Abstract
The attrition rate for anticancer drugs entering clinical trials is unacceptably high. For multiple myeloma (MM), we postulate that this is because of preclinical models that overemphasize the antiproliferative activity of drugs, and clinical trials performed in refractory end-stage patients. We validate the Vk*MYC transgenic mouse as a faithful model to predict single-agent drug activity in MM with a positive predictive value of 67% (4 of 6) for clinical activity, and a negative predictive value of 86% (6 of 7) for clinical inactivity. We identify 4 novel agents that should be prioritized for evaluation in clinical trials. Transplantation of Vk*MYC tumor cells into congenic mice selected for a more aggressive disease that models end-stage drug-resistant MM and responds only to combinations of drugs with single-agent activity in untreated Vk*MYC MM. We predict that combinations of standard agents, histone deacetylase inhibitors, bromodomain inhibitors, and hypoxia-activated prodrugs will demonstrate efficacy in the treatment of relapsed MM.
Introduction
For decades, conventional therapy for multiple myeloma (MM) used alkylating agents and glucocorticoids.1 Recently, an unprecedented collaboration between the pharmaceutical industry and academia has produced an explosion of investigational drugs with promising preclinical activity in human myeloma cell line (HMCL) models (documented in > 250 publications). Two novel classes of compounds, immunomodulatory drugs (IMiDs) and proteasome inhibitors, are now part of the standard therapy.2 Despite this outstanding success, the majority of investigational drugs have failed in the clinic, and only 5% of all anticancer drugs entering clinical trials receive regulatory approval, highlighting the pressing, general need for more predictive preclinical models.3,4 With very few exceptions (eg, thalidomide), all of the drugs evaluated in clinical trials in MM over the last half-century are active in cell line and xenograft models; however, only 4 classes of drugs with single-agent activity in patients with MM (glucocorticoids, alkylating agents, IMiDs, and proteasome inhibitors) have been identified. Therefore, the positive predictive value (PPV) of these models is low. As one example, single-agent vincristine is essentially inactive in MM patients,5 despite being among the most potent inducers of cell death in HMCLs in vitro (see Figure 1A and Table 1). This highlights a major drawback of drug assays that measure growth inhibition of cell lines in vitro or in vivo, which have the tendency of overestimating the antitumor activity of compounds that inhibit proliferation of actively cycling cells, but are likely ineffective in targeting the bulk of an indolent tumor in its native microenvironment (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).6 Notably, in the majority of MM patients at diagnosis, < 1% of MM cells are actively proliferating, and those few patients in which > 3% are proliferating have a very poor prognosis.7 To identify drugs that induce responses in patients with indolent BM-localized MM, it is intuitive to select drugs that induce responses in mice with indolent BM-localized MM.
In solid tumors, recent work using a KRAS-dependent genetically engineered mouse model (GEMM) has highlighted the ability of such models to predict clinical activity in lung cancer.8 In MM, the immunocompetent Vk*MYC GEMM has already demonstrated high biologic fidelity to the human disease, suggesting it is an ideal model to study the behavior of MM cells in the context of a native microenvironment and immune system.9 Rearrangements of the MYC locus are the most common genetic abnormality in human MM, present in more than one-half of advanced MM tumors, and almost all HMCLs.10 Activation of MYC is associated with the progression of monoclonal gammopathy to MM.9 This progression event is modeled when the Vk*MYC transgene is introduced into a strain of mice (C57Bl/6) that spontaneously develops a high rate of monoclonal gammopathy, resulting in mice that develop a high rate of MM.9 Vk*MYC MM cells are dependent on the BM microenvironment, have a very low proliferative index, and consistently do not grow in vitro. Furthermore, as in human patients, MM cells in Vk*MYC mice secrete a very high level of serum monoclonal Ig, resulting in a M-spike that is easily detected by serum protein electrophoresis (SPEP) and represents a clonal marker of tumor burden.9 To determine whether this biologic fidelity translates into clinical predictability, we report here studies of 37 preclinical and clinical therapeutics administered alone, and in combinations. These data establish a predictive blueprint of drug activity in MM, and provide a new paradigm for prioritizing therapeutic strategies guiding human clinical investigation.
Methods
Mice
All experiments were performed under approval of The Mayo Foundation Institutional Animal Care and Use Committee or the Peter MacCallum Cancer Center Animal Experimentation Ethics Committee, and conformed to all the regulatory Environmental Safety standards. The generation and initial characterization of the Vk*MYC mice has been reported elsewhere.9 The mice uniformly develop a monoclonal gammopathy starting at 30 weeks of age that progresses slowly over time associated with anemia, osteoporosis, and renal disease.
SPEP
Mice were periodically bled by tail grazing. Blood was collected into Microtainer tubes (BD Biosciences), allowed to coagulate at room temperature, and spun for 10 minutes at 2300g. Sera were diluted 1:2 in normal saline buffer and analyzed on a QuickGel Chamber apparatus using precasted QuickGels (Helena Laboratories) according to the manufacturer's instruction. Densitometric analysis was then performed using the clinically certified Helena QuickScan 2000 workstation which allows a precise quantization of the various serum fractions, including the measurements of gamma/albumin ratio.
