Abstract
Stroma induces treatment resistance in chronic lymphocytic leukemia (CLL), possibly because of alterations in the BCL-2 family of proteins, which are key regulators of apoptosis. We previously developed BH3 profiling, a functional assay that assesses mitochondrial depolarization in response to BH3-only peptides, to measure “apoptotic priming,” the proximity of a cell to the apoptotic threshold. In the present study, we use BH3 profiling to show that CLL cells from the PB are highly primed. Increased priming is associated with improved clinical response and, unexpectedly, with unmutated IGHV status. Coculturing CLL cells in vitro with stroma decreases priming. Using matched PB, BM, and lymph node compartment samples, we found in vivo that BM-derived CLL cells are the least primed. CLL cells cocultured with stroma were treated with the PI3K δ-isoform inhibitor CAL-101 (GS1101). CAL-101 caused CLL cell de-adhesion, leading to increased CLL cell priming. Stimulation of CLL cells with anti-IgM or CXCL12 caused decreased priming that could be reversed by CAL-101. Our results show that inhibition of stromal interactions leading to displacement of CLL cells into the blood by CAL-101 in vivo may increase CLL cell priming, suggesting a mechanism by which agents inducing lymphocyte redistribution might facilitate improved clinical response when used in combina-tion with other therapies.
Introduction
Chronic lymphocytic leukemia (CLL) is a lymphoid neoplasm characterized by the accumulation of malignant monoclonal lymphocytes in the peripheral blood (PB), BM, and lymph nodes (LNs).1 Although first-line therapy is often effective at inducing remission, patients inevitably relapse and the disease is incurable through conventional chemotherapy.2 One important cause of treatment resistance may be that CLL cells in the stromal microenvironment receive a variety of survival and proliferative signals, and heavy water–labeling studies have demonstrated that an appreciable fraction of the CLL clone may proliferate in the LNs.3 CLL cells isolated from LNs demonstrated a gene-expression profile of activated B cells and showed increased proliferation compared with samples from the same patient's PB.4 Stromal protection from apoptosis for CLL cells may arise from blockade of proapoptotic factors and stimulation of antiapoptotic factors in the BCL-2 family of proteins,5 although the pathophysiology of these complex interactions heretofore has been challenging to study.
CLL cells usually have a fundamental reliance on BCL-2 as protection from apoptosis through the intrinsic mitochondrial pathway.6 BH3 profiling is a functional assay developed in our laboratory to assess how close cells are to this threshold of apoptosis, a phenomenon we refer to as “priming.”7,8 This assay is based on measuring the permeabilization of mitochondria induced by peptides derived from the pro-death BH3 domains of pro-death BCL-2 family proteins. Unlike conventional techniques, BH3 profiling is able to simultaneously incorporate the contributions of more than a dozen BCL-2 family members by providing a physiologic readout of mitochondrial depolarization in response to a variety of BH3-only peptides. This readout integrates the effects of pro- and antiapoptotic protein expression levels, posttranslational modifications, and protein-protein interactions. Using BH3 profiling, we recently demonstrated that priming is a key determinant of chemosensitivity in acute leukemias and multiple myeloma.9
In the present study, we use BH3 profiling to characterize the priming of PB CLL cells from patients and to determine whether priming is associated with clinical outcomes. Previous work has shown that CLL cells exposed to a diverse array of stroma become resistant to treatment with both cytotoxic chemotherapy10 and BH3 mimetics such as ABT-737 or its oral analog, ABT-263.11 Using in vitro stromal cocultures, we tested the hypothesis that decreased mitochondrial priming may underlie such resistance. Using matched PB, BM, and LN samples from the same patients studied previously for differences in gene expression,4 we also investigated whether there may be differences in CLL cell priming in these different compartments in vivo.
We hypothesized that agents that antagonize stromal interactions might overcome stroma-mediated resistance in CLL by increasing priming. The δ-isoform specific PI3K inhibitor CAL-101 has been found recently to have promising preclinical12,13 and clinical activity14 in CLL. The drug appears to modulate the microenvironment in several ways, including inhibition of CLL cell chemotaxis toward CXCL12/13, down-regulation of chemokine secretion, and inhibition of the BCR pathway by decreasing phosphorylation of key downstream targets of PI3K such as AKT and MAPK (ERK).15 These pleotropic effects allow CAL-101 both to kill CLL cells directly and to sensitize CLL cells to treatment with other agents. An important clinical observation has been that most patients treated with CAL-101 and other agents targeting the BCR pathway have a rapid, but usually transient, lymphocytosis that is thought to be a lymphocyte redistribution phenomenon.14 Although the biology underlying this lymphocyte redistribution effect has been well studied, the mechanism of how the loss of stromal prosurvival signals sensitizes CLL cells to therapy is less clear. In the present study, we treated stroma-exposed CLL cells with CAL-101 to examine its effects on CLL cell adhesion and viability and then used BH3 profiling to determine whether increased mitochondrial priming by CAL-101 is an underlying mechanism of the reversal of stromal protection observed with this drug.
Methods
CLL cell purification
After obtaining informed consent, PB, BM, and LN samples were obtained from patients fulfilling diagnostic and immunophenotypic criteria for CLL at the Division of Hematologic Malignancies at the Dana-Farber Cancer Institute and the Hematology Branch of the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH). Consent for samples used in this study was obtained from patients in accordance with the Declaration of Helsinki on protocols that were reviewed and approved by the institutional review boards of the Dana-Farber/Harvard Cancer Center and the NIH. Mononuclear cells were isolated from blood and tissue samples by using Ficoll-Paque (GE Healthcare) density-gradient centrifugation. Samples were viably frozen in 10% DMSO (Sigma-Aldrich) in FBS (BD Biosciences), stored in liquid nitrogen, and later thawed for analysis. Single-cell suspensions were prepared for analysis, and CD19+ CLL cells generally accounted for > 85% of analyzed cells.
