic injection of CpG-Dotap or PIC (Figure 5). IL-12p40, IL-6, and TNF-α were produced at early time points after CpG-Dotap injection. The levels of these cytokines were reduced in IKL/L Rag−/− mice compared with IK+/+ Rag−/− and C57BL/6 mice. However, at later time points, IL-6 production was not affected by the absence of pDCs. Finally, a delay in G-CSF production was observed in IKL/L Rag−/− mice.
In contrast, no major differences were observed for any of these cytokines in response to TLR3 activation (Figure 5B), except for G-CSF, which was highly abundant in IKL/L Rag−/− mice.
pDCs are also known to produce inflammatory chemokines after activation.4,25 Having shown that pDCs are involved in the induction of NK responses, we next analyzed the production of chemokines that function in the chemoattraction of NK cells at different time points after CpG-Dotap stimulation. A strong defect in chemokine production was observed in IKL/L Rag−/− mice compared with the other mouse strains (Figure 5A). In contrast, no major differences were observed for any of these chemokines in response to PIC activation (Figure 5B).
As was shown for the type I IFN response, IKL/L Rag−/− mice injected with pDCs before CpG stimulation were able to mount a chemokine response (supplemental Figure 4A). Furthermore, the defect in chemokine production observed in IKL/L Rag−/− mice was not the result of a specific alteration of the TLR9 activation pathway because of the Ikaros mutation because purified cDCs from both IK+/+ Rag−/− and IKL/L Rag−/− mice produced similar levels of CCL3, CCL4, and CCL5 in vitro (supplemental Figure 4B). CCL2, CXCL9, and CXCL10 were not detectable under such conditions irrespective of the origin of the cDCs (data not shown), suggesting that the production of these chemokines requires interactions between different cell types, as has been previously suggested.26,27 CCL3 and CCL4 were produced by purified pDCs from IK+/+ Rag−/− mice (supplemental Figure 4C) in vitro, in agreement with previous studies of human pDCs.27,28
Alteration of innate immune cell recruitment in the absence of pDCs after TLR9 stimulation
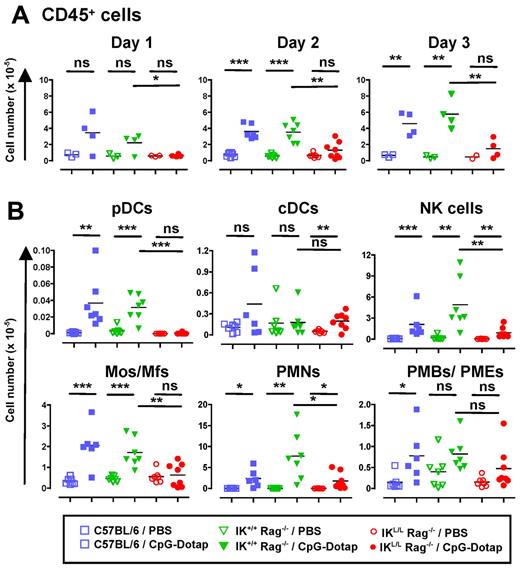
Considering the involvement of pDCs in chemokine production, we speculated that, after TLR9 triggering, these cells may function in the recruitment of innate immune cells to the site of inflammation. To test this hypothesis, we first analyzed cell recruitment to the peritoneal cavity after intraperitoneal injection of CpG-Dotap. A profound defect in CD45+ cell recruitment was observed in IKL/L Rag−/− mice 1 to 3 days after CpG-Dotap administration (Figure 6A). pDC recruitment was observed in C57BL/6 and IK+/+ Rag−/− mice 2 days after CpG-Dotap injection, supporting a role for these cells in such an activation model (Figure 6B). Importantly, in contrast to C57BL/6 and IK+/+ Rag−/− mice, no monocytes/macrophages were recruited to the peritoneum of IKL/L Rag−/− mice, demonstrating that pDCs are strictly required for the recruitment of these myeloid cells in response to CpG-Dotap. NK cell and PMN recruitment was also strongly reduced, but not fully abolished, in IKL/L Rag−/− mice compared with IK+/+ Rag−/− mice, arguing for a role for pDCs in the recruitment of these cells. In contrast, PMB and PME recruitment was not significantly affected by the absence of pDCs. The differences observed in cell recruitment between C57BL/6 and Rag2-deficient strains could be explained by the presence of lymphocytes because a great number of B cells were recruited in C57BL/6 mice (data not shown).