Drug studies
Drug effects on cell proliferation in HMCLs were measured by the CellTiter 96 Aqueous One Solution Cell Proliferation Assay (MTS; Promega), following manufacturer's instructions. For drug studies in mice, we chose for each drug at least 3 aged (> 1-year old) Vk*MYC mice with a densitometrically measured gamma/albumin ratio between 0.3 and 2.0 (median 0.5, SD 0.35; corresponding to a predominant M-spike between ∼ 10 and 70 g/L). Occasionally, we included mice with 2 or 3 M-spikes, which we have previously determined represent independent tumor clones, and we reported the response for each spike separately. For drug dosage and route of administration, we followed methods previously shown to be successful in xenograft studies (supplemental Table 1). SPEP at day 0 and day 14 after treatment was performed to measure reduction in the M-spike as a marker of tumor response, as done clinically (Figure 1A). Specifically, the gamma/albumin ratio posttreatment for each individual M-spike is normalized by the gamma/albumin ratio obtained at day 0. The presence of polyclonal Ig that comigrate with the M-spike on SPEP limits the ability to reliably identify a > 90% reduction in M-spike (corresponding clinically to very good partial response). Given the short duration of treatment, we did not use immunofixation or otherwise attempt to document complete response. In the absence of a response to the published effective in vivo dose, drug dosage was increased until clear signs of toxicity or weight loss were observed.
Flow cytometric and cell-cycle analysis
Mice were euthanized by CO2 inhalation. Lymphoid tissues were harvested, and a single-cell suspension was obtained by mechanical disruption between frosted glass slides. BM cells were collected by flushing out ilium, femur, tibia, humerus, and radius with PBS. After ACK lysis, on average, > 100 million BM nucleated cells were obtained from each mouse. Flow cytometric analysis, as previously described, allowed assessment of percentage of MM cells (B220-CD138+) in each tissue.9 B220 (RA3-6B2) and CD138 (281-2) Abs were from BD Pharmingen. Data were acquired on a 3-laser CyanADP (DakoCytomation) instrument and analyzed with FlowJo software (TreeStar). For cell-cycle analysis, plasma cells (PCs) were first labeled with anti-CD138 Ab, fixed in ethanol, and stained with 4',6-diamino-2-phenylindole (DAPI; Molecular Probes). DNA content and cell-cycle distribution were calculated using the Watson mathematical model in FlowJo software.
Transplantations
Transplant donors were selected from Vk*MYC mice with an M-spike ∼ 10-70 g/L and a BM plasmacytosis of > 5%. For initial transplantation experiments described in Figure 2 and supplemental Figure 2, total BM cells were flushed out in PBS from a tibia of donor Vk*MYC mouse and immediately injected (∼ 1 million cells/mouse) intracardially into 5- to 12-week-old congenic C57BL/6 wild-type recipient mice, either sublethally irradiated with 6 Gy or not, or into RAG1-2 null mice in C57BL/6 background. Irradiated recipient were provided with acidified water. A cohort of irradiated mice were also fed antibiotic water (Neomycin/polymixin) and Ensure (Abbott Australasia) for up to 1 month after transplantation. Recipient mice were bled every 2 weeks beginning 3 weeks after transplantation for SPEP analysis, and their time to engraftment (TTE), or time to first appearance of the M-spike, was calculated by Kaplan-Meier survival curve and log-rank test. For subsequent experiments, ∼ 2.5-5 × 105 BM or splenic cells from donor mice with MM were injected into irradiated or not C57BL/6 mice via the tail vein or intracardiac injection.
Statistics
To calculate positive and negative predictive values, response was defined as a > 50% reduction in the M-spike, as done clinically, with response rates of > 20% in clinical trials or the murine studies considered significant. Comparison of M-spike reduction with different drug treatments was done by 1-way ANOVA with Dunnett posttest versus vehicle/placebo performed using GraphPad Prism (GraphPad Software).
IHC
Four-millimeter sections from formalin fixed, paraffin embedded, and decalcified (for bone tissues only) tissue blocks were deparaffinized in xylene and boiled in 1mM EDTA pH = 8 prior to staining with goat anti-Ki67 (sc-7846; Santa Cruz Biotechnology), followed by alkaline phosphatase–conjugated secondary isotype-specific Abs (Jackson Immunoresearch Laboratories) and developed with nitroblue tetrazolium-5-bromo-4-chloro-3-indolyl phosphate (NBT-BCIP; Roche). Rat anti-CD138 (281-2; BD Pharmingen) and HRP-conjugated secondary Abs were then applied, followed by 3-3'-diamine benzidine (DAB; DAKO) detection, as previously published.9 Images were acquired on an Olympus BX41 microscope equipped with an Olympus QColor 3 digital camera and QCapture software (QImaging). For assessment of histone H3 acetylation, sections were deparaffinized, incubated with rabbit anti–mouse histone H3 (Millipore) primary Ab, followed by incubation with anti–rabbit HRP-conjugated secondary Abs and subsequent detection using DAB (Olympus BX51, 40× magnification, room temperature, SPOT RT3, SPOT Basic software). In situ apoptosis was assessed by TUNEL staining of deparaffanized sections according to kit instructions (S7100, Chemicon International, Kilsyth, Victoria). Briefly, sections were incubated in proteinase K (20 μg/mL) for 15 minutes, washed, and then incubated in H2O2 (3%) for 5 minutes. Sections were then incubated in equilibration buffer (75 μL/5 cm2) for 10 seconds at room temperature, blotted dry, then incubated with working strength TdT enzyme (55 μL/5 cm2) for 1 hour at 37°C, and stopped with working strength stop buffer for 10 minutes at room temperature. After being washed 3 times in PBS, sections were incubated with antidigoxigenin conjugate (65 μL/5 cm2) for 30 minutes at room temperature in humid chamber. Sections were washed, developed using DAB (75 μL/5 cm2), washed, counterstained, and imaged with an Olympus BX51 microscope.