Cell lines
The CD154+ L cell line was given to us by Dr Gerald Cohen (Leicester, United Kingdom) and was maintained in RPMI 1640 medium with 10% FBS, 2.05mM l-glutamine (HyClone), and penicillin-streptomycin (CellGro; Mediatech). The StromaNKTert cell line was purchased from the Riken Cell Bank and maintained in α-MEM with 1 μg/mL of hydrocortisone, 10% FBS, 10% human serum (Invitrogen,), 2.05mM l-glutamine, and penicillin-streptomycin. Nurse-like cells were established by suspending PBMCs from CLL patients in RPMI 1640 medium with 10% FBS and penicillin-streptomycin-glutamine to a concentration of 107 cells/mL (2 mL total) for 14 days in 24-well plates (Corning Life Sciences), as described previously.10
CLL cell and stromal cell cocultures
CLL cells were cultured for 24 hours under standardized conditions on stroma, as described previously.10 Briefly, stromal cells were seeded 1 day before each experiment onto 24-well plates at 3 × 105 cells/mL/well and incubated at 37°C in 5% CO2. Stromal cell confluence was confirmed by phase-contrast microscopy, and CLL cells were added onto the stroma at 3 × 106 cells/mL. For comparison, CLL cells were cultured either on parental stroma or in suspension at the same density. Cultures were then treated with drug for the specified time periods. CLL cells were removed for analysis by gentle pipetting with medium and were washed in PBS before analysis.
Cell viability testing and reagents
CLL cell viability was determined by annexin V–FITC (BD Biosciences) and propidium iodine (Sigma-Aldrich) by FACS. ABT-737 was provided by Abbott Pharmaceuticals and CAL-101 (GS-1101) was provided by Gilead Pharmaceuticals.
BH3 profiling
BH3 profiling was performed by either the plate-based fluorometry or FACS method, as described previously.8 Briefly, viably frozen samples from CLL patients were thawed and made into single-cell suspensions, then gently permeabilized using digitonin (0.002%). For the fluorometry-based method, 100μM JC-1 (Invitrogen) was added and cells were loaded onto a 384-well plate, with individual wells containing individual BH3-only peptides. The JC1-BH3 assays were conducted in triplicate on a Tecan Safire 2 with Ex 545 ± 20 nm and Em 590 ± 20 nm for 3 hours. For the FACS-based method, single-cell suspensions were exposed to human Fc Block (BD Pharmingen), followed by anti–CD19-V450 (BD Pharmingen) and anti–CXCR4-APC (BD Pharmingen). Cells were washed in PBS and then added into individual FACS tubes containing individual BH3-only peptides. Samples were incubated at room temperature for 60 minutes, with 100μM JC-1 added halfway through. Analysis was conducted on a BD FACSCanto II flow cytometer with lasers at 407, 488, and 633 nm. JC-1 was measured from the 488-nm laser using a 530/30-nm filter (FITC) and a 585/42-nm filter (PE), and the degree of mitochondrial depolarization was calculated using the surrogate of the change in the PE median. The depolarization reported in response to each BH3 peptide is normalized relative to the median percentage change in PE fluorescence of the JC-1 dye with a negative control, DMSO (0%), and a positive control, the mitochondrial uncoupling agent carbonyl cyanide 4-(trifluoromethoxy)-phenylhydrazone (100%; control data not shown).
Calcein-based adhesion assay
A confluent monolayer of CD154+ L or StromaNKTert cells was generated by plating 1 × 104 cells/well in 96-well plates for 24 hours. Single-cell suspensions of CLL cells at 0.5 × 106 cells/mL were then labeled with 1 μg/mL of Calcein-AM (Invitrogen) for 1 hour. Cells were then centrifuged and treated with drug or vehicle for 1-24 hours. Nonadherent cells were washed off, and CLL cell adhesion was quantified by fluorometry (Ex/Em = 485/520 nm).
Anti-IgM and CXCL12 stimulation
Primary CLL cells (107/mL) were incubated in complete RPMI medium at 37°C in 5% CO2 with 10 μg/mL of anti-IgM (MP Biomedicals) with or without 5μM CAL-101. After 18 hours, CLL cells were centrifuged and resuspended and BH3 profiling was performed. Analogous experiments were performed with stimulation by CXCL12 at 100 ng/mL (Millipore).
Data analysis and statistics
Results are shown with SEM and number of replicates as described in each figure. As appropriate for the analysis, the Student paired or unpaired t tests, Mann-Whitney U test, or linear regression analyses were used. Analyses were performed with Prism Version 5 software for PC (GraphPad). Flow cytometry data were analyzed using FACSDiva Version 6.1.1 software (BD Pharmingen). Clinical response was assessed using International Workshop on Chronic Lymphocytic Leukemia 2008 criteria,16 with responders defined as patients achieving a complete or partial response as best response. A 2-tailed P ≤ .05 was considered statistically significant unless otherwise noted.
Results
CLL cells are highly primed for apoptosis and usually BCL-2 dependent
BH3 profiling was performed on CLL cells obtained from the PB of 30 patients, most of whom were previously untreated, and none of whom had received recent therapy. Patient characteristics are detailed in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The BIM BH3 peptide interacts with high affinity with all antiapoptotic proteins.7 Therefore, we have found that the mitochondrial response to BIM BH3 peptide is a useful measure of a cell's proximity to the threshold of apoptosis, a property we refer to as “priming.” At a final concentration of 0.03μM, BIM BH3 peptide induced a significant amount of depolarization in most patient samples, with 22 of 30 (73.3%) samples having > 50% depolarization by 1 hour (Figure 1A). Compared with our prior studies that included nonmalignant human tissue and normal PBMCs,9 CLL cells were highly primed for apoptosis.
CLL cells are highly primed for apoptosis and usually BCL-2 dependent, and increased priming is associated with improved clinical response and unmutated IGHV. (A) PB CLL cells were BH3 profiled by FACS (n = 30) and the level of apoptotic priming for each sample was measured by quantifying the mitochondrial depolarization induced by the BIM BH3 peptide at a 0.03μM final concentration. Patient number, as detailed in supplemental Table 1, is depicted above each corresponding level of priming. (B) Examples of BH3 profiles from 3 individual patients showing pattern of relative dependence on BCL-2, MCL-1, and BCL-XL (see supplemental Table 1 for complete depolarization data for each patient by peptide). (C) Pretreatment samples from treatment-naive patients achieving a partial response (PR) or complete response (CR) by International Workshop on Chronic Lymphocytic Leukemia 2008 criteria are more primed than samples from patients with progressive disease during or within 6 months of completing frontline CLL therapy (P = .0333). (D) BH3 profiling shows that patients with unmutated IGHV status (n = 11) are significantly more primed than patients with mutated IGHV status (n = 12; P = .0074). (E) Percentage of VH homology to germline is positively correlated with level of priming (P = .0020; Spearman r: 0.480).