Defect in intraperitoneal cell recruitment in the absence of pDCs after intraperitoneal injection of CpG-Dotap. C57BL/6, IK+/+ Rag−/−, and IKL/L Rag−/− mice were injected intraperitoneally with either PBS or 200 μg of CpG-Dotap. After 1, 2, or 3 days, the total number of CD45+ intraperitoneal cells (A) and the number of cells in each indicated population at day 2 (B) were determined (Mos/Mfs indicates monocytes/macrophages). Cell subsets were characterized as shown in supplemental Figure 5. Each dot represents results obtained from individual mice, and bars represent the mean. Data represent the cumulative results of 3 experiments. ns indicates not significant (P > .05). *P < .05. **P < .01. ***P < .001.
Defect in intraperitoneal cell recruitment in the absence of pDCs after intraperitoneal injection of CpG-Dotap. C57BL/6, IK+/+ Rag−/−, and IKL/L Rag−/− mice were injected intraperitoneally with either PBS or 200 μg of CpG-Dotap. After 1, 2, or 3 days, the total number of CD45+ intraperitoneal cells (A) and the number of cells in each indicated population at day 2 (B) were determined (Mos/Mfs indicates monocytes/macrophages). Cell subsets were characterized as shown in supplemental Figure 5. Each dot represents results obtained from individual mice, and bars represent the mean. Data represent the cumulative results of 3 experiments. ns indicates not significant (P > .05). *P < .05. **P < .01. ***P < .001.
Overall, these data highlight an important function for pDCs as a key player in cell recruitment after TLR9 stimulation.
pDCs are required for the recruitment of monocytes and macrophages after pathogen stimulation
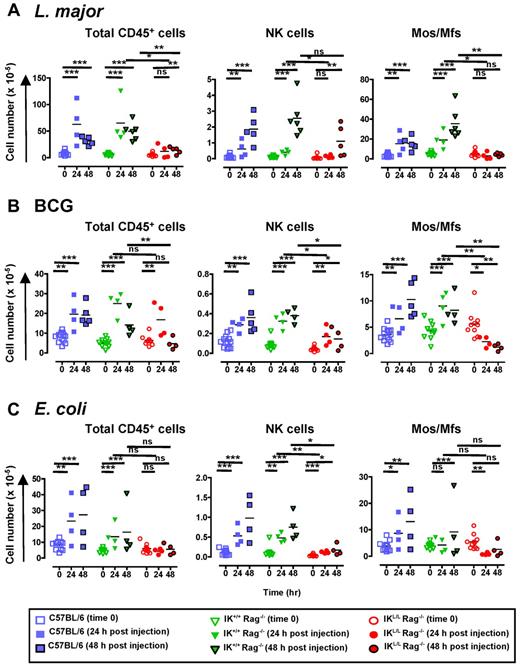
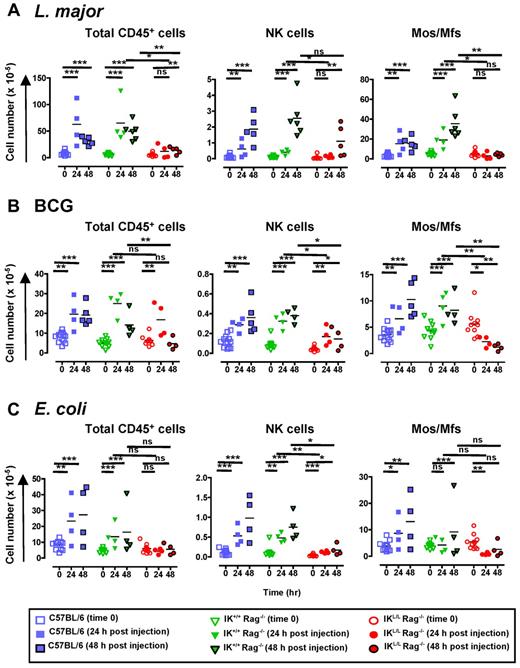
Because we observed a global defect in innate cell recruitment in IKL/L Rag−/− mice after TLR9 activation by CpG-Dotap, we next asked whether pDCs are also involved in cell recruitment during infection. To this end, we analyzed cell recruitment in the peritoneal cavity after intraperitoneal injection of heat-killed L major promastigotes, Mycobacterium bovis BCG, or heat-inactivated E coli. Both L major and BCG are recognized by TLR2, TLR4, and TLR9.18,29-31 Interestingly, TLR9-deficient mice are particularly susceptible to L major18,32 and Mycobacterium tuberculosis33 infection. In contrast, E coli primarily signals through TLR4,34 although bacterial DNA can also trigger TLR9.35