Hemoglobin and creatinine estimation
Venous blood samples were freshly obtained by retro-orbital sampling in EDTA-anticoagulant and hemoglobin estimates performed using the Advia 120 automated hematology system (Siemens Healthcare Diagnostics). Seventy microliters of plasma were separated by centrifugation from the same samples and analyzed for creatinine concentration using the Advia 1200 automated biochemistry system (Siemens Healthcare Diagnostics).
Micro-CT analysis
Tibias obtained from tumor-burdened and non–tumor-bearing control mice were fixed for 24 hours in 2% paraformaldehyde solution and then stored in 70% ethanol before imaging. Imaging was performed at St Vincents Institute (Melbourne, Australia) on the SkyScan1076 in vivo micro-computed tomography (micro-CT; x-ray potential 50 peak kilovoltage [KVp]; Kontich). Image acquisition and estimation of trabecular bone volume was performed as previously described.11
Transmission electron microscopy
Kidneys were dissected from tumor-burdened and non–tumor-bearing control mice. Tissue was fixed in 2% paraformaldehyde, 2.5% glutaraldehyde in 0.08M Sorensen phosphate buffer (PBS) for 2 hours, then washed in 3 × 10 minutes changes of PBS. Postfixation was with 2% osmium tetroxide in PBS followed by dehydration through a graded series of alcohols, 2 acetone rinses, and embedding in Spurrs resin.12 Sections ∼ 80-nm thick were cut with a diamond knife (Diatome) in an Ultracut-S ultramicrotome (Leica) and contrasted with uranyl acetate and lead citrate. Images were captured with a Megaview II cooled CCD camera (Soft Imaging Solutions; Olympus) in a JEOL 1011 transmission electron microscope (TEM). Images are representative of at least 3 cortical glomeruli per specimen.
Results
Drug response in Vk*MYC MM reflects single-agent drug activity in human MM
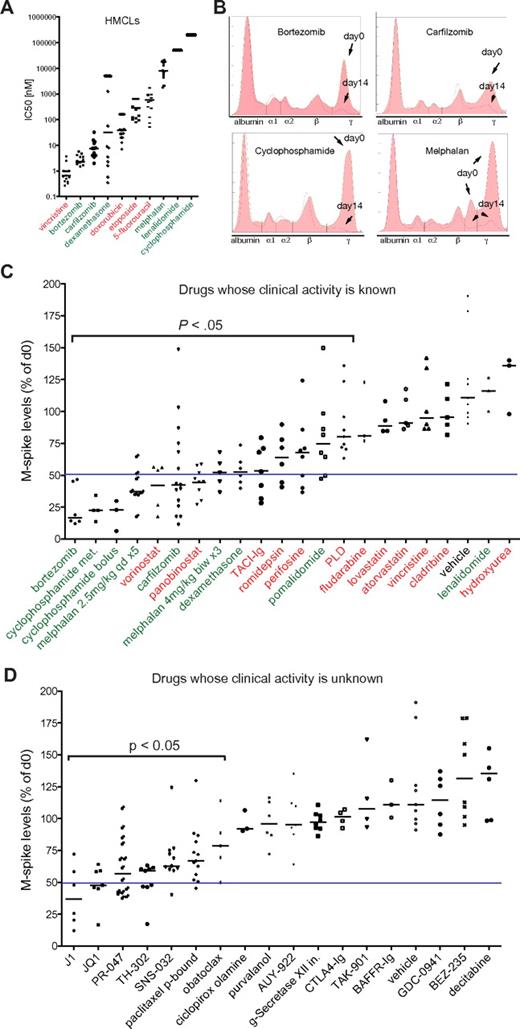
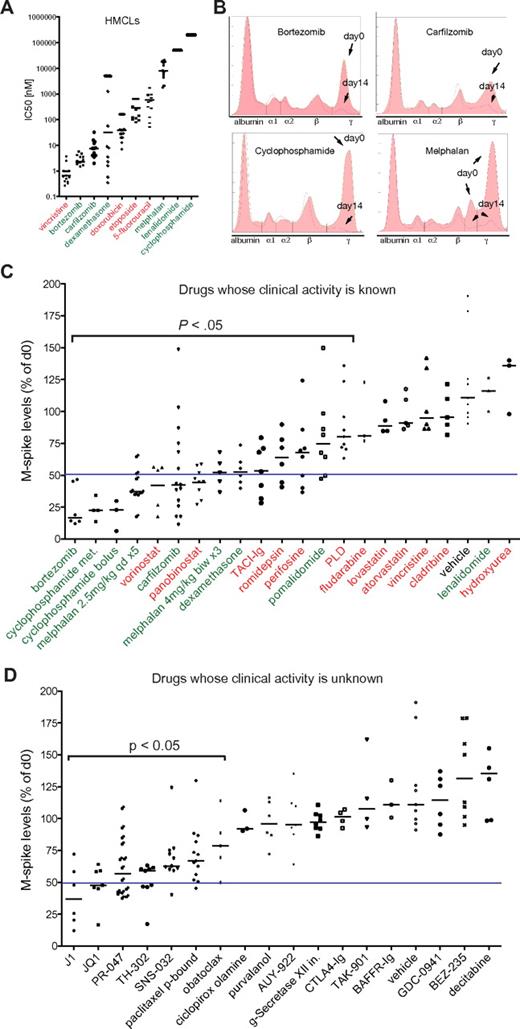
To assess the clinical predictive value of drug response in Vk*MYC mice, we selected aged (> 1 year old) mice with variable levels of M-spikes of at least 10 g/L to mimic the heterogeneity observed in patients, and measured the reduction in the M-spike by SPEP after 2 weeks of treatment (Figure 1B) with a series of drugs that have been characterized for their single-agent activity in MM patients (refer to supplemental Table 1 for references, dose, schedule, route, preclinical, and clinical activity of all the drugs tested in Vk*MYC mice). In all cases, we used well-established dosage and schedule of drugs that had been shown to be effective in xenograft models of MM, and we focused on response rate defined as a > 50% reduction in the M-spike. Among agents that have been evaluated in clinical trials, we identified 6 classes with activity in this model (alkylating agents, glucocorticoids, proteasome inhibitors, IMiDs, histone deacetylase inhibitors [HDACi] and TACI-Ig) although only the first 4 demonstrated clinical activity in MM patients with single-agent response rates > 20%, for a positive predictive value of 67%. Some responses were also seen to perifosine, which interacts with membrane phospholipids affecting a broad spectrum of pathways and that we were not able to assign to a specific drug class. More importantly, among 7 classes of drugs with documented preclinical activity in other MM models, but inactive in Vk*MYC