Abstract
Immune thrombocytopenia (ITP) results from decreased platelet production and accelerated platelet destruction. Impaired CD4+ regulatory T-cell (Treg) compartment and skewed Th1 and possibly Th17 responses have been described in ITP patients. The trigger for aberrant T-cell polarization remains unknown. Because monocytes have a critical role in development and polarization of T-cell subsets, we explored the contribution of monocyte subsets in control of Treg and Th development in patients with ITP. Unlike circulating classic CD14hiCD16− subpopulation, the CD16+ monocyte subset was expanded in ITP patients with low platelet counts on thrombopoietic agents and positively correlated with T-cell CD4+IFN-γ+ levels, but negatively with circulating CD4+CD25hiFoxp3+ and IL-17+ Th cells. Using a coculture model, we found that CD16+ ITP monocytes promoted the expansion of IFN-γ+CD4+ cells and concomitantly inhibited the proliferation of Tregs and IL-17+ Th cells. Th-1–polarizing cytokine IL-12, secreted after direct contact of patient T-cell and CD16+ monocytes, was responsible for the inhibitory effect on Treg and IL-17+CD4+ cell proliferation. Our findings are consistent with ITP CD16+ monocytes promoting Th1 development, which in turn negatively regulates IL-17 and Treg induction. This underscores the critical role of CD16+ monocytes in the generation of potentially pathogenic Th responses in ITP.
Introduction
Classic differentiation of naive CD4+ T cells into different T helper (Th) subsets, including Th1, Th2, and Th17, occurs in lymphoid tissues after contact with antigen-presenting cells that produce polarizing cytokines. These Th subsets in turn orchestrate diverse immune responses also mediated by production of distinct cytokines. Because aberrant Th1 or Th17 activities have the potential to trigger chronic inflammatory and autoimmune diseases,1,2 effector Th responses in healthy persons are under tight regulation mediated in part by CD4+ regulatory T cells (Tregs) that are thymic-derived or naive T cell–inducible.3 Understanding how Th1/Th17/Treg differentiation and expansion are controlled is likely to provide an explanation of how inflammation may be sustained in pathologic environments.
More recently, human monocytes were shown to trigger and polarize Th responses4,5 as well as to both stimulate and suppress T-cell responses during infection and in autoimmune diseases.5,6 Monocytes, which are generally regarded as precursors of tissue macrophages and dendritic cells,7 can be phenotypically divided based on surface expression of CD14 (lipopolysaccharide receptor) and CD16 (low affinity Fcγ receptor III) expression into subsets, each with distinct functional activities. The major monocyte subpopulation characterized by high CD14 but no CD16 expression (CD14hiCD16−), also referred to as classic monocytes, have higher phagocytic activity.8 The minor CD16+ cells produce higher TNF after stimulation and expand under infectious or inflammatory conditions.9,10 With regards to the control of Th differentiation and reactivation, the specific role of the monocyte subsets has not been fully characterized.
Immune thrombocytopenia (ITP) is an autoimmune bleeding disease resulting from decreased platelet production as well as accelerated platelet destruction mediated in part by autoantibody-based destruction mechanisms.11 ITP patients harbor activated platelet-autoreactive T cells with increasing cytokine imbalance toward IL-2 and IFN-γ12–14 as well as altered Treg numbers and function.15–20 A shift toward stimulatory monocytes with enhanced FcγR-mediated phagocytic capacity further supports a generalized immune dysregulation in ITP.21 More recently, studies reported increased Th17 cells or IL-17 cytokine in ITP patients,22–24 implicating a possible role for Th17 cells in ITP immunopathology, although 2 reports did not detect any difference.25,26 Among the treatment options available to ITP patients, the recently licensed thrombopoietic agents, by increasing platelet production, have yielded overall durable responses in patients with persistent, chronic, and/or refractory ITP while on treatment.27 Interestingly, improved Treg function in ITP patients was associated with increased platelet counts after the use of these agents,28 despite apparent lack of immunomodulatory activity associated with such agents. Similarly, improved Treg compartment was reported in ITP patients with a platelet response to rituximab,18 and treatment with high-dose dexamethasone was shown to increase the frequency of circulating Tregs17 as well as decrease Th1 cells.26
In the present study, we explored the role of monocyte subsets in polarization of Th subsets in patients with ITP. Our findings are consistent with ITP CD16+ monocytes promoting Th1 development, which in turn negatively regulates IL-17 and Treg induction, thus providing a potential mechanism for the formation of potentially pathogenic Th cells in this autoimmune disease.
Methods
Human samples
All the studies were approved by the Institutional Review Boards of the New York Blood Center and the Weill Cornell Medical College. Peripheral blood was obtained from patients with chronic ITP (defined > 1 year since diagnosis) and closely age-matched normal healthy volunteers on informed consent. Most patients were on treatment with thrombopoietic agents, including Promacta and Romiplostim, as well as the investigational small-molecule thrombopoietin receptor agonist Shionogi S-888711, with various platelet count responses at the time of the study visit.
Cell surface, Foxp3 and intracellular cytokine, and CFSE staining
All expression analyses were performed by flow cytometry using BD FACSCanto with Diva software (BD Biosciences). Cell surface monocyte phenotypic analysis was performed using whole blood (100 μL) after staining with human anti-CD14 (peridinin chlorophyll protein, clone MϕP9; BD Biosciences) and anti-CD16 (FITC, clone NKP15). For intracellular detection of Foxp3, whole blood (150 μL) was first incubated with CD4-peridinin chlorophyll protein and CD25-allophycocyanin (BD Biosciences) and then stained with Foxp3 (clone PCH101, eBioscience; and an isotype control) following the manufacturer's instructions. Intracellular IFN-γ staining was performed using sorted CD4+CD25− T cells after stimulation with 0.25 μg/mL phorbol myristate acetate (PMA; Sigma-Aldrich) and 0.375 μg/mL ionomycin (Sigma-Aldrich) in the presence of GolgiPlug (BD Biosciences) for 5 hours. Cells were then restained with anti-CD4, fixed and permeablized with Cytofix/Cytoperm according to the manufacturer's instructions (BD Biosciences), followed by staining with anti–IFN-γ antibody (BD Biosciences). Because IL-17 expression levels in sorted PMA/ionomycin stimulated CD4+CD25− T cells in healthy persons are low (< 1%-2%), we analyzed intracellular IL-17 expression in CD4+ cells using anti-CD4 and anti–IL-17A (both BD Biosciences) in whole blood after stimulation with PMA/ionomycin in the presence of GolgiPlug for 5 hours.
To follow proliferative responses of T cells, PBMCs or purified T cells were resuspended at a final concentration of 5 × 106/mL before staining with 1 μg/mL CFSE (Invitrogen) PBS solution for 5 minutes at room temperature, according to the manufacturer's instructions. After staining anti-CD3 and anti-CD4, the proliferating T-cell fractions were defined as the CFSELo population by flow cytometry.