The inoculation of C57BL/6 and IK+/+Rag−/− mice with each of these pathogens induced a marked recruitment of CD45+ cells to the peritoneal cavity (Figure 7), including pDCs (data not shown). This increase in cell number was mainly the result of the recruitment of PMNs and monocytes/macrophages (Figure 7; supplemental Figure 6). In contrast, a profound defect in the recruitment of CD45+ cells was observed in IKL/L Rag−/− mice, except at day 1 after BCG inoculation. The main cell population affected in IKL/L Rag−/− mice was monocytes/macrophages, as evidenced by the total lack of recruitment to the peritoneal cavity observed in these mice after pathogen injection. Moreover, a significant decrease in monocyte/macrophage numbers was observed in IKL/L Rag−/− mice in response to BCG or E coli stimulation (Figure 7), which probably reflects a leak/death of residential macrophages that, in the absence of pDCs, was not compensated by the recruitment of inflammatory macrophages. A strong reduction in NK-cell recruitment was also observed in the absence of pDCs after stimulation with both BCG and E coli and, to a lesser extent, after L major injection (Figure 7). In contrast, the recruitment of cDCs, PMNs, and PMBs/PMEs was not affected in IKL/L Rag−/− mice (supplemental Figure 6).
Defect in intraperitoneal cell recruitment in response to pathogens in the absence of pDCs. C57BL/6, IK+/+ Rag−/−, and IKL/L Rag−/− mice were injected intraperitoneally with 109 heat-killed L major promastigotes (A), 2 × 107 CFU of BCG (B), or 5 × 108 CFU of heat-inactivated E coli (C). Intraperitoneal cells were collected before or 24 and 48 hours after injection, and the number of CD45+ cells (left panel), NK cells (middle panel), and monocytes/macrophages (Mos/Mfs, right panel) were determined. NK cells and monocytes/macrophages were characterized as shown in supplemental Figure 5. Each dot represents the cell number obtained from individual mice, and bars represent the mean. Data shown are the cumulative results of 3 experiments. ns indicates not significant (P > .05). *P < .05. **P < .01. ***P < .001.
Defect in intraperitoneal cell recruitment in response to pathogens in the absence of pDCs. C57BL/6, IK+/+ Rag−/−, and IKL/L Rag−/− mice were injected intraperitoneally with 109 heat-killed L major promastigotes (A), 2 × 107 CFU of BCG (B), or 5 × 108 CFU of heat-inactivated E coli (C). Intraperitoneal cells were collected before or 24 and 48 hours after injection, and the number of CD45+ cells (left panel), NK cells (middle panel), and monocytes/macrophages (Mos/Mfs, right panel) were determined. NK cells and monocytes/macrophages were characterized as shown in supplemental Figure 5. Each dot represents the cell number obtained from individual mice, and bars represent the mean. Data shown are the cumulative results of 3 experiments. ns indicates not significant (P > .05). *P < .05. **P < .01. ***P < .001.
Taken together, these data demonstrate that, even when other signaling pathways are triggered with TLR9, pDCs play a crucial role in the recruitment of innate immune cells.