MM (vincristine, hydroxyurea, fludarabine, cladribine, pegylated liposomal doxorubicin, statins, lenalidomide) only the latter has a response rate > 15% as a single agent in MM patients for a negative predictive value of 86% (Figure 1C-D, Table 1, supplemental Table 1). The response to IMiDs was modest in this model. Thalidomide has been shown to require a human liver to metabolize the drug into a compound with activity against MM.13 It did not show activity in the Vk*MYC model (data not shown). Although lenalidomide has been reported to have cytostatic anti-MM activity at high doses in vitro (Figure 1A), and to show only very modest prolongation of survival in xenograft models of MM,14,15 it also did not produce any responses in the Vk*MYC model. Only the more potent pomalidomide, administered at superphysiologic doses, showed partial responses (Figure 1C), consistent with a species-specific pharmacologic effect of the IMiDs.
Assessment of drug response in Vk*MYC MM. (A) IC50 (nM) of the indicated drugs obtained by MTT assay in various HMCLs. Each dot represents a cell line, and bars show median values plotted on a log10 scale. In green or red are labeled drugs with single-agent response rates in MM clinical trials above or below 20%, respectively. (B) SPEP and densitometric profile of Vk*MYC mice treated with known active antimyeloma drugs. The day 0 (filled) and day 14 (pink line) traces have been overlaid. The various serum fractions on the densitometry plot are labeled. Arrows point to M-spikes before (day 0) and after (day 14) treatment. (C) The response of Vk*MYC mice to drugs with known (green, red) or (D) unknown (black) activity. Drug activity is quantified by measuring M-spike levels at day 14 after treatment normalized to day 0. Each dot represents an individual M-spike. Bars show median M-spike levels at day 14 normalized to day 0. A blue line in each dot plot arbitrarily separates active drugs (> 50% M-spike reduction, on the left) from inactive ones (< 50% M-spike reduction, on the right). The P value of active drugs compared with vehicle is shown. PLD indicates pegylated liposomal doxorubicin.
Assessment of drug response in Vk*MYC MM. (A) IC50 (nM) of the indicated drugs obtained by MTT assay in various HMCLs. Each dot represents a cell line, and bars show median values plotted on a log10 scale. In green or red are labeled drugs with single-agent response rates in MM clinical trials above or below 20%, respectively. (B) SPEP and densitometric profile of Vk*MYC mice treated with known active antimyeloma drugs. The day 0 (filled) and day 14 (pink line) traces have been overlaid. The various serum fractions on the densitometry plot are labeled. Arrows point to M-spikes before (day 0) and after (day 14) treatment. (C) The response of Vk*MYC mice to drugs with known (green, red) or (D) unknown (black) activity. Drug activity is quantified by measuring M-spike levels at day 14 after treatment normalized to day 0. Each dot represents an individual M-spike. Bars show median M-spike levels at day 14 normalized to day 0. A blue line in each dot plot arbitrarily separates active drugs (> 50% M-spike reduction, on the left) from inactive ones (< 50% M-spike reduction, on the right). The P value of active drugs compared with vehicle is shown. PLD indicates pegylated liposomal doxorubicin.