CLL cells are highly primed for apoptosis and usually BCL-2 dependent, and increased priming is associated with improved clinical response and unmutated IGHV. (A) PB CLL cells were BH3 profiled by FACS (n = 30) and the level of apoptotic priming for each sample was measured by quantifying the mitochondrial depolarization induced by the BIM BH3 peptide at a 0.03μM final concentration. Patient number, as detailed in supplemental Table 1, is depicted above each corresponding level of priming. (B) Examples of BH3 profiles from 3 individual patients showing pattern of relative dependence on BCL-2, MCL-1, and BCL-XL (see supplemental Table 1 for complete depolarization data for each patient by peptide). (C) Pretreatment samples from treatment-naive patients achieving a partial response (PR) or complete response (CR) by International Workshop on Chronic Lymphocytic Leukemia 2008 criteria are more primed than samples from patients with progressive disease during or within 6 months of completing frontline CLL therapy (P = .0333). (D) BH3 profiling shows that patients with unmutated IGHV status (n = 11) are significantly more primed than patients with mutated IGHV status (n = 12; P = .0074). (E) Percentage of VH homology to germline is positively correlated with level of priming (P = .0020; Spearman r: 0.480).
CLL cells putatively rely on at least 3 different antiapoptotic proteins for their survival: BCL-2,6,17 MCL-1,17 and BCL-XL.11 Certain BH3 peptides used in our assay interact selectively with these antiapoptotic proteins. For example, the BAD BH3 peptide interacts with BCL-2 and BCL-XL, the NOXA BH3 peptide interacts with MCL-1, and the BAD and HRK BH3 peptides interact with BCL-XL.7 The degree of depolarization induced by these BH3-only peptides can therefore be used to compare the relative functional importance of each of these antiapoptotic proteins in maintaining survival of CLL cells.6 In other words, cells that rely preferentially on BCL-2, MCL-1, or BCL-XL for their survival will undergo significant mitochondrial depolarization when exposed to the BH3-only peptides BAD, NOXA, and BAD and HRK, respectively. We found that most CLL patient samples showed relatively increased depolarization from BAD BH3 peptide, suggesting primary dependence on BCL-2 (n = 22), but a minority of cases also showed some reliance on MCL-1 and BCL-XL, as suggested by relatively higher depolarization in response to NOXA and HRK peptides, respectively (Figure 1B and supplemental Table 1).
Level of apoptotic priming is associated with the quality of clinical response
Given the relationship between mitochondrial priming and clinical response observed in other cancers,9 we sought to evaluate this relationship in CLL. We hypothesized that patients with samples showing higher levels of apoptotic priming would have a superior clinical response to therapy. Ten patients in our study had a baseline pretreatment BH3 profile and were subsequently treated and followed for clinical response. Of these 10 patients, those achieving a partial response or complete response had significantly increased apoptotic priming compared with patients who had stable or progressive disease (P = .033; Figure 1C). Despite the heterogeneity of the treatments used (supplemental Table 1), mitochondrial priming nonetheless predicted treatment outcome. Interestingly, the 3 patients who achieved complete response had cells that were among the most primed for apoptosis. These findings support the mitochondrial basis of chemosensitivity in CLL, and suggest the potential utility of BH3 profiling of PB CLL cells as a predictive marker in CLL.
Unmutated IGHV status is associated with increased apoptotic priming
We also sought to determine whether apoptotic priming was associated with traditional prognostic factors in the CLL patients being studied. Priming was not associated with Rai stage, ZAP-70 status, or FISH cytogenetics (supplemental Figure 1); however, a significant association was observed with the mutation status of IGHV. We found that unmutated IGHV status was associated with increased mitochondrial priming (P = .0074; Figure 1D). When analyzed by linear regression, priming increased as the percentage homology to the germline VH domain increased (P = .0020; Figure 1E). This finding may at first appear somewhat paradoxical, given that favorable clinical response is associated with increased priming and IGHV unmutated patients are known to carry a poorer prognosis than their mutated counterparts. However, we believe that it provides an insight into an important physiologic distinction based on IGHV status (see Discussion section).
Primary CLL cells cocultured with stroma are resistant to the BH3-mimetic ABT-737
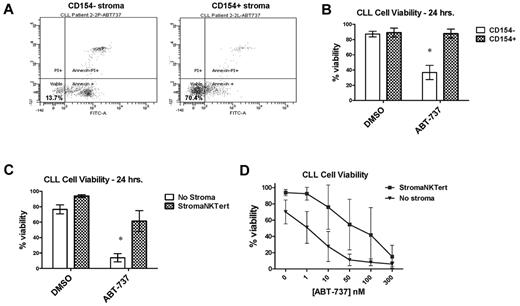
Given prior observations that interactions with stroma impair chemosensitivity10 and our finding that poorly primed CLL cells respond poorly to chemotherapy, we next investigated whether decreased mitochondrial apoptotic priming might be an important mechanism underlying stroma-mediated chemoresistance. CLL cells cocultured in a simulated LN–like microenvironment with CD154+ L-cell murine fibroblasts were previously shown to induce resistance to the potent BH3-mimetic drug ABT-737.11 Using these same CD154+ murine fibroblasts, we replicated this finding in an independent cohort of CLL patients (a representative patient is shown in Figure 2A and aggregate data in Figure 2B; P = .0017). We next investigated whether ABT-737 resistance could also be conferred by a human stromal cell line, StromaNKTert. CLL cells were again found to be highly resistant to ABT-737 when cocultured with StromaNKTert cells (P = .0004; Figure 2C). We also evaluated whether there was a dose dependency of ABT-737 resistance in stroma and, indeed, the stroma was able to generate resistance to ABT-737 in a dose-dependent fashion (Figure 2D).
Primary CLL cells cocultured for 24 hours with mouse or human stroma are resistant to the BH3-mimetic ABT-737. (A) Representative primary FACS data of PB-derived CLL cells cocultured with either CD154 + or CD154− stroma shows that the CD154+ cells significantly maintain CLL cell viability (annexin V and propidium iodide negative) in response to 100nM ABT-737. (B) Aggregate data for CLL cells cocultured ± 100nM ABT-737 with CD154 stroma confirms stromal protection (n = 4), with means depicted as horizontal bars ± SEM; P ≤ .05 between each group. (C) Aggregate data for CLL cells cocultured ± 100nM ABT-737 with or without the human stromal cell line StromaNKTert confirms the strongly protective effect of human stroma (n = 7). (D) Aggregate data from dose-response curves on 5 CLL patient samples cocultured ± ABT-737 with or without StromaNKTert. Both drugs kill consistently in a dose-dependent manner in the absence of stroma; induction of apoptosis by both drugs is significantly inhibited in the presence of stroma.
