In this issue of Blood, Baba and colleagues characterize a residual pool of CD4+CD25+Foxp3+ regulatory T cells (Tregs) surviving a nonmyeloablative conditioning regimen that undergo robust homeostatic expansion to limit the full potential of adoptive T-cell therapy (ACT) for the treatment of cancer.1
Adoptive T-cell therapy is perhaps the most potent form of cancer immunotherapy and is capable of inducing durable complete responses in patients with advanced solid and hematologic malignancies refractory to standard therapies.2,3 Whether the infused populations of T cells are derived from tumor-infiltrating lymphocytes or peripheral blood T cells genetically engineered to express an exogenous tumor-specific receptor, multiple clinical trials have established that prior lymphodepletion is required for optimal clinical responses. The immunologic impact of host preconditioning using chemo- or radiation therapy is complex. However, preclinical studies in mice have elucidated many of the key mechanisms underlying the augmented efficacy of T-cell therapies after lymphodepletion.4
For example, host conditioning removes endogenous T cells that compete with transferred tumor-reactive T cells for the activating homeostatic cytokines IL-7 and IL-15.5 Lymphodepletion may also cause mucosal injury allowing gut-associated microbes to enter the circulation and activate toll-like receptors expressed on host dendritic cells and transferred T cells.6 Finally, chemotherapy and irradiation preconditioning depletes Tregs,7 providing for a more favorable balance of effector T cells to Tregs in vivo after cell transfer.8 Recently, a detailed analysis from 5 different ACT clinical trials using a variety of preconditioning regimens revealed a significant correlation between the depth and duration of Treg depletion and the likelihood of patients achieving an objective clinical response.9 Although these findings are highly suggestive that the kinetics of Treg reconstitution after lymphodepletion may influence the clinical outcome of adoptive immunotherapy, the retrospective nature of these data precludes the establishment of a causal link. In their current work, Baba and colleagues now fill this gap in our knowledge and provide compelling evidence in mice that Tregs that survive and expand after lymphodepletion have a deleterious impact on the efficacy of ACT.
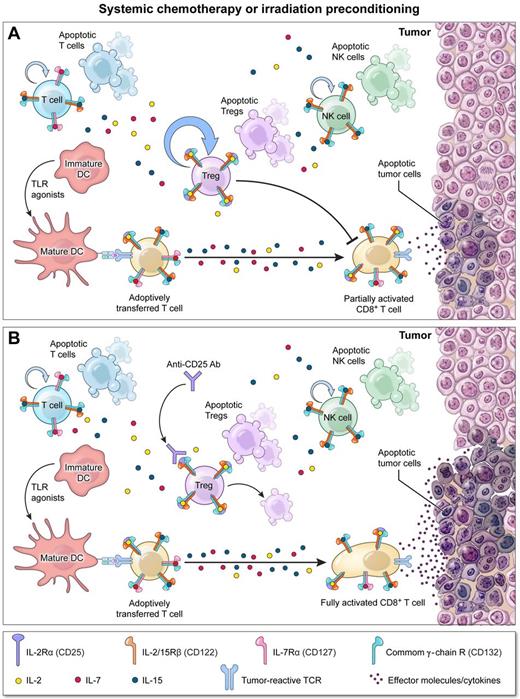
In both tumor challenge and treatment models, the authors confirmed that lymphodepletion before ACT can significantly augment tumor destruction. Importantly, by performing a careful assessment of T-cell reconstitution after lymphodepletion, the authors demonstrated that Tregs out-proliferate Foxp3− T cells (see figure, panel A), possibly resulting from the increased affinity that naturally occurring Tregs possess for both self-antigens and IL-2. As a consequence, the fractional percentage of Tregs relative to nonregulatory CD4+ and CD8+ T cells became enriched as tumor-bearing hosts reconstituted. Despite the highly inflammatory milieu present after irradiation,6 an environment that can subvert the suppressive capacity of Tregs in some models, reconstituting Tregs remained as potent as Tregs isolated from nonirradiated hosts in inhibiting tumor-specific CD4+ and CD8+ T-cell effector responses (see figure, panel A). To explore the functional impact of this “stubborn” pool of residual Tregs on ACT, the authors depleted these cells using PC61, a depleting antibody targeting the IL-2 receptor α chain (CD25). While PC61 treatment by itself had no impact on tumor growth after irradiation, in combination with ACT it significantly improved tumor regression compared with animals receiving cell transfer alone (see figure, panel B). Based primarily on these data, the authors conclude that reconstituting Tregs after lymphodepletion limit the full potential of ACT therapy.