Discussion
Among DC subsets, pDCs represent a unique cell type that is specialized for the rapid production of high levels of type I IFNs after viral infection.1 In addition to their indirect, pleiotropic role mediated by the release of type I IFNs, it has been suggested that pDCs may also play a direct role in the induction or control of immune responses. In the present study, we developed a new, constitutively pDC-deficient mouse strain, the IKL/L Rag−/− mouse, which permits long-term analysis of immune responses in the absence of pDCs. Importantly, in IKL/L Rag−/− mice, hematopoietic cells, such as DCs, are present at normal levels in the different organs and are functional.
The role of the different DC subsets in NK-cell activation is still unclear. NK cells play a crucial role in antiviral immunity,36 whereas pDCs are pivotal in the initiation of innate responses and, particularly, during the early stage of viral infection.10 Therefore, we analyzed NK-cell responses in pDC-deficient IKL/L Rag−/− mice after injection of CpG-Dotap and PIC, which mimic viruses retained in the endosomal compartment37 and viral double-stranded RNA, respectively.
In contrast to IK+/+ Rag−/− mice, IKL/L Rag−/− mice failed to develop efficient NK-cell responses after CpG-Dotap injection, whereas marked NK-cell activation in response to PIC was observed in both strains of mice. These results demonstrate that, when directly targeted through TLR9, pDCs play a nonredundant role in the induction of NK-cell activity. The pDC-derived factors involved in NK-cell activation remain to be determined. Type I IFNs production was abolished in IKL/L Rag−/− mice in response to CpG-Dotap, in correlation with the failure to activate NK cells, which supports the hypothesis that NK-cell activation is type I IFN-dependent.21,23,38 It has been previously suggested that pDCs are essential for the production of IFN-γ by NK cells because they are required for the induction of IL-12 by cDCs.22 However, IL-12 was produced in IKL/L Rag−/− mice in response to CpG-Dotap. TNF-α, another NK cell-activating cytokine,21,23 was also produced in the absence of pDCs. Type I IFNs seem to be the only NK-activating factors whose production is strictly dependent on pDCs. Altogether, our results support the hypothesis that pDCs are the main source of type I IFNs, which is required for IL-15–dependent NK-cell activation by cDCs38 after TLR9 activation.
Because previous studies suggested an involvement of pDCs in chemokine production in response to virus or to TLR stimulation,4,26,27,39 we determined whether the absence of pDCs in IKL/L Rag−/− mice would impact the systemic production of chemokines after TLR9 triggering. We observed a dramatic effect of pDC absence on the production of CCL2, CCL3, CCL4, CCL5, CXCL9, and CXCL10 in response to CpG-Dotap, thus demonstrating that pDCs are required for chemokine production after TLR9 triggering. Importantly, normal chemokine secretion was induced in IKL/L Rag−/− mice in response to PIC, demonstrating that, as observed for NK-cell response, different pathways could be used depending on the activation signals.
We observed a profound defect in NK-cell recruitment in pDC-deficient mice in the peritoneal cavity after an intraperitoneal injection of CpG-Dotap. Our results are in agreement with the observation that CpG-activated pDCs injected into the mouse peritoneum were able to recruit NK cells40 and confirm the potential role for human pDCs in NK-cell recruitment.26,39,41 This defect in NK-cell recruitment could be explained by the fact that these cells expressed CCR2, CCR5, and CXCR3,42 which respond to CCL2, CCL3, CCL4, CCL5, CXCL9, and CXCL10. All of these chemokines were absent in CpG-Dotap–stimulated IKL/L Rag−/− mice compared with control Rag−/− mice. The alteration of NK-cell recruitment was also observed in response to microbial stimulants, such as L major, BCG, and E coli. Nevertheless, NK-cell recruitment was not completely abolished in IKL/L Rag−/− mice, suggesting that NK cells can also migrate in response to pDC-independent signals. This is in accordance with the fact that NK-cell activity is not strictly dependent of pDCs and type I IFN production during Leishmania infection.43
Similar to NK cells, we observed a reduction in PMN recruitment in IKL/L Rag−/− mice in response to CpG-Dotap. This result is consistent with the production of the PMN-attracting chemokine CXCL8 by human pDCs.26-28 However, PMNs accumulated normally in the peritoneum of IKL/L Rag−/− mice after inoculation with pathogens that trigger various signaling pathways. Thus, in response to infectious agents, additional signals to TLR9, such as tissue damage,44 or pathogen components, such as LPS,45 may modulate PMN recruitment in a pDC-independent manner.