Cell isolation and purification
PBMCs were separated from peripheral blood by Ficoll (GE Healthcare) density centrifugation and subjected for most experiments to cell sorting (purity > 95%) using either MoFlo (Beckman Coulter) or Aria (BD Biosciences) instruments. For depletion studies, magnetic bead based depletion in addition to cell sorting was used. Because CD16 surface marker is also expressed on NK cells, for the microbead-based (all from Miltenyi Biotec) depletion experiments, PBMCs were first depleted of NK cells using CD56 microbeads followed by depletion of CD16+ monocytes using CD16 microbeads following the manufacturer's instructions (> 95% purity). For cell sorting-based depletion studies, PBMCs were stained with human NK surface marker CD56 (PE, clone NCAM16.2) and CD16 (FITC; clone NKP15), and cells were gated on monocyte fraction based on forward and side scatter pattern before sorting out the CD16+CD56− cells. For purification of T cell and specific monocyte subsets, PBMCs were first subjected to magnetic bead selection using CD16 Microbeads, and both CD16+ and CD16− enriched cell populations were collected separately. The CD16+ enriched PBMCs were used to isolate CD16+ monocyte subset by sorting out CD16+CD56− cells (purity > 95%) after staining with anti-CD16 and anti-CD56 and under the gating for the monocyte fraction based on the forward and side scatter pattern. The CD16− enriched PBMCs were stained with peridinin chlorophyll protein-conjugated CD14 (clone MϕP9; BD Biosciences), FITC-conjugated CD16 and Alexa-700–conjugated anti-CD3 (clone UCHT1; eBioscience), and sorted to obtain purified CD3+ T cells and CD14hiCD16− population (purity > 95% for both subpopulations).
T-cell stimulation
CFSE-labeled total or monocyte subset-depleted PBMCs were resuspended in culture medium containing RPMI 1640 (Invitrogen) supplemented with 5% Human AB serum (Valley Biomedical), 2mM glutamine (Invitrogen), 100 U penicillin and streptomycin (Invitrogen), and 0.055mM 2-mercaptoethanol (Sigma-Aldrich) and stimulated with 1 μg/mL soluble anti-CD3 antibody (1 μg/mL; clone HIT3α) in U-bottom 96-well plates for 7 days before analysis for intracellular cytokine production. For coculture studies, CFSE-labeled purified T cells (1.25 × 105 cells/mL) were mixed with autologous sorted CD14hiCD16− monocytes or CD16+ monocytes at a ratio of 2:1 in the presence of soluble anti-CD3 for 7 days. For the antibody blocking studies, anti–IFN-γ (5 μg/mL) anti–IL-12 (1 μg/mL), or anti–IL-10 (0.5 μg/mL) or isotope-matched controls (5 μg/mL; R&D Systems) were added at the start of the cultures to CFSE-labeled purified T cells (1.25 × 105 cells/mL) cocultured with autologous sorted CD14hiCD16− monocytes (2:1 ratio) without or with addition of autologous CD16+ monocytes (at a ratio equal to CD14hiCD16− monocytes) in the presence of soluble anti-CD3 for 7 days as before.
For detection of intracellular IL-17 or IFN-γ expression, cultured cells that had been stimulated with anti-CD3 antibody for 7 days were restimulated with PMA/ionomycin and analyzed for intracellular expression of IL-17A or IFN-γ antibody as described above. To determine whether the divided CD4+IL-17 cells were naive or memory cells, we also included the antibody directed against CD45RO (BD Biosciences) in some experiments. For analysis of Foxp3 expression, the cells that had been stimulated with anti-CD3 were subjected to direct intracellular staining for Foxp3.
Transwell studies
In the transwell culture system (Corning), purified T cells and CD14hiCD16− monocytes were cocultured at a ratio of 2:1, respectively, in the upper compartment. Autologous CD16+ monocytes were added to the upper or lower compartment at a ratio equal to CD14hiCD16− monocytes. In some experiments, a 2:1 ratio of T cell and CD16+ monocytes mixture was added to the lower compartment. For T-cell stimulation, soluble anti-CD3 antibody was added to both the upper and lower compartments. On day 7, cells in both the upper and lower compartments were restimulated with PMA/ionomycin, but only the upper compartment cells were harvested and analyzed for intracellular expression of IL-17A and IFN-γ in CD4+ subpopulation by flow cytometry.
Statistical analysis
Data are expressed as mean values ± SEM. Statistical significance of differences between groups was determined by Mann-Whitney test, association studies were Spearman correlation test, and statistical significance of differences of paired data were determined by paired t test or Wilcoxon signed ranks test. Statistical analyses were performed using PASW Statistics Version 18 software (IBM Inc).
Results
CD16+ monocytes are inversely correlated with platelet counts, Treg frequency, and CD4+IL-17+ cells, and positively associated with CD4+IFN-γ+ cells in ITP patients
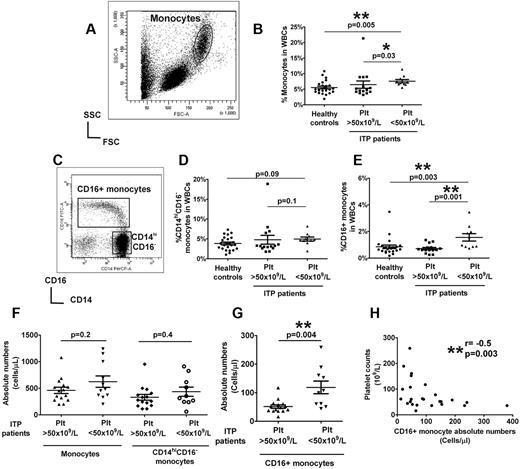
To control for the effects of ITP-related treatment drugs, we selected a cohort of ITP patients who had been on treatment solely with thrombopoietic agents for at least 1 month, but with various platelet responses (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). About half of the patients were splenectomized, but the overall platelet counts were comparable in splenectomized and nonsplenectomized groups (supplemental Figure 1). Patients with platelet counts of < 50 × 109/L had slightly higher total circulating monocyte frequencies compared with patients with elevated platelet counts and healthy controls (Figure 1A-B). Unlike the CD14hiCD16− subset (Figure 1C-D), significantly increased CD16+ monocyte frequency was detected in patients with low platelet counts compared with healthy controls (Figure 1C,E). In contrast, in patients with higher platelet counts, the proportions of CD16+ monocytes were comparable with levels in healthy controls (Figure 1C,E). Similarly, the absolute numbers of CD16+ monocytes, but not total monocytes or CD14hiCD16− subset, were significantly higher in patients with platelet counts of < 50 × 109/L compared with patients with higher platelet counts (Figure 1F-G). Furthermore, an inverse correlation between CD16+ monocytes, but not CD14hiCD16− subset (supplemental Figure 2), with platelet counts was detected (Figure 1H). These data indicate that CD16+ monocytes are expanded in patients with low platelet counts but that their numbers are normalized as platelet counts increase.
