Hereditary thrombotic thrombocytopenic purpura (TTP) may be rare, but it is forever. What is the future for our patients?
Hereditary TTP, caused by ADAMTS13 mutations resulting in a severe deficiency of ADAMTS13 activity, is also known as Upshaw-Schulman syndrome. In 1960, Schulman et al reported an 8-year-old girl who had repeated episodes of thrombocytopenia that responded to plasma infusion; they concluded that she had a “congenital deficiency of a platelet-stimulating factor.”1 In 1978, Upshaw et al reported a 29-year-old woman who had repeated episodes of TTP.2 These 2 patients were among the 4 patients with chronic relapsing TTP studied by Moake et al in 1982, demonstrating the presence of unusually large von Willebrand factor (VWF) multimers and postulating a missing factor required for VWF cleavage.3 The missing factor was initially identified as a VWF-cleaving protease in 1997 in a patient with hereditary TTP4 and subsequently characterized in 2001 as ADAMTS13 in a study of 4 families with hereditary TTP.5 This is a wonderful scientific story, but then the next question is, what happens to people with a lifetime deficiency of ADAMTS13?
The experience of Fujimura et al with 43 patients from Japan documents the heterogeneity of hereditary TTP.6 Eighteen (42%) had severe neonatal hemolysis requiring exchange transfusion; 25 (58%) were diagnosed as children; 15 (35%) were 15 to 45 years old when they were diagnosed, 7 in association with pregnancy; 3 (7%) were 51 to 63 years old when TTP suddenly developed. Fujimura et al postulated that some ADAMTS13 mutations may result in a mild, late-onset phenotype, without manifestations of overt TTP unless additional factors, such as pregnancy or infection, are present.6
These observations are extended by Lotta et al in this issue of Blood.7 They describe 29 patients with hereditary TTP in 25 families from 4 centers across Europe. Using an assay with sensitivity to 0.5%, 26 patients had measurable ADAMTS13 activity (range, 0.5%-6.8%); 31 different ADAMTS13 mutations were identified. The severity of the deficiency of ADAMTS13 activity correlated with younger age of the first TTP episode, a higher frequency of TTP episodes, and the use of regular plasma prophylaxis. ADAMTS13 mutations affecting the highly conserved N-terminal domains of ADAMTS13 were associated with lower residual ADAMTS13 activity and more severe clinical features. These data provide a basis for understanding the genetic heterogeneity of hereditary TTP, similar to the heterogeneity of hemophilia related to residual factor VIII or IX activity. However, Lotta et al also observed exceptions; some patients with higher ADAMTS13 activity had early onset and frequent recurrences of acute episodes. These observations emphasize that multiple additional factors are important for triggering acute episodes of microvascular thrombosis, similar to other thrombotic disorders. Pregnancy appears to be a particular hazard for women with hereditary TTP.6,8
Until 3 months ago I thought that hereditary TTP was too rare for clinicians to care about. I had not recognized a patient with hereditary TTP since 1974.9 Then we diagnosed hereditary TTP in a teenage girl and subsequently in both of her younger teenage sisters. All 3 girls had required exchange transfusion at birth for severe hemolysis and thrombocytopenia; the oldest sister had a focal seizure and small cerebral thrombosis on her first day of life that promptly resolved. At that time, these episodes were attributed (with some uncertainty) to their other clinically apparent hereditary disorder, elliptocytosis. Subsequently all 3 girls have had excellent health except for 3 episodes of transient, asymptomatic thrombocytopenia: 2 in the youngest sister, 1 in the oldest. They are all outstanding students and athletes. My conversations with this family around their kitchen table have been all about forecasting the future. We've discussed potential problems and developed management plans for the near future, but what about the next 75 years? Other than for pregnancies, should plasma infusions be given only when there is evidence of TTP? What are the relative benefits and risks of regular prophylactic plasma infusions?10 What about the girls' risks for kidney disease, hypertension, and cardiovascular disease; should these be anticipated and can they be prevented? The patient reported by Schulman et al1 developed renal failure and became dialysis dependent.10 Experimental data suggest that ADAMTS13 deficiency can accelerate the development of atherosclerosis.11 These observations emphasize the potential dangers in the decades ahead for my patients.
To answer these questions about the future of patients with hereditary TTP, systematic lifetime follow-up of many patients will be required. To achieve this, an international Hereditary TTP Registry (www.ttpregistry.net; NCT 01257269) was established in 2009; 83 patients from 74 families in 18 countries have been enrolled. Genetic data and detailed clinical observations from long-term follow-up will determine the risk for renal, cardiac, or cognitive problems in patients with hereditary TTP and will help to determine appropriate management.
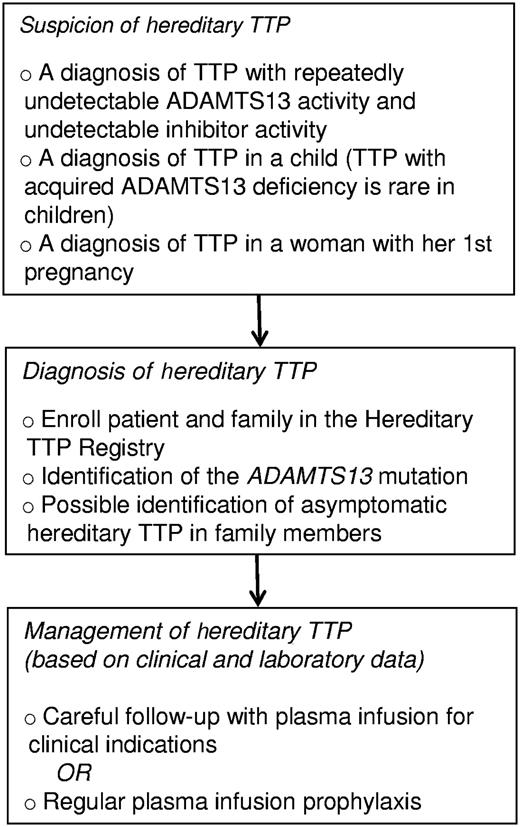
My recent experience has changed my opinion. Now I am convinced that hereditary TTP is an important, though rare, problem for clinicians. The first problem for clinicians is recognition (see figure). There are several situations that should stimulate suspicion for the diagnosis of hereditary TTP: the occurrence of thrombocytopenia and hemolytic anemia in a newborn infant; recurrent episodes of acute thrombocytopenia in a child; the diagnosis of TTP in a child, because acquired TTP is rare in children; TTP occurring during an initial pregnancy.6,8 Once suspected, the definitive genetic diagnosis of hereditary TTP is available. The next step is to determine appropriate management.10 Regular plasma infusions have logistical problems and potential risks that may be minimized by future availability of recombinant ADAMTS13. Management may be guided by our increasing knowledge of the genetic heterogeneity of hereditary TTP.6,7 These data will allow us to translate research into practice and forecast the future with increasing confidence.
Detection and management of patients with hereditary TTP. The Hereditary TTP Registry (www.ttpregistry.net; NCT 01257269, clinicaltrials.gov) is an observational study that provides genotyping and systematic long-term follow-up.
Detection and management of patients with hereditary TTP. The Hereditary TTP Registry (www.ttpregistry.net; NCT 01257269, clinicaltrials.gov) is an observational study that provides genotyping and systematic long-term follow-up.
Conflict-of-interest disclosure: The author serves as a consultant for Baxter Healthcare for the development of rADAMTS13 as a potential treatment for TTP and is a co-investigator for the Hereditary TTP Registry (www.ttpregistry.net; NCT 01257269), which is partially supported by a grant from Baxter Healthcare. ■