Primary CLL cells cocultured for 24 hours with mouse or human stroma are resistant to the BH3-mimetic ABT-737. (A) Representative primary FACS data of PB-derived CLL cells cocultured with either CD154 + or CD154− stroma shows that the CD154+ cells significantly maintain CLL cell viability (annexin V and propidium iodide negative) in response to 100nM ABT-737. (B) Aggregate data for CLL cells cocultured ± 100nM ABT-737 with CD154 stroma confirms stromal protection (n = 4), with means depicted as horizontal bars ± SEM; P ≤ .05 between each group. (C) Aggregate data for CLL cells cocultured ± 100nM ABT-737 with or without the human stromal cell line StromaNKTert confirms the strongly protective effect of human stroma (n = 7). (D) Aggregate data from dose-response curves on 5 CLL patient samples cocultured ± ABT-737 with or without StromaNKTert. Both drugs kill consistently in a dose-dependent manner in the absence of stroma; induction of apoptosis by both drugs is significantly inhibited in the presence of stroma.
Stromal coculture leads to decreased CLL cell priming in vitro
To evaluate in vitro whether stroma-mediated resistance to BH3-mimetic drugs was related to alterations in apoptotic priming, CLL cells from the same PB samples used in Figure 2B-C (as well as additional patient samples) were cocultured for 24 hours with a variety of stroma. Three different types of stroma were studied to ensure that the effects observed were not because of the idiosyncrasies of any one particular cell line. The CD154+ murine fibroblast L-cell line was used to simulate a LN–like microenvironment; the human stromal cell line StromaNKTert was used to more closely mimic human BM stroma. To provide an even more realistic simulation of the in vivo stromal microenvironment, we also grew nurse-like cells from CLL patient PB samples, as described previously.18
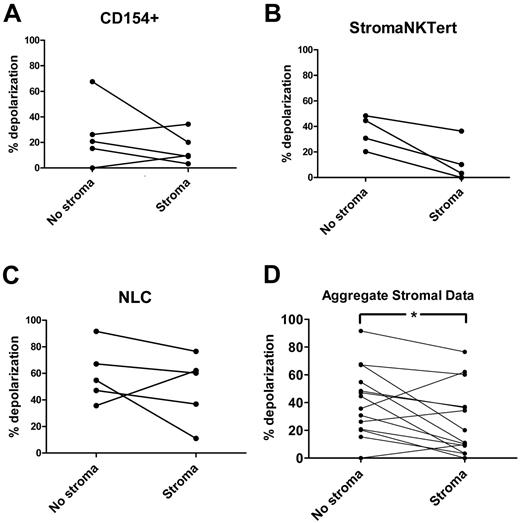
Using the BIM BH3 peptide as a general measure of mitochondrial priming, stroma exposure induced decreased mitochondrial depolarization in CLL cells exposed to all 3 types of stroma (Figure 3A-C). An aggregate pairwise comparison of matched CLL samples cultured in the presence or absence of all 3 stroma types confirmed that stroma decreases mitochondrial priming significantly (P = .020; Figure 3D). These results suggest that alterations in mitochondria correspond to the drug resistance observed in Figure 2.
In vitro, BH3 profiling demonstrates that primary CLL cells cocultured for 24 hours with a variety of stroma are less primed to undergo apoptosis. Priming of CLL cells from individual patients cocultured without and with CD154+ L-cell (A; n = 5), StromaNKTert (B; n = 4), or primary human nurse-like cells (C; n = 5) stroma for 24 hours, measured by 0.03μM BIM BH3 peptide (complete BH3 profiling data from individual patients are provided in supplemental Figure 2). All BH3 profiles are via the FACS-based method, except for 2 samples in panel B and 3 samples in panel C, which were via the plate-based method (see Methods). (D) Aggregate data from all 3 stroma (n = 14) reveals significantly decreased priming in stroma-exposed CLL cells (P = .020 by paired t test).
In vitro, BH3 profiling demonstrates that primary CLL cells cocultured for 24 hours with a variety of stroma are less primed to undergo apoptosis. Priming of CLL cells from individual patients cocultured without and with CD154+ L-cell (A; n = 5), StromaNKTert (B; n = 4), or primary human nurse-like cells (C; n = 5) stroma for 24 hours, measured by 0.03μM BIM BH3 peptide (complete BH3 profiling data from individual patients are provided in supplemental Figure 2). All BH3 profiles are via the FACS-based method, except for 2 samples in panel B and 3 samples in panel C, which were via the plate-based method (see Methods). (D) Aggregate data from all 3 stroma (n = 14) reveals significantly decreased priming in stroma-exposed CLL cells (P = .020 by paired t test).
ABT-737 can be used like a BH3 peptide by adding the drug directly to mitochondria in gently permeabilized CLL cells just before BH3 profiling. This method allows the measurement of the effects of stroma on direct mitochondrial sensitivity to ABT-737 without interference from issues such as cellular drug entry. When used in this fashion, ABT-737 also induced less depolarization in the presence of stroma in most patient samples (supplemental Figure 2), providing further evidence of the importance of BCL-2 and/or BCL-XL in conferring stroma-mediated protection from apoptosis.
BM-derived CLL cells are less primed than those from the PB in vivo
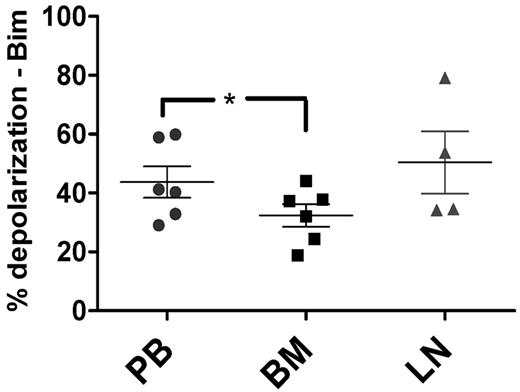
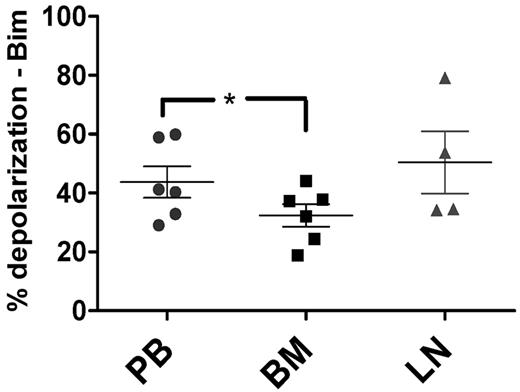
To confirm the physiologic relevance of our in vitro stromal coculture experiments, we next performed BH3 profiling on CLL cells derived in vivo from matched patient samples from the PB (n = 6), BM (n = 6), and LNs (n = 4). These compartment samples were from the same patients recently studied by gene-expression analysis.4 Mononuclear cells from all 3 types of samples were isolated, viably frozen immediately after sample acquisition, and thawed just before BH3 profiling to ensure comparable cell viability between samples. Using the amount of mitochondrial depolarization induced by the BIM BH3 peptide as a measure of apoptotic priming, we found that BM-derived CLL cells were significantly less primed than PB-derived CLL cells (P = .011; Figure 4). Pairwise comparisons confirmed this finding, whereas comparisons of PB or BM with LNs showed only statistically insignificant trends (supplemental Figure 3).