Circulating monocyte subsets in ITP patients are associated with platelet numbers. (A) Representative dot plot of the forward and side scatter in PBMCs showing the gating strategy for analysis of monocyte population. (B) Percent total monocytes based on forward and side scatter gating as a fraction of white blood cells (WBCs) in healthy controls and ITP patients with more > or < 50 × 109/L. (C) Expression of CD14 and CD16 expression in the monocyte population is shown by the representative dot plot, and the gating strategy for analysis of CD16+ and CD14hiCD16− subsets is indicated. Frequencies of CD14hiCD16− (D) and CD16+ (E) subsets in WBCs analyzed by flow cytometry in the same patient and healthy control cohort as in panel B. The absolute numbers in whole blood of total monocytes and CD14hiCD16− cells (F) as well as CD16+ subsets (G) as calculated based on complete blood counts are shown for patients with above and below platelet counts of 50 × 109/L. All P values were calculated using the Mann-Whitney t test. (H) Correlation between absolute CD16+ monocyte numbers in ITP patients and their platelet counts as calculated by Spearman correlation test showing a negative association between CD16+ monocytes and platelet counts. No significant correlation was seen between the classic CD14hiCD16− monocytes and platelet counts (see supplemental Figure 2).
Circulating monocyte subsets in ITP patients are associated with platelet numbers. (A) Representative dot plot of the forward and side scatter in PBMCs showing the gating strategy for analysis of monocyte population. (B) Percent total monocytes based on forward and side scatter gating as a fraction of white blood cells (WBCs) in healthy controls and ITP patients with more > or < 50 × 109/L. (C) Expression of CD14 and CD16 expression in the monocyte population is shown by the representative dot plot, and the gating strategy for analysis of CD16+ and CD14hiCD16− subsets is indicated. Frequencies of CD14hiCD16− (D) and CD16+ (E) subsets in WBCs analyzed by flow cytometry in the same patient and healthy control cohort as in panel B. The absolute numbers in whole blood of total monocytes and CD14hiCD16− cells (F) as well as CD16+ subsets (G) as calculated based on complete blood counts are shown for patients with above and below platelet counts of 50 × 109/L. All P values were calculated using the Mann-Whitney t test. (H) Correlation between absolute CD16+ monocyte numbers in ITP patients and their platelet counts as calculated by Spearman correlation test showing a negative association between CD16+ monocytes and platelet counts. No significant correlation was seen between the classic CD14hiCD16− monocytes and platelet counts (see supplemental Figure 2).
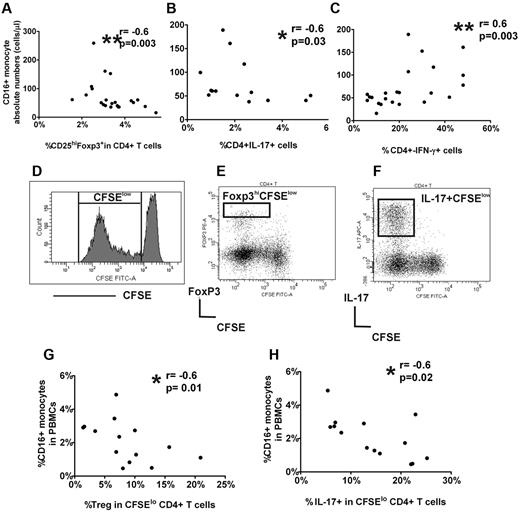
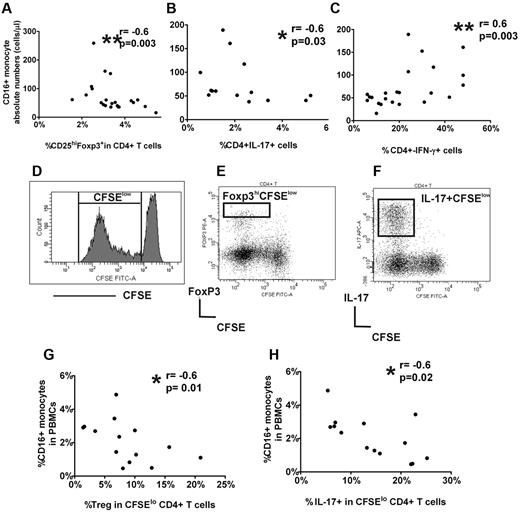
To determine whether monocyte numbers were associated with altered CD4+ subsets, we performed correlation studies between monocyte subsets and the percent circulating CD25hiFoxp3+ cells in CD4+ cells as well as frequencies of IL-17+ and IFN-γ+ CD4+ cells after short (5-hour) stimulation of whole blood or sorted CD4+CD25− cells with PMA and ionomycin. CD16+ monocyte counts were negatively associated with CD25hi+Foxp3+ in CD4+ cell frequencies (Figure 2A) as well as with the percentage of IL-17+CD4+ cells (Figure 2B) but were positively correlated with the frequency of CD4+IFN-γ+ cells (Figure 2C). No association was found between CD4+ subsets and CD14hiCD16− monocyte subset (supplemental Figure 3). Altogether, the data indicate an inverse relationship between platelet counts and CD16+ monocytes in patients with ITP and that with increasing CD16+ monocyte numbers, circulating Treg frequencies decrease as do IL-17+CD4+ cells, whereas the percentage of IFN-γ+ Th cells increase.
CD16+ monocyte subset in ITP patients is associated with peripheral Treg frequency and Th responses. Correlation between absolute CD16+ monocyte and (A) frequency of peripheral CD25hiFopx3+ in CD4+ cells, (B) percent intracellular IL-17 expression in CD4+ cells after 5-hour PMA/ionomycin stimulation of whole blood, and (C) percent intracellular IFN-γ expression CD4+ cells after 5-hour PMA/ionomycin stimulation of sorted CD4+CD25− cells. As indicated by P values, CD16+ monocytes are negatively correlated with CD25hiFopx3+ as well as CD4+IL-17+ cell frequencies but positively with CD4+IFN-γ+ cells. Gating strategies for enumeration of CD4+CD25hiFopx3+, CD4+IL-17+, and CD4+IFN-γ+ cells are shown in the supplemental Figure 3. (D) PBMCs labeled with CFSE were stimulated for 7 days, and the expression of CFSE in CD4+ cells within CD3 population is shown by the representative histogram. The frequency of CFSElo cells within CD4+ cells was used throughout the study to calculate the percentage proliferation of CD4+ cells. The gating strategy to analyze the frequency of Foxp3hi (E) and IL-17+ (F) cells in divided (CFSElo) CD4+ subset in stimulated PBMC cultures. For analysis of “% Treg in CFSElo CD4+ cells,” only the high Foxp3 expressing cells were included (see supplemental Figure 4). (G) Correlation of percent CD16+ monocytes in PBMCs at the start of the cocultures and the frequency of Foxp3hi (Tregs) in CD4+ divided CFSElo cells, and (H) percent IL-17+ cells in CFSEloCD4+ cells at the end of the stimulated PBMC cultures of ITP patients, indicating an inverse relationship between CD16+ monocytes and Treg and CD4+IL-17+ frequencies.