We found promising activity of the pan-HDACi panobinostat and vorinostat, and of the class I–selective HDACi romidepsin. Responses were also seen with TACI-Ig, which is a decoy receptor for BAFF and APRIL, but not with BAFFR-Ig, consistent with the APRIL-BCMA axis being crucial for plasma cell (PC) survival.16 Interestingly, although none of these drugs induced responses as single agents in clinical trials of MM patients relapsed and/or refractory to alkylating agents, glucocorticoids, proteasome inhibitors, and IMiDs, there was nonetheless some evidence of clinical efficacy in this advanced patient population (stable disease and minor responses).17 Together with elegant preclinical data indicating synergistic activity of HDACi combined with bortezomib,18 this provided sufficient encouragement for the initiation of 2 large, international phase 3 randomized clinical trials exploring this combination in patients with relapsed/refractory MM. Preliminary data from one of these trials have reported an increased response rate (56% vs 41%, P < .0001) and progression-free survival (7.6 months vs 6.8 months, P < .01) for the combination of vorinostat and bortezomib compared with bortezomib alone.19
Using the Vk*MYC model to predict the clinical efficacy of experimental drugs in MM
Encouraged by the therapeutic fidelity of the Vk*MYC model, we evaluated the antitumor response in Vk*MYC mice of drugs currently in phase 1-3 clinical trials in MM as well as in early stages of development (Figure 1D). As expected, the melphalan prodrug J1 (activated by cellular peptidases), and the oral proteasome inhibitor PR-047 showed robust anti-MM activity. We found remarkable activity of the bromodomain inhibitor JQ1 that inhibits MYC transcriptional activity presumably by inhibiting the recruitment of the transcriptional elongation complex pTEFb (composed of CDK9 and cyclin T1).20 SNS-032, an inhibitor of CDK2, CDK7, and CDK9 also shows partial activity, which together with JQ1 suggests a role for CDK9 inhibition in the treatment of MM. The hypoxia-activated alkylator prodrug TH-302 demonstrated significant activity, consistent with the hypoxic condition of MM cells in their native microenvironment.21 Protein-bound paclitaxel was also active, consistent with a reported 15% to 34% response rate in untreated MM to single-agent paclitaxel.22 The remaining agents tested, belonging to a wide variety of classes, did not have marked activity in the Vk*MYC mice, despite demonstrated activity in other MM preclinical models (Table 1, supplemental Table 1).
Transplanted Vk*MYC MM provides a model of relapsed refractory MM
To perform larger, controlled studies on genetically homogeneous tumors, we transplanted the total BM from one tibia of 118 individual Vk*MYC mice with MM into 5 recipient wild-type mice by intracardiac injection. The median TTE of the tumor (or first appearance of the M-spike) was 26 weeks after transplantation in 269 recipients pretreated with sublethal (6 Gy) irradiation, compared with 44 weeks in 161 nonirradiated recipients, whereas the TTE into RAG1/2 null mice was somewhere in between (supplemental Figure 2). Eleven Vk*MYC MM (10%) did not engraft.
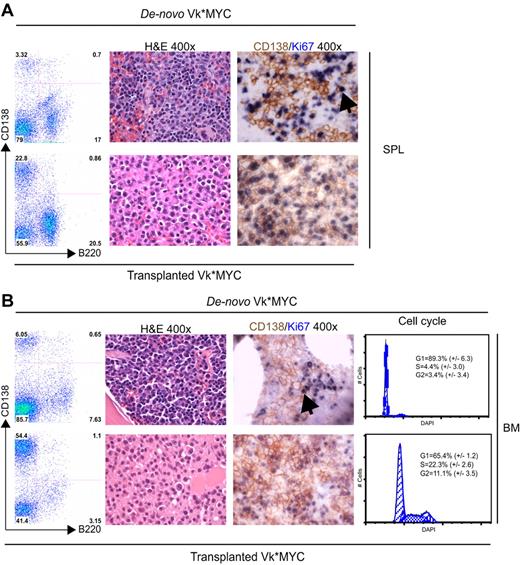
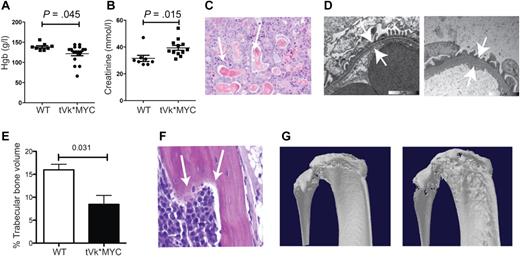
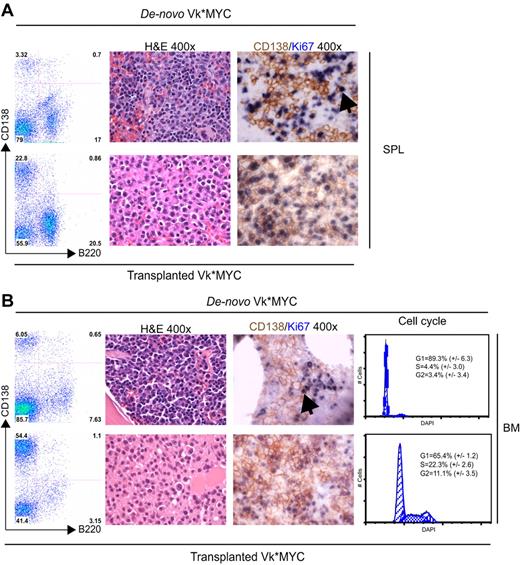
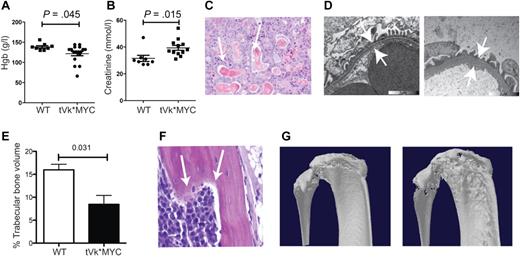
After transplantation, we observed a substantially increased tumor burden both in the BM and in extramedullary sites (spleen, lymph nodes, and thymus, that appear grossly enlarged; Figure 2A-B). There was a corresponding increase in proliferation, CD138/ki67 double staining identified frequent double-positive, proliferating PCs in the transplanted but not de novo, Vk*MYC mice. Consistently, cell-cycle analysis identified 33% cycling PCs in the transplanted but only 7% in de novo Vk*MYC mice (Figure 2B left panel). Typical of human MM, transplanted Vk*MYC (tVk*MYC) mice developed anemia, renal impairment (elevated creatinine, tubular cast deposition, diffuse thickening of the glomerular basement membrane demonstrable on TEM, heavy clonal proteinuria (Figure 3A-D), and bone destruction by micro-CT (Figure 3E-G).