In vivo, priming is decreased in BM-derived CLL cells compared with PB-derived CLL cells. BM-derived CLL cells (n = 6) were found to be significantly less primed than their PB (n = 6) counterparts using BIM BH3 peptide at 0.01μM (P = .011), with means depicted as horizontal bars ± SEM and P ≤ .05 as indicated by asterisks. LN-derived (n = 4) CLL cells trended toward having increased priming compared with their BM-derived counterparts (P = .11).
In vivo, priming is decreased in BM-derived CLL cells compared with PB-derived CLL cells. BM-derived CLL cells (n = 6) were found to be significantly less primed than their PB (n = 6) counterparts using BIM BH3 peptide at 0.01μM (P = .011), with means depicted as horizontal bars ± SEM and P ≤ .05 as indicated by asterisks. LN-derived (n = 4) CLL cells trended toward having increased priming compared with their BM-derived counterparts (P = .11).
Given the inevitable admixture of more highly primed PB-derived CLL cells into the BM-derived samples during aspiration, we were curious as to whether a particular subset of BM-derived CLL cells were especially responsible for the decreased priming we observed. The chemokine receptor CXCR4 has a high level of expression on the surface of PB-derived CLL cells and decreased surface expression in LN- and BM-derived CLL cells.4,19 Therefore, CXCR4 surface expression is one marker that may distinguish PB-derived CLL cells from CLL cells resident in the microenvironment. We found that CXCR4 surface expression was significantly lower on BM-derived compared with PB-derived CLL cells; however, no clear relationship between apoptotic priming and CXCR4 status was observed for BM-derived CLL cells.
PI3K antagonism may facilitate release of CLL cells sequestered in stroma and overcome stroma-mediated resistance
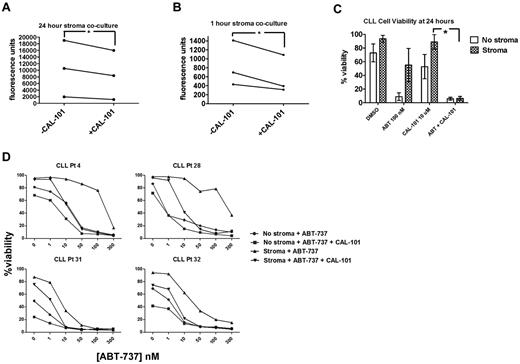
A promising new approach to targeting the microenvironment as a therapeutic modality in CLL is PI3K inhibitors such as CAL-101 (GS1101), which have been found to cause a transient lymphocytosis soon after the initiation of therapy in the majority of patients.14 We examined the effects of CAL-101 on the adhesion, viability, and priming of CLL cells exposed to stroma in vitro. We first evaluated whether a significant decrease in adhesion could be observed in CLL cells cocultured with StromaNKTert and treated with CAL-101. CLL cells were significantly less adherent in the presence of CAL-101 at 24 hours (Figure 5A). Because CAL-101 can induce some cell death by 24 hours, we wanted to ensure that the decreased CLL cell adhesion we observed was not primarily because of cell death. Therefore, we also tested a shorter time point and, interestingly, CLL cells showed significantly less adherence even after only 1 hour of CAL-101 treatment (Figure 5B), well before significant CLL cell death would occur.
CAL-101 releases CLL cells sequestered in stroma to overcome stroma-mediated resistance. PB-derived CLL cells were labeled with Calcein-AM and cocultured on StromaNKTert for 24 hours ± 10μM CAL-101, rinsed by gentle pipetting, and quantified by whole-well fluorometry. The number of adherent cells is proportional to the fluorescence units on the y-axis. Significantly more CLL cells were adherent at 24 hours (A) than at 1 hour (B), and significantly decreased CLL cell adherence was observed in the presence of 10μM CAL-101 at both 24 hours (A) and at 1 hour (B; 1-tailed P = .045 and 0.032, respectively). (C) PB-derived CLL cells were cocultured in the presence or absence of StromaNKTert for 24 hours with drug treatments as depicted in the graph, and viability assessed by annexin V/propidium iodide. The mean percent viability for 6 patients is depicted along with SEM. Patients demonstrated stroma-mediated resistance to either 100nM ABT-737 or 10μM CAL-101 alone, but this resistance was overcome by the combination of the 2 drugs in all patients. (D) Dose-response curves for CLL cells from 4 individual patients cultured in the presence of ABT-737 for 24 hours ± StromaNKTert and ± CAL-101. ABT-737 alone or in combination with CAL-101 showed dose-dependent killing in the absence of stroma. Resistance to ABT-737 alone and CAL-101 alone was observed in the presence of StromaNKTert, but this could be overcome by adding CAL-101 at 10μM to concentrations of ABT-737 as low as 10nM.
CAL-101 releases CLL cells sequestered in stroma to overcome stroma-mediated resistance. PB-derived CLL cells were labeled with Calcein-AM and cocultured on StromaNKTert for 24 hours ± 10μM CAL-101, rinsed by gentle pipetting, and quantified by whole-well fluorometry. The number of adherent cells is proportional to the fluorescence units on the y-axis. Significantly more CLL cells were adherent at 24 hours (A) than at 1 hour (B), and significantly decreased CLL cell adherence was observed in the presence of 10μM CAL-101 at both 24 hours (A) and at 1 hour (B; 1-tailed P = .045 and 0.032, respectively). (C) PB-derived CLL cells were cocultured in the presence or absence of StromaNKTert for 24 hours with drug treatments as depicted in the graph, and viability assessed by annexin V/propidium iodide. The mean percent viability for 6 patients is depicted along with SEM. Patients demonstrated stroma-mediated resistance to either 100nM ABT-737 or 10μM CAL-101 alone, but this resistance was overcome by the combination of the 2 drugs in all patients. (D) Dose-response curves for CLL cells from 4 individual patients cultured in the presence of ABT-737 for 24 hours ± StromaNKTert and ± CAL-101. ABT-737 alone or in combination with CAL-101 showed dose-dependent killing in the absence of stroma. Resistance to ABT-737 alone and CAL-101 alone was observed in the presence of StromaNKTert, but this could be overcome by adding CAL-101 at 10μM to concentrations of ABT-737 as low as 10nM.