CD16+ monocyte subset in ITP patients is associated with peripheral Treg frequency and Th responses. Correlation between absolute CD16+ monocyte and (A) frequency of peripheral CD25hiFopx3+ in CD4+ cells, (B) percent intracellular IL-17 expression in CD4+ cells after 5-hour PMA/ionomycin stimulation of whole blood, and (C) percent intracellular IFN-γ expression CD4+ cells after 5-hour PMA/ionomycin stimulation of sorted CD4+CD25− cells. As indicated by P values, CD16+ monocytes are negatively correlated with CD25hiFopx3+ as well as CD4+IL-17+ cell frequencies but positively with CD4+IFN-γ+ cells. Gating strategies for enumeration of CD4+CD25hiFopx3+, CD4+IL-17+, and CD4+IFN-γ+ cells are shown in the supplemental Figure 3. (D) PBMCs labeled with CFSE were stimulated for 7 days, and the expression of CFSE in CD4+ cells within CD3 population is shown by the representative histogram. The frequency of CFSElo cells within CD4+ cells was used throughout the study to calculate the percentage proliferation of CD4+ cells. The gating strategy to analyze the frequency of Foxp3hi (E) and IL-17+ (F) cells in divided (CFSElo) CD4+ subset in stimulated PBMC cultures. For analysis of “% Treg in CFSElo CD4+ cells,” only the high Foxp3 expressing cells were included (see supplemental Figure 4). (G) Correlation of percent CD16+ monocytes in PBMCs at the start of the cocultures and the frequency of Foxp3hi (Tregs) in CD4+ divided CFSElo cells, and (H) percent IL-17+ cells in CFSEloCD4+ cells at the end of the stimulated PBMC cultures of ITP patients, indicating an inverse relationship between CD16+ monocytes and Treg and CD4+IL-17+ frequencies.
In vitro proliferative responses of Tregs and IL-17+CD4+ cells are associated with CD16+ monocytes in ITP patients
To begin to dissect the possible role of CD16+ monocytes in control of Treg and Th responses, we used a vitro T-cell stimulation culture system in which PBMCs were stimulated with soluble anti-CD3 antibody for 7 days. To monitor the proliferating activity of the cells, PBMCs were stained with CFSE dye at the start of the cultures. As expected, Th cells (CD3+CD4+ cells) proliferated after stimulation with soluble anti-CD3 as indicated by diluted CFSE dye (Figure 2E). It has previously been shown that only the high level expression of Foxp3 correlates with Treg population in anti-CD3 stimulated PBMCs.29 Consistent with the known inhibitory function of Tregs on T-cell proliferation, we found that, with increasing frequency of Foxp3hiCFSElo, there was a decrease in proliferation of CD4+ cells as indicated by lower frequency of total divided CD4+ cells in both ITP and healthy controls (supplemental Figure 4D-E). For analysis of Th responses, the anti-CD3 stimulated PBMCs were further stimulated with PMA and ionomycin for 5 hours with Golgi transport inhibition on day 7 to detect cytoplasmic expression of cytokines. Almost all IL-17+ cells were CFSElo (Figure 2F) and were positive for CD4+ and the memory marker, CD45RO (supplemental Figure 5), indicating that, after soluble CD3 stimulation, the large proportion of divided IL-17+ cells are memory Th cells, corroborating previous reports that memory T cells are the main IL-17–secreting T cells.30 An inverse relationship between CD16+ monocyte frequency in PBMCs at the start of the cultures and frequency of Foxp3hiCFSElo and IL-17+CFSElo in CD4+ cells were found in ITP patients (Figure 2G-H) but not in healthy controls (supplemental Figure 4F-G). Although we did not examine the CD4+IFN-γ responses, the results from our in vitro cultures assays are consistent with data obtained with whole blood and further support a negative association between CD16+ monocytes and proliferative responses of Treg and IL-17+CD4+ cells in ITP patients.
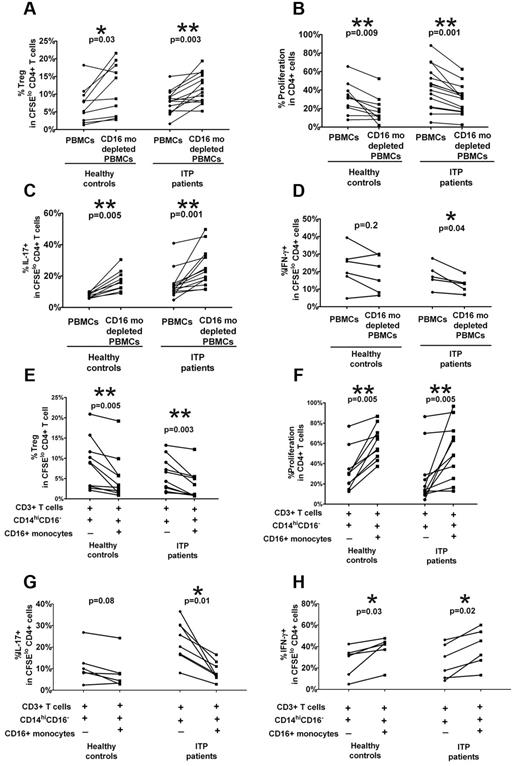
CD16+ monocyte depletion increases Treg and CD4+IL-17 expansion and decreases IFN-γ+ Th proliferation in ITP patients
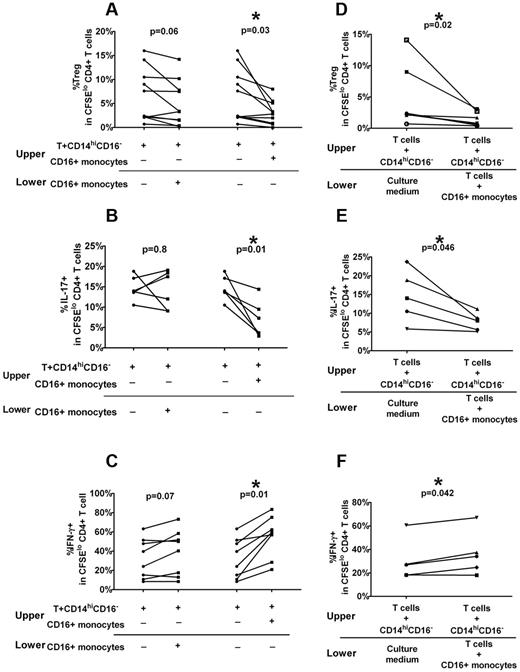
To investigate whether CD16+ monocytes can regulate Treg and Th expansion, we performed monocyte depletion studies by cell sorting or magnetic bead separation. A statistically significant increase in the frequency of CFSElo CD4+Foxp3+ cells and a concomitant decrease in CD4+ cell proliferation, which is consistent with increased Treg frequency, were found when autologous CD16+ cells were depleted in ITP patients as well as healthy controls (Figure 3A-B). Furthermore, a reciprocal increase in CFSElo CD4+IL-17+ cells (Figure 3C), but decrease in CFSElo IFN-γ+ Th frequency (Figure 3D), was detected on depletion of the CD16+ subset in ITP patients alone. Based on the PBMC stimulation studies (Figure 2) and depletion studies (Figure 3A-D), it appears that CD16+ monocytes have an inhibitory effect on Treg and IL-17+ Th development not only in ITP patients but also in healthy controls, although the effect in healthy controls is only detectable when the subset is depleted. Similarly, the CD16+ subset has a more pronounced stimulatory effect on IFN-γ+ Th proliferation in ITP patients than in healthy controls. Altogether, the data indicate that CD16+ monocyte subset in ITP patients have a polarizing ability to affect Th and Treg development that is more pronounced in ITP patients compared with healthy controls.