Comparison of de novo with transplanted Vk*MYC mice. (Left panel) A flow cytometric analysis of SPL (A) and BM (B) from a matching de novo Vk*MYC and transplant recipient mouse highlighting higher tumor burden and extramedullary MM in tVk*MYC mice. (Middle panel) Immunostaining of (A) SPL and (B) BM sections from de novo and tVk*MYC mice identify a high fraction of proliferating (Ki67+, blue) MM (CD138+, brown) cells in tVk*MYC mice only. Arrows highlight the rare double-positive cells in the de novo Vk*MYC mice. (B left panel) Cell-cycle analysis on CD138+ cells from BM of de novo and tVk*MYC mice. Values (%) ± SD from 3 independent experiments are given.
Comparison of de novo with transplanted Vk*MYC mice. (Left panel) A flow cytometric analysis of SPL (A) and BM (B) from a matching de novo Vk*MYC and transplant recipient mouse highlighting higher tumor burden and extramedullary MM in tVk*MYC mice. (Middle panel) Immunostaining of (A) SPL and (B) BM sections from de novo and tVk*MYC mice identify a high fraction of proliferating (Ki67+, blue) MM (CD138+, brown) cells in tVk*MYC mice only. Arrows highlight the rare double-positive cells in the de novo Vk*MYC mice. (B left panel) Cell-cycle analysis on CD138+ cells from BM of de novo and tVk*MYC mice. Values (%) ± SD from 3 independent experiments are given.
Characterization of transplanted Vk*MYC mice. (A) Peripheral blood hemoglobin and (B) plasma creatinine levels from tVk*MYC mice compared with nontumor-bearing controls. (C) Renal cast nephropathy, typical of autopsy findings from terminal mice bearing aggressive tVk*MYC MM. (D) Transmission electron microscopy of the glomerular basement membrane (GBM) from a normal C57BL/6 mouse compared at the same magnification to GBM from a tumor-bearing mouse. Note diffuse GBM thickening indicating glomerulopathy. (E) Tibial trabecular bone volume of tVk*MYC myeloma-bearing mice (n = 3) compared with irradiated, age-matched wt controls (n = 3). (F) Histologic evidence of bone lysis in the femoral shaft of a tVk*MYC mouse. (G) Representative micro-CT scans demonstrating cortical lytic lesions in the tibial plateau and fibula from mice bearing the same clone.
Characterization of transplanted Vk*MYC mice. (A) Peripheral blood hemoglobin and (B) plasma creatinine levels from tVk*MYC mice compared with nontumor-bearing controls. (C) Renal cast nephropathy, typical of autopsy findings from terminal mice bearing aggressive tVk*MYC MM. (D) Transmission electron microscopy of the glomerular basement membrane (GBM) from a normal C57BL/6 mouse compared at the same magnification to GBM from a tumor-bearing mouse. Note diffuse GBM thickening indicating glomerulopathy. (E) Tibial trabecular bone volume of tVk*MYC myeloma-bearing mice (n = 3) compared with irradiated, age-matched wt controls (n = 3). (F) Histologic evidence of bone lysis in the femoral shaft of a tVk*MYC mouse. (G) Representative micro-CT scans demonstrating cortical lytic lesions in the tibial plateau and fibula from mice bearing the same clone.