We also sought to evaluate whether the de-adherence of the CLL cells from stroma that we observed in response to PI3K inhibition could lead to enhanced killing of CLL cells. To avoid studying only direct killing of CLL cells by CAL-101, we identified CLL patient samples relatively resistant to both ABT-737 and CAL-101 as single agents in the presence of stroma. CAL-101 restored sensitivity to ABT-737 in the presence of stroma (Figure 5C). We next performed dose-response experiments with ABT-737 in combination with a fixed dose of 10μM CAL-101. We found that, although stroma-exposed CLL cells from these patients were resistant to CAL-101 as a single agent, they experienced a dose-dependent increase in killing with ABT-737 (Figure 5D).
PI3K inhibition may help overcome stroma-mediated treatment resistance in CLL cells by increasing apoptotic priming
Given the decreased apoptotic priming of stroma-exposed CLL cells both in vitro and in vivo, we next evaluated whether PI3K inhibition increased the sensitivity of CLL cells released from the stroma by increasing priming. PB-derived CLL cells were cultured with or without StromaNKTert cells for 24 hours, and CLL cell viability was assessed by annexin-propidium iodide. BH3 profiling was also performed on a subset of these same samples treated identically. In light of our previous observation that CAL-101 is effective at mobilizing CLL cells out of stroma, we hypothesized that CAL-101 would also induce CLL cell apoptosis and increase mitochondrial priming after 24 hours of culture in the presence of stroma.
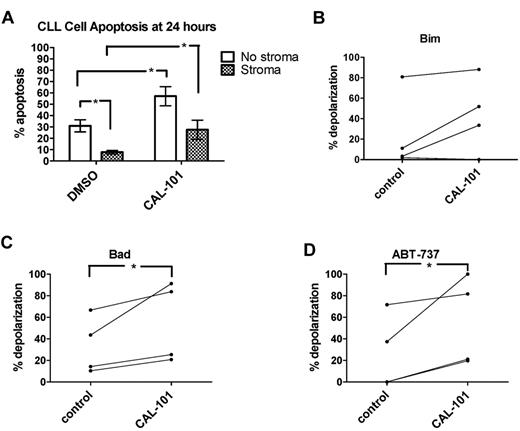
Untreated CLL cells typically undergo measurable spontaneous apoptosis in ex vivo culture over 24 hours.20 In the present study, stromal coculture of CLL cells led to protection from apoptosis in untreated cells (Figure 6A) and 10μM CAL-101 was able to partially overcome this resistance, causing significantly more CLL cell apoptosis in stroma-exposed CAL-101–treated cells compared with untreated stroma-exposed cells. BH3 profiling revealed a trend toward increased mitochondrial priming (as measured by BIM BH3 peptide) at 24 hours in stroma-exposed CLL cells treated with CAL-101 compared with untreated cells (1-tailed P = .075; Figure 6B). Both the BAD BH3 peptide and ABT-737 used as a peptide induced significantly more mitochondrial depolarization in the CAL-101–treated CLL cells (1-tailed P = .046 and P = .047, respectively; Figure 6C). In summary, treatment with CAL-101 causes de-adherence of CLL from stroma accompanied by increased mitochondrial priming and increased sensitivity to BCL-2 antagonism.
PI3K inhibition may help overcome stroma-mediated resistance by increasing CLL cell priming. PB-derived CLL cells from 8 individual patients were cocultured for 24 hours ± StromaNKTert and ± 10μM CAL-101. (A) Mean CLL cell percent apoptosis as measured by annexin V/propidium iodide is depicted along with SEM for all 8 patient samples. In 2-way ANOVA analysis, stroma provided significant protection from spontaneous apoptosis in the absence of CAL-101. In the absence of stroma, CAL-101 induced significantly more apoptosis than control. In the presence of stroma, CAL-101 was also able to induce significantly more apoptosis than control. (B) A trend toward increased priming by BIM BH3 peptide was observed in stroma-exposed CLL cells treated with CAL-101 compared with controls (1-tailed P = .0749). (C-D) BAD BH3 peptide and ABT-737 used as a peptide both induced significantly increased mitochondrial depolarization in stroma-exposed CLL cells treated with CAL-101 compared with control (we predicted this result based on the results shown in panel A, and therefore 1-tailed P values were used: P = .0462 and P = .0468 for BAD and ABT-737, respectively).
PI3K inhibition may help overcome stroma-mediated resistance by increasing CLL cell priming. PB-derived CLL cells from 8 individual patients were cocultured for 24 hours ± StromaNKTert and ± 10μM CAL-101. (A) Mean CLL cell percent apoptosis as measured by annexin V/propidium iodide is depicted along with SEM for all 8 patient samples. In 2-way ANOVA analysis, stroma provided significant protection from spontaneous apoptosis in the absence of CAL-101. In the absence of stroma, CAL-101 induced significantly more apoptosis than control. In the presence of stroma, CAL-101 was also able to induce significantly more apoptosis than control. (B) A trend toward increased priming by BIM BH3 peptide was observed in stroma-exposed CLL cells treated with CAL-101 compared with controls (1-tailed P = .0749). (C-D) BAD BH3 peptide and ABT-737 used as a peptide both induced significantly increased mitochondrial depolarization in stroma-exposed CLL cells treated with CAL-101 compared with control (we predicted this result based on the results shown in panel A, and therefore 1-tailed P values were used: P = .0462 and P = .0468 for BAD and ABT-737, respectively).