CD16+ monocytes alter Treg and Th proliferative responses in patients with ITP. PBMCs from healthy controls and ITP patients were depleted of CD16+ monocyte subsets by cell sorting or by magnetic bead selection. PBMCs before and after depletion were stained with CFSE and stimulated with anti-CD3. At day 7, intracellular expression of Foxp3, IL-17, and IFN-γ was detected by cytometry. The percentage of (A) Foxp3hi as well as (B) total CD4+ proliferation as measured by frequency of divided CFSElo CD4+ cells and (C) IL-17 and (D) IFN-γ+ cells in divided CFSElo CD4+ T cells before and after depletion of CD16+ monocytes. The P values were calculated by paired t test and indicate that depletion of CD16+ cells improves Treg and CD4+IL-17+ Th development in both healthy controls and ITP patients. In contrast, the absence of CD16+ monocytes inhibits CD4+IFN-γ+ proliferative responses in ITP patients. (E-H) The addition of CD16+ monocytes to cocultures of T cells and CD14hiCD16− cells alters Treg and Th proliferative responses. Autologous CD3+ T cells, CD14hiCD16− and CD16+ monocyte subsets, were purified from PBMCs of healthy controls and ITP patients by cell sorting. After CFSE labeling, the T cells were cocultured with CD14hiCD16− cells with or without CD16+ monocytes (as indicated by + and −) in the presence of anti-CD3 for 7 days. The percentage of (E) Foxp3hi (F) total CD4+ proliferation as the percent (G) IL-17 and (H) IFN-γ+ cells in divided CFSElo CD4+ T cells before and after addition of CD16+ monocytes. The indicated P values were calculated by paired t test. Whereas the addition of CD16+ monocytes inhibited the proliferative responses of Tregs and CD4+ IL-17+ cells, it stimulated the expansion of CD4+IFN-γ+ cells more significantly in ITP patients than in healthy controls.
CD16+ monocytes alter Treg and Th proliferative responses in patients with ITP. PBMCs from healthy controls and ITP patients were depleted of CD16+ monocyte subsets by cell sorting or by magnetic bead selection. PBMCs before and after depletion were stained with CFSE and stimulated with anti-CD3. At day 7, intracellular expression of Foxp3, IL-17, and IFN-γ was detected by cytometry. The percentage of (A) Foxp3hi as well as (B) total CD4+ proliferation as measured by frequency of divided CFSElo CD4+ cells and (C) IL-17 and (D) IFN-γ+ cells in divided CFSElo CD4+ T cells before and after depletion of CD16+ monocytes. The P values were calculated by paired t test and indicate that depletion of CD16+ cells improves Treg and CD4+IL-17+ Th development in both healthy controls and ITP patients. In contrast, the absence of CD16+ monocytes inhibits CD4+IFN-γ+ proliferative responses in ITP patients. (E-H) The addition of CD16+ monocytes to cocultures of T cells and CD14hiCD16− cells alters Treg and Th proliferative responses. Autologous CD3+ T cells, CD14hiCD16− and CD16+ monocyte subsets, were purified from PBMCs of healthy controls and ITP patients by cell sorting. After CFSE labeling, the T cells were cocultured with CD14hiCD16− cells with or without CD16+ monocytes (as indicated by + and −) in the presence of anti-CD3 for 7 days. The percentage of (E) Foxp3hi (F) total CD4+ proliferation as the percent (G) IL-17 and (H) IFN-γ+ cells in divided CFSElo CD4+ T cells before and after addition of CD16+ monocytes. The indicated P values were calculated by paired t test. Whereas the addition of CD16+ monocytes inhibited the proliferative responses of Tregs and CD4+ IL-17+ cells, it stimulated the expansion of CD4+IFN-γ+ cells more significantly in ITP patients than in healthy controls.
Addition of CD16+ monocytes inhibits Treg and CD4+IL-17 proliferative responses while promoting IFN-γ+ Th expansion in ITP patients
The depletion studies (Figure 3A-D) were performed with PBMC populations consisting of multiple cell types. To examine the interaction of monocytes and T cells in isolation, we sorted populations of monocytes and T cells from ITP patients and healthy controls and analyzed Treg and Th proliferative responses in our in vitro assay. CFSE-labeled purified CD3+ cells were cultured at 2:1 ratio with sorted autologous CD14hiCD16− cells alone or sorted autologous CD14hiCD16− cells, followed by stimulation with anti-CD3 antibody for 7 days before analysis for Foxp3 and intracellular cytokine production. As shown in Figure 3E, the addition of ITP CD16+ monocytes resulted in a significant decrease in the levels of autologous CFSElo Foxp3+CD4+ cells (P = .002) coupled to an increase in CD4+ proliferation (Figure 3F) as well as a decrease in proportions of CFSElo Th17+CD4+ cells (Figure 3G) combined with an increase in divided IFN-γ+ CD4+ cells (Figure 3H). The results further confirm that, in ITP patients, CD16+ monocytes have an inhibitory effect on IL-17+ Th and Treg development with a concomitant stimulatory effect on IFN-γ+ Th expansion.
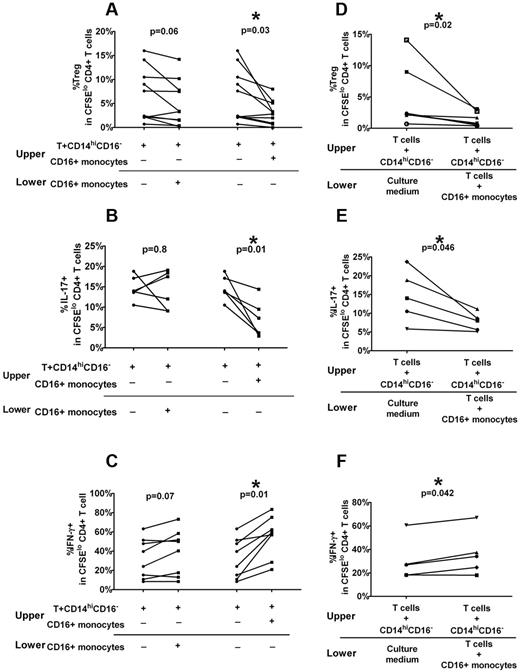
Role of cell-cell contact and soluble factors in CD16+ monocyte modulation of Treg and Th proliferation in ITP patients
To determine whether the mechanism of ITP CD16+ inhibitory effect on Treg and Th development requires direct cell-cell contact between T cells and monocytes, we performed transwell studies in which CD16+ monocytes were separated from T cells by a permeable membrane that prevents direct contact but allows movement of soluble factors. Sorted T cells stained with CFSE and CD14hiCD16− monocytes were added to a membrane-containing insert and placed above wells containing autologous purified CD16+ monocytes. As comparison, the CD16+ monocytes were cultured together with the T cells and CD14hiCD16− monocytes in the upper chamber. As expected, addition of CD16+ monocytes to the cocultures of T cell and CD14hiCD16− monocytes inhibited Treg (Figure 4A) and IL-17+CD4+ (Figure 4B) cell proliferation, but promoted IFN-γ+ Th proliferation (Figure 4C). In contrast, the addition of CD16+ monocytes to a compartment separated from direct contact with T cells and CD14hiCD16− monocytes abolished the inhibitory effect on IL17+CD4+ (Figure 4B) and to a lesser Treg proliferation (Figure 4A) and the stimulatory effect on IFN-γ+ Th proliferation (Figure 4C) was no longer significant. These data indicate that direct cell contact is necessary for robust inhibitory effect of CD16+ monocytes on IL-17+CD4+ and Treg development.