tVk*MYC MM as a model for single-agent and combination drug treatment
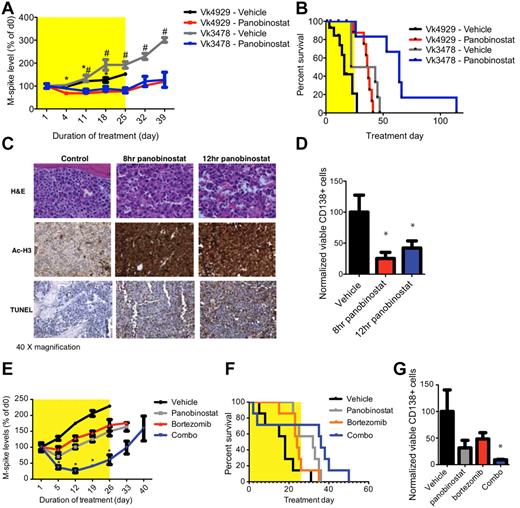
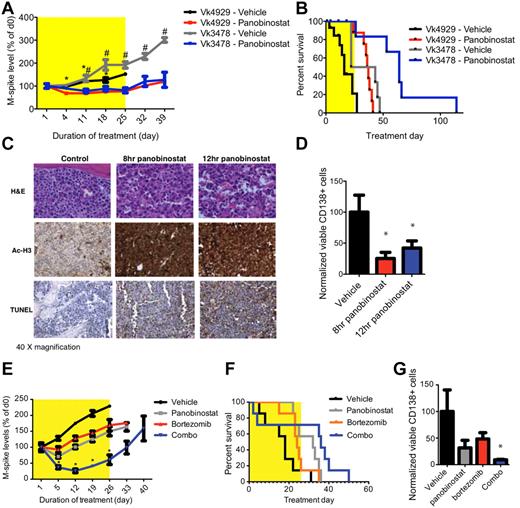
Based on the demonstrated anti-MM effects of single-agent panobinostat and bortezomib in Vk*MYC mice, we sought to investigate their therapeutic effects in the tVk*MYC model. While the paraprotein level increased steadily in tVk*MYC mice receiving vehicle, it remained stable and significantly lower during treatment with panobinostat (25 mg/kg for 4 days followed by 15 mg/kg for 3 weeks; P < .05, Figure 4A). Moreover, survival of tVk*MYC mice was significantly greater in those treated with panobinostat than those receiving vehicle (increased by 20.5/31.5 days, Figure 4B). Immediate and dramatic induction of apoptosis in panobinostat-treated mice was associated with histone H3 acetylation and loss of viable CD138+ PCs (Figure 4C-D). We further investigated combination therapy of panobinostat with bortezomib in tVk*MYC MM. Single-agent panobinostat (20 mg/kg, days 1-2, reduced to 10 mg/kg onward to reduce toxicity) or bortezomib appeared to slow the increase in paraprotein levels (Figure 4E), and only minor responses were observed. The combination of both agents synergistically reduced paraprotein levels and increased survival (Figure 4F). Moreover, FACS analysis of BM from combination treated mice showed a significantly reduced proportion of viable CD138+ PCs than untreated controls (Figures 4G, P < 0.05).
Drug activity in tVk*MYC mice. (A) C57BL/6 mice bearing transplanted Vk*MYC tumor (Vk4929 or -3478) were treated with panobinostat alone (4929, n = 16; 3478, n = 6; 25 mg/kg for 4 days followed by 15 mg/kg for 3 weeks) or vehicle control (4929, n = 15; 3478, n = 6; D5W, dextrose water) for a total period of 4 weeks. Serum paraprotein was assessed on day 1 and then weekly for 6 weeks and presented as mean change from levels on day 0 (mean ± SEM). *P < .05 vs Vk4929 vehicle treated. #P < .05 vs Vk3478 vehicle treated. (B) Survival of Vk4929 or -3478 mice treated with panobinostat alone or vehicle (D5W). Median survival of mice treated with panobinostat alone was 36.5 days and 64 days compared with vehicle control-treated mice of 16 days and 32.5 days in Vk4929 or -3478 mice, respectively (P < .05). (C) H&E, acetylated histone H3 and TUNEL-stained BM sections from tVk*MYC 4929 tumor after 8 hours and 12 hours treatment with panobinostat (25 mg/kg) compared with vehicle control (D5W). (D) FACS analysis of viable CD138+/B220− PCs in the BM of Vk4929 mice after 8 hours and 12 hours treatment with panobinostat (25 mg/kg) compared with vehicle control (D5W). Values are normalized to the percentage of PCs in vehicle control treated BM (100%). *P < .05. (E) Vk4929 mice were treated with panobinostat (20 mg/kg, days 1-2, reduced to 10 mg/kg onwards to reduce toxicity, n = 7), bortezomib (0.5 mg/kg, twice weekly, n = 7), the combination of both agents (n = 7) or vehicle control (D5W, n = 7) for 4 weeks. Serum paraprotein was assessed on day 1 and then weekly for 6 weeks and presented as mean change from levels on day 0 (mean ± SEM). *P < .05 vs vehicle control. (F) Survival of Vk4929 mice treated with panobinostat, bortezomib, the combination of both agents and vehicle control (D5W). Median survival of mice were as follows: panobinostat alone 32 days; bortezomib alone 24 days; the combination of both agents 36 days; vehicle control-treated mice 18 days. (G) FACS analysis of viable CD138+/B220− PCs in the BM of Vk4929 mice after 5 days of treatment with panobinostat (10 mg/kg daily), bortezomib (0.5 mg/kg, days 1 and 4), the combination of both agents or vehicle control (D5W). Values are normalized to the percentage of PCs in vehicle control treated BM (100%). *P < .05 vs vehicle control.