BCR pathway activation decreases priming, which may be reversed by CAL-101
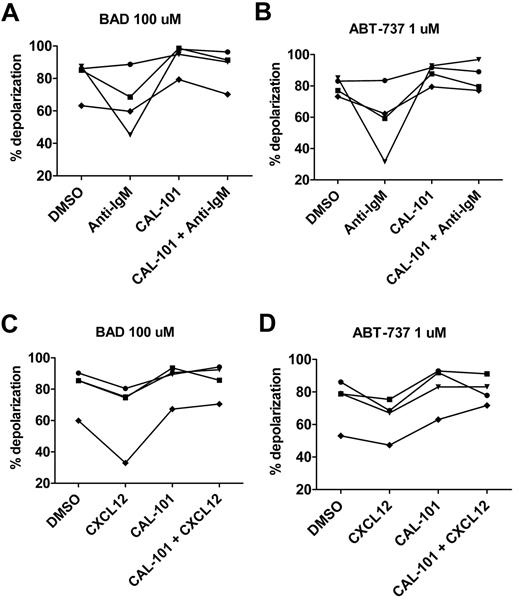
To examine possible mechanisms underlying stroma-mediated inhibition of CLL cell priming, we performed a series of functional experiments aimed at evaluating the effects of stimulating the BCR signaling pathway on mitochondrial priming. It is has been shown previously that BCR stimulation using anti-IgM can lead to protection of CLL cells from apoptosis and that this protection can be overcome by blocking the BCR pathway with CAL-101.15 In the present study, we show that anti-IgM treatment decreases mitochondrial depolarization to both BAD BH3 peptide and ABT-737 used directly on mitochondria through permeabilized cells and, in both cases, the level of priming can be increased through treatment with CAL-101 (Figure 7A-B). Similarly, stimulation of CXCR4 by its ligand CXCL12 can lead to both homing of CLL cells into protective niches18 and to activation of PI3K within the CLL cell.21 As with anti-IgM treatment, we found that CLL cells treated with CXCL12 had less mitochondrial depolarization in response to BAD BH3 peptide and ABT-737 in permeabilized cells and that priming could be restored by CAL-101 (Figure 7C-D), suggesting that the BCR pathway itself is a possible mechanistic link between PI3K inhibition and the augmentation of apoptotic priming.
BH3 profiling demonstrates that primary CLL cells stimulatedinvitro with anti-IgM or CXCL12 are less primed to undergo apoptosis and that priming can be restored with CAL-101. (A-B) PB-derived CLL cells from 4 individual patients were stimulated with 10 μg/mL of anti-IgM and BH3 profiling was performed at 18 hours. As measured by BAD peptide at 100μM and ABT-737 used like a peptide at 1μM in permeabilized cells, priming was decreased in the presence of anti-IgM, but could be restored in the presence of 5μM CAL-101. (C-D) Analogous experiments revealed that priming was also decreased in the presence of stimulation with 100 ng/mL of CXCL12, but that priming could be restored in the presence of CAL-101.
BH3 profiling demonstrates that primary CLL cells stimulatedinvitro with anti-IgM or CXCL12 are less primed to undergo apoptosis and that priming can be restored with CAL-101. (A-B) PB-derived CLL cells from 4 individual patients were stimulated with 10 μg/mL of anti-IgM and BH3 profiling was performed at 18 hours. As measured by BAD peptide at 100μM and ABT-737 used like a peptide at 1μM in permeabilized cells, priming was decreased in the presence of anti-IgM, but could be restored in the presence of 5μM CAL-101. (C-D) Analogous experiments revealed that priming was also decreased in the presence of stimulation with 100 ng/mL of CXCL12, but that priming could be restored in the presence of CAL-101.
Discussion
BH3 profiling uses a functional approach to determine the proximity of malignant cells to the threshold of apoptosis and to elucidate the relative importance of different antiapoptotic proteins in cell survival. BCL-2 expression is known to be elevated in CLL cells, which theoretically should make the cells less susceptible to undergoing apoptosis because of an increased antiapoptotic reserve; however, it is also well-known that CLL cells in the PB die readily by apoptosis in response to a variety of therapeutic agents. This apparent paradox of high levels of antiapoptotic proteins in highly chemosensitive cells highlights the inherent limitations of examining single protein expression alone.
In the present study, we used BH3 profiling to show that CLL cells are generally highly primed to undergo apoptosis, although individual CLL patient samples exhibit a range of priming levels. In the subset of our patients who underwent frontline treatment, patients with unprimed CLL cells were less likely to achieve an objective response to therapy. Unprimed CLL cells start farther from the threshold of apoptosis, and therefore chemotherapeutic agents cannot push these cells far enough to cross this threshold to cause cell death. Our patients who achieved complete response showed very high levels of priming. These results are consistent with our prior work showing a significant association of priming with the quality of clinical response in other hematologic malignancies.9 Our present results suggest that BH3 profiling could potentially be used to identify a subset of CLL patients less likely to respond to traditional chemotherapeutic agents that kill mainly through apoptotic mechanisms. Further investigation will be required to determine whether such patients with unprimed CLL cells would be better off being treated with agents that are thought to kill by immunologic or other means, such as Ab-based or chimeric Ag receptor–based therapies or highly selective targeted agents.
This study confirms our prior work showing that CLL cells usually have a fundamental reliance on BCL-2 for survival,6 although a minority of samples in this study also showed important functional protection from MCL-1 and BCL-XL. CLL patients whose cells are relatively more BCL-2 dependent would be expected to respond well to BH3-mimetic drugs such as the BCL-2/BCL-XL–specific ABT-263 (the oral analog of ABT-737), whereas patients whose cells are more MCL-1 dependent would be less likely to respond to such an agent, which does not target MCL-1. Indeed, a recent report on the phase 1 study of ABT-263 (Navitoclax) in patients with relapsed refractory CLL provided some preliminary evidence in patients in whom a high BIM:BCL-2 or BIM:MCL-1 ratio may be correlated with improved clinical response to the drug.22 Given its potential to identify mechanisms of acquired resistance to apoptosis in patients who progress on such drugs, BH3 profiling may be an even more robust predictive marker for clinical response. We are currently testing this hypothesis.
The association of increased priming with unmutated IGHV status initially seems counterintuitive because increased priming is associated with increased chemosensitivity and it is well-established that patients with unmutated IGHV have a more aggressive form of CLL than their mutated counterparts.23 However, there is also evidence that CLL cells from unmutated IGHV patients have increased BCR signaling and have a tendency to proliferate more than mutated IGHV cells, as evidenced by relatively shorter telomere length.24,25 Furthermore, it was recently reported that unmutated CLL cells are more prone to undergo spontaneous apoptosis than mutated CLL cells.26 Our finding herein of increased priming of unmutated CLL cells suggests that these unmutated CLL cells are inherently at least as sensitive as mutated CLL cells to the death signaling induced by chemotherapy. This result may help to explain why unmutated IGHV status, although conferring a shorter time to first treatment and poorer overall survival, does not alter the clinical response rates to frontline chemoimmunotherapy.27 Therefore, our findings indicate that the poor prognosis of unmutated CLL, including inferior progression-free survival, may have more to do with increased cell kinetics rather than with a decreased propensity to undergo apoptosis in response to therapy.