Optimal effects of CD16+ monocytes on Treg and Th proliferative require direct contact with T cells and release of soluble factors. Autologous CD3+ T cells, CD14hiCD16− and CD16+ monocyte subsets, from ITP patients were purified from PBMCs by cell sorting. Using a transwell plate, CFSE-labeled T cells were cocultured with CD14hiCD16− cells in the upper compartment, and CD16+ monocytes were added in the same compartment or in a separate lower compartment. After 7 days of stimulation in the presence of anti-CD3, the percent of (A) Foxp3hi as well as (B) IL-17 and (C) IFN-γ+ cells in divided CFSElo CD4+ T cells before and after addition of CD16+ monocytes in the lower compartment (left panel) or to upper compartment is shown. CD16+ monocytes, when separated from cocultures of T cells plus CD14hiCD16− (left panel), are not as effective in altering Treg and Th responses (P > .05, paired t test) compared with that when added to the same compartment as T+CD14hiCD16− cells (P < .05). (D) Sorted ITP T cells and CD14hiCD16− cells were placed in the upper compartment and autologous CD16+ monocyte without (“Culture medium”) or with T cells were added to the lower compartment of the transwell system. After 7 days of stimulation in the presence of anti-CD3, only the cells in the upper compartment were harvested, and the levels of divided CD4+Foxp3 as well as (E) CD4+IL-17+ and (F) CD4+ IFN-γ+ cells were analyzed. As indicated by the P values (paired t test), CD16+ monocytes in direct contact with T cells can affect polarization of Th cells in a separate compartment.
Optimal effects of CD16+ monocytes on Treg and Th proliferative require direct contact with T cells and release of soluble factors. Autologous CD3+ T cells, CD14hiCD16− and CD16+ monocyte subsets, from ITP patients were purified from PBMCs by cell sorting. Using a transwell plate, CFSE-labeled T cells were cocultured with CD14hiCD16− cells in the upper compartment, and CD16+ monocytes were added in the same compartment or in a separate lower compartment. After 7 days of stimulation in the presence of anti-CD3, the percent of (A) Foxp3hi as well as (B) IL-17 and (C) IFN-γ+ cells in divided CFSElo CD4+ T cells before and after addition of CD16+ monocytes in the lower compartment (left panel) or to upper compartment is shown. CD16+ monocytes, when separated from cocultures of T cells plus CD14hiCD16− (left panel), are not as effective in altering Treg and Th responses (P > .05, paired t test) compared with that when added to the same compartment as T+CD14hiCD16− cells (P < .05). (D) Sorted ITP T cells and CD14hiCD16− cells were placed in the upper compartment and autologous CD16+ monocyte without (“Culture medium”) or with T cells were added to the lower compartment of the transwell system. After 7 days of stimulation in the presence of anti-CD3, only the cells in the upper compartment were harvested, and the levels of divided CD4+Foxp3 as well as (E) CD4+IL-17+ and (F) CD4+ IFN-γ+ cells were analyzed. As indicated by the P values (paired t test), CD16+ monocytes in direct contact with T cells can affect polarization of Th cells in a separate compartment.
To test whether soluble factors released through direct contact of T cells and CD16+ monocytes are required for the inhibitory effect, we added T cells to the CD16+ monocytes but kept these cocultures separate from the cocultures of T cells and CD14hiCD16− in the upper chamber. The frequencies of various CD4+CFSElo populations in T cells isolated from the upper chamber were then tested. We found, in the upper compartment, significant inhibition of Treg (Figure 4D) and IL-17+CD4+ (Figure 4E) cell proliferation and increase in CFSElo CD4+ IFN-γ+ frequency (Figure 4F), suggesting that cytokines or factors secreted after contact between T cells and CD16+ may be responsible for the polarizing effect of CD16+ cells on Treg and Th development.
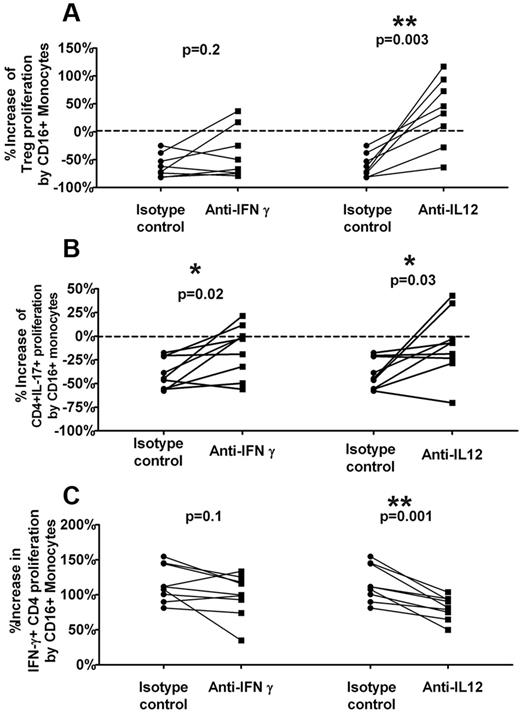
Th1-polarizing cytokines participate in CD16+ monocyte-mediated inhibition of Treg and CD4+IL-17+ development
Our studies so far indicate that CD16+ monocytes can inhibit IL-17+CD4+ expansion while promoting IFN-γ+CD4+ proliferation in ITP patients by cell-cell contact and soluble mediators. This raises the possibility that the increase in IFN-γ+ Th proliferation may be responsible for suppression of IL-17+CD4+ development given the reciprocal effect of Th1 cytokines on Th17 development.5,31,32 To test whether Th1-skewing cytokines can alter the IL-17 response, we used blocking antibodies to IL-12 and IFN-γ. Sorted T cells and CD14hiCD16− were cocultured with or without sorted CD16+ monocytes in the presence of anti–IFN-γ, anti–IL-12(p40), or isotype controls at the start of the cultures. The inhibitory effect of CD16+ monocytes on Treg and CD4+IL-17+ proliferative responses as well the stimulatory effect on CD4+IFN-γ+ expansion was then measured under blocking antibody or isotype control conditions. In the presence of anti–IL-12, the overall inhibitory activity of CD16+ monocytes on Treg (Figure 5A) and CD4+IL-17+ (Figure 5B) proliferation as well as its stimulatory effect on CD4+IFN-γ+ expansion (Figure 5C) was significantly reduced. Specifically, the addition of anti–IL-12 completely reversed the inhibition of Treg proliferation by CD16+ monocyte in 6 of 8 patients and reduced it by about half in the other 2 patients (Figure 5A). Furthermore, anti–IL-12 decreased the inhibitory effect of CD16+ monocytes on CD4+IL-17+ proliferative responses by almost 80% (Figure 5B) while lowering the CD16+ monocyte stimulation of CD4+IFN-γ+ expansion by ∼ 30% (Figure 5C), consistent with the role of these Th1 skewing cytokines in CD16+ monocyte-mediated suppression of Th17 responses.