Depletion of stubborn Tregs surviving lymphodepletion augments adoptive T-cell therapy (ACT). (A) Systemic chemo- or radiation therapy before ACT enhances the function of adoptively transferred T cells by removing cellular “sinks” for homeostatic cytokines, reducing the number of regulatory T cells (Tregs), and activating host dendritic cells by triggering the release of endogenous Toll-like receptor (TLR) agonists through mucosal injury. However, surviving Tregs undergo robust homeostatic expansion limiting the full antitumor potential of transferred T cells. (B) Concurrent depletion of these “stubborn” Tregs using antibodies targeting CD25, the IL-2Rα chain, unleashes transferred T-cell function providing for optimal tumor regression.
Depletion of stubborn Tregs surviving lymphodepletion augments adoptive T-cell therapy (ACT). (A) Systemic chemo- or radiation therapy before ACT enhances the function of adoptively transferred T cells by removing cellular “sinks” for homeostatic cytokines, reducing the number of regulatory T cells (Tregs), and activating host dendritic cells by triggering the release of endogenous Toll-like receptor (TLR) agonists through mucosal injury. However, surviving Tregs undergo robust homeostatic expansion limiting the full antitumor potential of transferred T cells. (B) Concurrent depletion of these “stubborn” Tregs using antibodies targeting CD25, the IL-2Rα chain, unleashes transferred T-cell function providing for optimal tumor regression.
PC61 has historically been used as a strategy to remove Tregs in preclinical animal models. However, results using this antibody are confounded by the fact that CD25 is not exclusively expressed on Tregs. Most notably, acutely activated effector CD4+ and CD8+ T cells express CD25. Indeed daclizumab, a humanized monoclonal antibody targeting CD25, is used clinically as an immunosuppressive reagent in patients with autoimmune and refractory graft-versus-host diseases specifically because it depletes effector T cells. Moreover, CD25 has also been reported to be expressed by other immune cells, including B cells, dendritic cells, and monocytes. As these other cellular subsets can have both suppressive as well as immune-stimulatory properties, the relative contribution of Treg depletion might be more precisely dissected in the future using genetic approaches. For example, conditional depletion of cells expressing the Treg lineage-specific transcription factor Foxp3 using the “DE-REG” mouse could unequivocally establish the contributions of Tregs after lymphodepletion. Despite these considerations, the authors show in their model system that PC61 efficiently depleted Tregs while having no discernible impact on other cell populations in multiple tissues throughout the body. Thus, further depletion of Tregs was likely the major contributor of the augmented tumor treatment observed after PC61 administration.
Taken together, the data provided by Baba et al provide deeper insight into the complex perturbations in immune cell homeostasis after ablative host conditioning and provide further impetus to develop conditioning strategies that more potently suppress Treg reconstitution. Clinically, the use of fully myeloablative conditioning using TBI and hematopoietic stem cell rescue before ACT has been used to robustly deplete all host lymphocytes, including Tregs.8,9 Although such a strategy has been associated with improved response rates,2 the toxicity and complexity of this regimen can be a barrier to its widespread adoption. Recombinant immunotoxins targeting cells expressing CD25, such as denileukin diftitox and LMB-2, have been used clinically to deplete Tregs in patients with cancer, but results have been marginal.10 In addition, as noted above, such an approach runs the risk of depleting tumor-reactive effector T cells. Thus, reagents that disarm the suppressive capacity of Tregs without having detrimental effects on effector T cells might be the best way to effectively translate the findings reported here. For example, combining ACT with blocking antibodies against cytotoxic T-lymphocyte antigen 4 (CTLA-4) might positively impact outcomes not only by inhibiting Tregs but also augmenting the effector functions of the adoptively transferred T cells. In support of such an approach, a post hoc analysis suggests a trend toward improved survival in patients previously treated with anti–CTLA-4 before receiving lymphodepletion and ACT.2 For this reason, a prospective trial testing the addition of anti–CTLA-4 with ACT would be warranted.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■
REFERENCES
National Institutes of Health