Whereas large numbers of monocytes and macrophages accumulated in the peritoneal cavity of C57BL/6 and IK+/+ Rag−/− mice after stimulation with CpG-Dotap, L major, BCG, or E coli, this recruitment was profoundly affected in IKL/L Rag−/− mice. These results are consistent with the lack of production of CCL2, CCL3, CCL4, and CCL5 in IKL/L Rag−/− mice, which mediate monocyte/macrophage attraction through interactions with CCR2 and CCR5.46 Thus, our data clearly demonstrate the crucial role that pDCs play in monocyte/macrophage recruitment to the site of inflammation, probably via the modulation of chemokine production. Indeed, recent studies report that type I IFNs regulate monocyte/macrophage and PMN recruitment during infection with MCMV,47 influenza,48 and Listeria monocytogenes49 through the regulation of chemokine production.
After the inoculation of BCG and E coli into the peritoneal cavity of IKL/L Rag−/− mice, we observed a significant decrease in monocyte/macrophage numbers. It should be mentioned that CCL5, which was not produced by pDC-deficient mice on TLR9 triggering, is required to prevent apoptosis of macrophages during infection with influenza virus.50 Macrophage apoptosis could have also occurred in C57BL/6 and IK+/+ Rag−/− mice, masked by the continuous arrival of inflammatory macrophages. To our knowledge, our study is the first to demonstrate the pivotal role played by pDCs in monocyte/macrophage migration and survival, leading to accumulation of these cells at sites of inflammation. This function of pDCs is of particular interest given the crucial role played by monocytes and macrophages in many infectious diseases and, in particular, within L major– or M tuberculosis–induced pathologies.51,52
In conclusion, in the present study, we describe the power of the new IKL/L Rag−/− mouse model to analyze the role of pDCs in immune responses. Taken together, our results demonstrate that the alteration of innate responses observed in IKL/L Rag−/− mice were only the result of the absence of pDCs and not intrinsic cell defects resulting from the Ikaros mutation. This new model offers the advantage of constitutive depletion of pDCs without toxin treatment, allowing studies on innate immunity in the complete absence of pDCs in the periphery, although it cannot be used to evaluate the impact of pDCs in adaptive responses. Using this model, we established that, under physiologic conditions, pDCs are key players in the initiation of innate responses to TLR9 stimulation. Importantly, this study demonstrates that the role of pDCs is not limited to the production of type I IFNs but is also crucial for the migration/activity of NK cells, monocytes, and macrophages, all of which are vital to innate immunity. Considering the ability of pDCs to accumulate at the site of infection in various human and mouse pathologies,4 our work highlights the importance of pDCs in the induction of innate responses and inflammation against TLR9-stimulating pathogens.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank M. Rojas for technical assistance and M. Albert and J. Di Santo for the gift of β2m−/− and Ragγc−/− mice, respectively.
This work was supported by Ligue Nationale Contre le Cancer (équipe labellisée 2010) and Banque Privée Européenne.
Authorship
Contribution: C.G., C.L., and G.D. conceived and designed the experiments, analyzed data, and wrote the paper; C.G., J.M., G.P., and G.D. performed experiments; N.D. provided parasites; H.K.W.L. acquired and analyzed multispectral imaging; S.C. and P.K. provided Ikaros mice; and C.L. and G.D. supervised the work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gilles Dadaglio, Unité de Régulation Immunitaire et Vaccinologie, Inserm U1041, Institut Pasteur, 25 rue du Docteur Roux, F-75015 Paris, France; e-mail: gilles.dadaglio@pasteur.fr.
References
Author notes
C.L. and G.D. are co–senior authors.