IL-12 reverses CD16+ monocyte-mediated inhibition of Treg and CD4+IL-17+ proliferation. Sorted T cells were cocultured with CD14hiCD16− with or without CD16+ monocytes in the absence or presence of neutralizing anti–IFN-γ and IL-12 or isotype control antibodies. After 7 days of stimulation with anti-CD3, the frequencies of Foxp3hi, IL-17, and IFN-γ–positive cells in divided CD4+ T cells was analyzed. Percent increase in proliferation of (A) Treg, (B) CD4+IL-17+, and (C) CD4+IFN-γ cells by CD16+ monocytes was calculated as 1 − (% CFSElo CD4+ (Foxp3hi or IL-17+ or IFN-γ) in cocultures of T cells plus CD14hiCD16− plus CD16+ monocytes)/(% CFSElo CD4+(Foxp3hi or IL-17+ or IFN-γ) in cocultures of T cells plus CD14hiCD16−) in the presence of isotype control (“Control”) or the specific antibodies (anti–IFN-γ and anti–IL-12) are shown. Addition of anti–IL-12 decreases the inhibition of CD16+ monocytes on Treg and CD4+IL-17+ proliferative responses as well as its stimulatory effect on CD4+ IFN-γ+ expansion (P < .05, all paired t test). Anti–IFN-γ also has a significant inhibitory effect on proliferation of CD4+IL-17+ (P = .02).
IL-12 reverses CD16+ monocyte-mediated inhibition of Treg and CD4+IL-17+ proliferation. Sorted T cells were cocultured with CD14hiCD16− with or without CD16+ monocytes in the absence or presence of neutralizing anti–IFN-γ and IL-12 or isotype control antibodies. After 7 days of stimulation with anti-CD3, the frequencies of Foxp3hi, IL-17, and IFN-γ–positive cells in divided CD4+ T cells was analyzed. Percent increase in proliferation of (A) Treg, (B) CD4+IL-17+, and (C) CD4+IFN-γ cells by CD16+ monocytes was calculated as 1 − (% CFSElo CD4+ (Foxp3hi or IL-17+ or IFN-γ) in cocultures of T cells plus CD14hiCD16− plus CD16+ monocytes)/(% CFSElo CD4+(Foxp3hi or IL-17+ or IFN-γ) in cocultures of T cells plus CD14hiCD16−) in the presence of isotype control (“Control”) or the specific antibodies (anti–IFN-γ and anti–IL-12) are shown. Addition of anti–IL-12 decreases the inhibition of CD16+ monocytes on Treg and CD4+IL-17+ proliferative responses as well as its stimulatory effect on CD4+ IFN-γ+ expansion (P < .05, all paired t test). Anti–IFN-γ also has a significant inhibitory effect on proliferation of CD4+IL-17+ (P = .02).
Discussion
In the present study, we have investigated the role of monocyte subsets in control of Treg and Th development in patients with ITP on treatment with thrombopoietic agents. In contrast to the circulating classic CD14hiCD16− subpopulation, the CD16+ subset positively correlated with CD4+IFN-γ+ cell frequencies. Furthermore, an inverse relationship was seen between patient CD16+ monocyte numbers and peripheral Treg frequency and CD4+IL-17+ cells. Using an in vitro proliferation assay, the ITP CD16+ monocyte subset inhibited both Treg and CD4+IL-17+ cell expansion while promoting CD4+IFN-γ+ cell proliferation. The inhibitory effect of CD16+ monocytes on Treg and CD4+IL-17+ development in ITP patients was mediated by Th1-polarizing cytokines secreted by direct cell contact between CD16+ monocytes and T cells. This suggests that induction of IFN-γ+ CD4+ responses by CD16+ monocytes may be responsible for suppression of IL-17+CD4+ as well as Treg development. These data are consistent with a role for circulating CD16+ monocyte subsets in controlling the critical balance In Th-polarized immune responses in ITP patients.
The key finding of the present study is the identification of CD16+ blood monocytes as the subset that can modulate IL-17+ and IFN-γ+ CD4+ as well as Treg development in patients with ITP and, to a lesser extent, in healthy controls. The differences between ITP patients and healthy controls may be the result of an increase in activation state of this monocyte subset in ITP patients. As such, stimulation through TLR-7, which is preferentially expressed on CD16+ monocytes at least in nonhuman primates,33 was associated with increased IL-12 secretion in ITP patients, but not in healthy controls, indicating that patients' cells are at a heightened activation state.34 Although our study has focused on the role of CD16+ monocyte subset, a few studies have also explored the role of CD14+ monocytes in modulation of Th17 development in the setting of autoimmunity. For example, in type 1 diabetes, circulating CD16− monocytes from patients secrete more Th17-associated cytokines, such as IL-1β and IL-6, compared with those from healthy controls, and patient monocytes induced more IL-17–secreting cells compared with healthy control monocytes.35 In rheumatoid arthritis patients, CD14+ monocytes derived from inflamed joints spontaneously and specifically promote Th17 responses.5 It remains unclear whether the CD14hiCD16− subpopulation in ITP patients can drive Th17 development. Interestingly, in the rheumatoid arthritis patient study, monocytes from peripheral blood did not induce Th17 responses,5 raising the possibility that monocytes derived from sites of disease pathology, such as the bone marrow in ITP, may be responsible for the pronounced effects on Th17 development. A limitation of our study is that we did not analyze the lymphoid organs, such as the spleen where T-cell development and proliferation mostly occurs. Interestingly, splenectomy did not appear to alter the conclusions of our study, at least at the level of monocyte subset frequencies (supplemental Figure 1).