In recent years, there has been an increasing realization that stromal support facilitates CLL cell survival and resistance to conventional chemotherapy,10 and the mechanistic underpinnings of these interactions are now being elucidated. Our present results indicate that the physiologic basis for the reduced chemosensitivity of stroma-exposed CLL cells is reduced mitochondrial priming. In the present study, we have shown in vitro that stroma is able to induce decreased CLL cell priming (Figure 3) and in vivo that BM, but not LN-derived CLL cells, are less primed than PB-derived CLL cells (Figure 4).
These findings are of particular current relevance considering recent results of clinical trials of ABT-263.22 In several cases, the investigators found that even when there was no evidence of disease elsewhere, residual CLL cells were found diffusely in the BM. We have shown herein that mitochondria from BM-resident CLL cells are specifically less sensitive to BCL-2 antagonism by either the BAD BH3 peptide or ABT-737. Our results therefore indicate that the basis for the BM resistance of CLL cells in trials of ABT-263 (and likely other therapies) lies in how the priming of their mitochondria is altered by stromal interactions.
The results of the present study provide mechanistic insights into how PI3K inhibition can antagonize the protection afforded to CLL cells by stroma. We found that CAL-101 was effective at reversing the major effects of stroma on CLL cells: adhesion, decreased mitochondrial priming, and decreased sensitivity to therapies that inhibit BCL-2 (Figures 5 and 6). Furthermore, it was recently shown that anti-IgM stimulation of CLL cells may phosphorylate BIM,28 thereby protecting the cells from apoptosis. Using BH3 profiling of CLL patient samples stimulated with anti-IgM, we show herein that BCR stimulation may inhibit apoptosis through decreased priming (Figure 7A-B) and that stimulation of CLL cells with CXCL12 produces a strikingly similar effect (Figure 7C-D). Interestingly, apoptotic priming of these anti-IgM- or CXCL12-treated CLL cells can be increased by CAL-101 treatment (Figure 7A-D), suggesting that restoring the priming of stroma-exposed CLL cells is one mechanism underlying the efficacy of the drug in CLL. We are currently investigating whether other promising drugs targeting the BCR pathway, such as BTK inhibitors, demonstrate similar effects on apoptotic priming.
Our present data also suggest that the lymphocyte redistribution observed in patients treated with BCR pathway antagonists such as CAL-101 may not be an epiphenomenon, but in fact may be a key to the efficacy of the drugs. By releasing CLL cells from stroma, CAL-101 may allow them to emerge from the antiapoptotic stromal milieu and escape tonic BCR stimulation, thereby increasing their mitochondrial priming and making them more susceptible to both spontaneous and treatment-induced apoptosis. Our results not only support current efforts to test combinations of PI3K inhibition with conventional chemotherapy, they also suggest that combinations of PI3K inhibition with BCL-2 inhibition might deepen responses to BCL-2 inhibition so that even relatively resistant reservoirs such as BM-resident CLL cells are effectively killed.
Many, if not most, chemotherapeutics kill via the mitochondrial apoptosis pathway that is controlled by the BCL-2 family of proteins. BH3 profiling provides a functional assessment of the apoptotic pathway of CLL cells. This information has the potential to be used as a predictive tool, perhaps eventually even to prospectively guide personalization of CLL therapy. Moreover, understanding how the mitochondrial apoptotic pathway is altered by environmental cues helps us to identify rational combinations to overcome treatment resistance and improve the response to therapy and ultimately the survival of patients with CLL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients who generously donated their samples for this study; Jeremy Ryan, Trang Vo, Patrick Bhola, Jan Burger, Abdel Kareem Azab, and Irene Ghobrial for general technical support and advice; Triona Ni Chonghaile and Jennifer Davids for close review of the manuscript; John F. Daley II, Suzan Lazo-Kallanian, and Robert W. Smith for technical support with flow cytometry; Prof Gerald M. Cohen for generously providing the CD154+ L-cell line; and Abbott Laboratories for providing ABT-737.
This work was funded by the FLAMES Fund of the Pan-Mass Challenge, the National Institutes of Health (RO1CA129974), a Ruth L. Kirschstein National Research Service Award T32 National Cancer Institute grant, and the Friends of the Dana-Farber Cancer Institute. M.S.D. is a Leukemia & Lymphoma Society Special Fellow in Clinical Research and a recipient of an National Institutes of Health Loan Repayment Program award. A.W. and W.H.W. are supported by the Intramural Research Program of the National Heart, Lung, and Blood Institute, the National Institutes of Health, and the National Cancer Institute, respectively. C.J.W. is a Damon Runyon Cancer Research Foundation Clinical Investigator and a recipient of an American Association of Cancer Research/Stand Up To Cancer Innovative Research Grant. J.R.B. is a Leukemia & Lymphoma Society Scholar in Clinical Research and a Scholar of the American Society of Hematology. A.L. is a Leukemia & Lymphoma Society Scholar and a Scholar of the American Society of Hematology.
National Institutes of Health
Authorship
Contribution: M.S.D. designed and performed the experiments, analyzed the data, designed the figures, and wrote the manuscript; J.D. performed the experiments and analyzed the data; A.W. and W.H.W. provided patient samples, helped with the experimental design, and reviewed the manuscript; B.J.L. provided CAL-101 (GS-1101) and helped with the experimental design; L.W., C.J.W., and J.R.B. provided patient samples, helped with the data interpretation, and reviewed the manuscript; and A.L. helped with the experimental design, supervised the study, analyzed the data, and revised the manuscript.
Conflict-of-interest disclosure: B.J.L. is an employee of Gilead Sciences. J.R.B. was a paid consultant for Calistoga Pharmaceuticals before its acquisition by Gilead Sciences. A.L. is a cofounder of, and formerly a paid advisor to, Eutropics Pharmaceuticals, which has purchased a license to BH3 profiling from Dana-Farber Cancer Institute. A.L. and the Dana-Farber Cancer Institute have a patent relating to the use of BH3 profiling in predicting chemosensitivity. The remaining authors declare no competing financial interests.
Correspondence: Anthony Letai, MD, PhD, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; e-mail: anthony_letai@dfci.harvard.edu.