In our patient cohort, the CD16+ subset was expanded in patients with low platelet counts on treatment with thrombopoietic agents, whereas those with higher platelet numbers had normalized CD16+ counts (Figure 1E). Although these data suggest that platelet counts under thrombopoietic agent therapy dictate the number of CD16+ monocytes, our present study did not address how or why CD16+ monocytes are expanded in our patients with low platelet counts or if they are related to the effect of the treatment. Longitudinal studies to follow off treatment ITP patients as the disease progresses will be needed to determine whether platelet counts correlate with CD16+ monocyte numbers, and experimental models can question whether the increase in CD16+ monocytes simply results from low platelet counts. CD16+ cells are expanded under infectious or inflammatory conditions,9,10 although the exact mechanism for increase in CD16+ monocytes is not known. The activation state of platelets in ITP was reported to be inversely correlated with platelet counts,36 and it may be that, in patients with low platelet counts, platelet-derived factors either directly or indirectly through activation of other mediators play a role in increased peripheral numbers of CD16+ monocytes. For example, the chemokine CX3CL1/fractalkine known for its ability to promote the survival of CD16+ monocytes37 was elevated, albeit not statistically significant, in blood and bone marrow of ITP patients.38
The expansion of CD16+ monocytes (our study) and/or their potential heightened activation state34 in ITP may be responsible for the previously described skewed Th1 responses,12–14,26 which was also confirmed in this study. We did not analyze platelet antigen-specific Th1 responses, but our working hypothesis is that the increase in Th1 responses will ultimately augment antigen-specific Th1 responses through positive feedback mechanisms. The skewed Th1 responses together with increased numbers of CD16+ monocytes may lead to increased platelet phagocytosis, which can be directly tested by platelet phagocytosis assays. Interestingly, mouse CX3CR1hiLy6Clo cells, which are thought to represent the mouse counterpart of CD16+ monocytes, were recently shown to be responsible for antibody-mediated platelet destruction.39 Treatment with steroids reduces CD14+ monocyte phagocytosis in ITP patients.21 However, it remains unknown whether treatment with thrombopoietic agents can lower platelet phagocytosis, although responders to thrombopoietic treatment have improved Treg function28 and a normalized CD16+ compartment (this study).
Depending on culturing conditions, monocytes can differentiate into various macrophage and dendritic cell types which differ markedly in their ability to influence Th differentiation. For example, a combination of GM-CSF and IL-4 differentiates monocytes into immature dendritic cells which, after maturation, favor Th1 differentiation.40 However, addition of TGF-β to such a GM-CSF and IL-4 cocktail combination induces monocytes to become Langerhans cells, which preferentially induce Th17 rather than Th1 development.41 In this study, we avoided the use of any exogenous cytokines in our cultures and relied solely on the interactions of anti-CD3 activated T cells with monocytes, either in PBMCs or as a sorted population, to study Th proliferative responses. Although CD16+ monocytes constitute only ∼ 5%-10% of total monocytes in healthy persons, they can be further subdivided into the intermediate monocytes (CD14hiCD16+, analogous to CD64+CD16−) and the nonclassic subpopulation (CD14dimCD16hi analogous to CD64−CD16+).8 We were unable to functionally separate these 2 subpopulations with respect to their ability to suppress Treg or IL-17+ Th development (supplemental Figure 6). Thus, in contrast to depletion of total CD16+ cells, depletion of CD14hiCD16+ or CD14dimCD16hi did not markedly alter IL-17+ Th development (supplemental Figure 6), suggesting that the ability to affect Th17 development may be functionally redundant in these 2 subsets. In support of this, the accessory function of CD64−CD16+ and CD64+CD16− monocytes in stimulating T cells in allogeneic mixed lymphocyte reactions is similar.42
A striking observation in this study was the concomitant stimulatory effect of CD16+ monocytes on CD4+IFN-γ+ Th expansion and their inhibitory effect on CD4+IL-17 as well as Treg proliferative responses. This is consistent with the previously described reciprocal effect of Th1 cytokines on Th17 development5,31,32 and corroborate previous reports that Th1-polarizing conditions block Treg proliferation.43 Our working hypothesis is that ITP CD16+ monocytes promote Th1 development, which in turn negatively regulates both IL-17 as well as Treg induction. The transwell studies suggest that ITP CD16+ monocytes require cell-contact with CD4+ T cells for their Th polarizing effects (Figure 4A-C). These data indicate an important role for cell-to-cell signals, such as costimulation or adhesion as part of this inhibitory mechanism, although the exact nature of these signals, beyond the secretion of IL-12, remains to be determined. The requirement for cell-cell contact was also reported for induction of Th17 responses by TLR-stimulated monocyte.44 In our transwell studies, CD16+ monocytes altered the Treg and Th proliferative responses of T cells in a separate compartment if cocultured with T cells in the presence of anti-CD3 stimulation (Figure 4D-F). A likely explanation is that direct contact between CD16+ monocyte and activated T cells resulted in secretion of soluble factors, including cytokines that caused Treg and Th polarization. Using antibody-blocking experiments, albeit with whole IgG fragments instead of F(ab′)2, we identified IL-12 as a cytokine mediating the Th/Treg polarizing activity of CD16+ monocytes. Indeed, cross-linking CD154 on T cells and CD40 on antigen-presenting cells can result in the production of IL-12 by antigen-presenting cells and that Th-1 derived IFN-γ can trigger IL-12 production by antigen-presenting cells synergistically with CD40 signals.45,46 It should be noted that purified Tregs can also suppress monocyte cytokine responses,47 although we think that, in our cultures of total T cells, the numbers of Tregs are too few to have a major impact on monocyte cytokine expression.
Human Th17 cells can also express IFN-γ without the simultaneous loss of IL-17. These IL-17+/IFN-γ+ cells, intermediate between Th17 and Th1, have been previously described in many disease states.48,49 Similarly, Foxp3+ cells can simultaneously coexpress IL-17.50 Our studies were not designed to determine the relative proportion of these intermediate cell types, although future studies are designed to understand the role of different monocyte subsets on relative induction of Th1, Th17, Treg and Th1/17 and Treg/Th17 cells in ITP.
In conclusion, ITP CD16+ monocyte subsets have a stimulatory effect on proliferation of IFN-γ Th responses while concomitantly inhibiting Treg and IL-17+ Th development. Based on our data, our working model predicts that direct interaction of ITP CD16+ monocytes with T cells is responsible for expansion of IFN-γ+CD4+ responses and that the induced Th1-polarizing conditions is responsible for suppression of IL-17+CD4+ and Treg development. These data highlight the importance of the innate immune system in controlling potentially pathogenic Th responses cells in ITP and suggest that specific monocyte subsets may be a critical target in ITP to control inflammatory responses.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the National Heart, Lung, and Blood Institute (grant HL096497-01, K.Y.).
National Institutes of Health
Authorship
Contribution: H.Z. conceived the idea, performed research, analyzed data, and drafted the manuscript; W.B. and X.L. performed research and analyzed data; A.M., C.S., and N.H. recruited and consented patients; J.B. selected and recruited patients and edited the paper; and K.Y. designed, directed, and wrote the paper.
Conflict-of-interest disclosure: J.B. receives clinical research support from the following companies: Amgen, Cangene, GlaxoSmithKline, Genzyme, IgG of America, Immunomedics, Ligand, Eisai, Inc, Shionogi, and Sysmex. His family owns stock in Amgen and GlaxoSmithKline. He has participated in Advisory Boards for Amgen, GlaxoSmithKline, Ligand, Shionogi, and Eisai. He also had a 1-day consult with Portola. The remaining authors declare no competing financial interests.
Correspondence: Karina Yazdanbakhsh, Laboratory of Complement Biology, New York Blood Center, 310 E 67th St, New York, NY 10065; e-mail: kyazdanbakhsh@nybloodcenter.org.