Drug activity in tVk*MYC mice. (A) C57BL/6 mice bearing transplanted Vk*MYC tumor (Vk4929 or -3478) were treated with panobinostat alone (4929, n = 16; 3478, n = 6; 25 mg/kg for 4 days followed by 15 mg/kg for 3 weeks) or vehicle control (4929, n = 15; 3478, n = 6; D5W, dextrose water) for a total period of 4 weeks. Serum paraprotein was assessed on day 1 and then weekly for 6 weeks and presented as mean change from levels on day 0 (mean ± SEM). *P < .05 vs Vk4929 vehicle treated. #P < .05 vs Vk3478 vehicle treated. (B) Survival of Vk4929 or -3478 mice treated with panobinostat alone or vehicle (D5W). Median survival of mice treated with panobinostat alone was 36.5 days and 64 days compared with vehicle control-treated mice of 16 days and 32.5 days in Vk4929 or -3478 mice, respectively (P < .05). (C) H&E, acetylated histone H3 and TUNEL-stained BM sections from tVk*MYC 4929 tumor after 8 hours and 12 hours treatment with panobinostat (25 mg/kg) compared with vehicle control (D5W). (D) FACS analysis of viable CD138+/B220− PCs in the BM of Vk4929 mice after 8 hours and 12 hours treatment with panobinostat (25 mg/kg) compared with vehicle control (D5W). Values are normalized to the percentage of PCs in vehicle control treated BM (100%). *P < .05. (E) Vk4929 mice were treated with panobinostat (20 mg/kg, days 1-2, reduced to 10 mg/kg onwards to reduce toxicity, n = 7), bortezomib (0.5 mg/kg, twice weekly, n = 7), the combination of both agents (n = 7) or vehicle control (D5W, n = 7) for 4 weeks. Serum paraprotein was assessed on day 1 and then weekly for 6 weeks and presented as mean change from levels on day 0 (mean ± SEM). *P < .05 vs vehicle control. (F) Survival of Vk4929 mice treated with panobinostat, bortezomib, the combination of both agents and vehicle control (D5W). Median survival of mice were as follows: panobinostat alone 32 days; bortezomib alone 24 days; the combination of both agents 36 days; vehicle control-treated mice 18 days. (G) FACS analysis of viable CD138+/B220− PCs in the BM of Vk4929 mice after 5 days of treatment with panobinostat (10 mg/kg daily), bortezomib (0.5 mg/kg, days 1 and 4), the combination of both agents or vehicle control (D5W). Values are normalized to the percentage of PCs in vehicle control treated BM (100%). *P < .05 vs vehicle control.
Induction of bortezomib-resistant Vk*MYC MM with suboptimal treatment
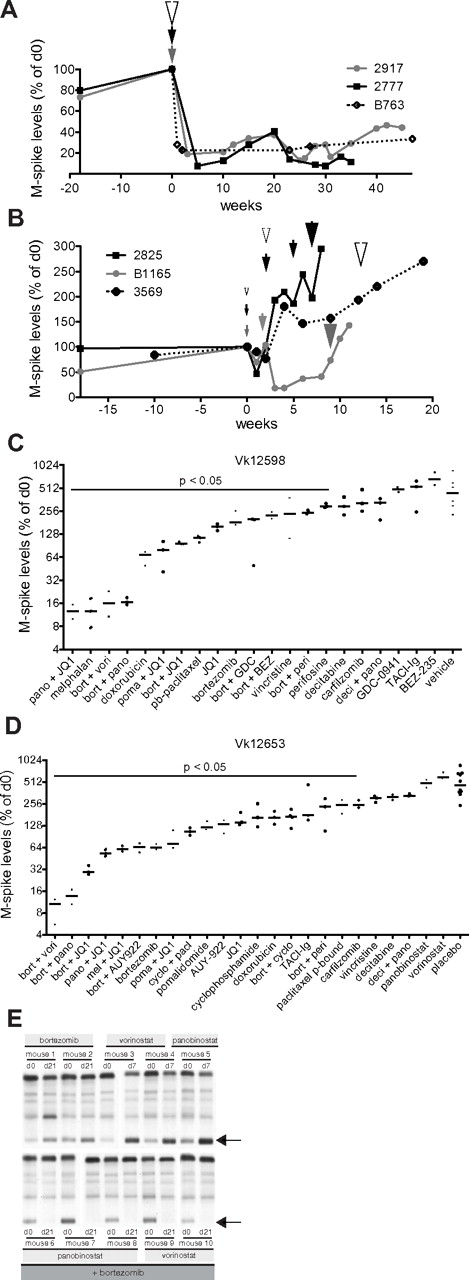
The development of bortezomib resistance in patients with MM is clinically challenging. Therefore, we studied the induction of bortezomib resistance in vivo using Vk*MYC MM. When we treated Vk*MYC mice with full-dose bortezomib (1.0 mg/kg, days 1, 4, 8, 11), we observed complete response (CR) that lasted from 30 weeks to > 60 weeks. Using a slightly lower dose (0.5 mg/kg), we continued to observe CR; however, the time to progression (TTP) was reduced to 10 weeks. On serial retreatment, the mice achieved CR each time; however, the TTP became progressively shorter (Figure 5A). Interestingly, a completely different disease course was observed after suboptimal treatment (0.15 mg/kg), which induced only a partial response (PR), promptly followed by an accelerated disease course associated with resistance to full-dose bortezomib (1 mg/kg; Figure 5B). On necropsy, bortezomib refractory MM cells were always identified in multiple lymphoid organs, highlighting their aggressive extramedullary nature (supplemental Figure 3). This dramatic result argues strongly for increasing the interval between treatments rather than decreasing the dosage of each treatment when attempting to alleviate the toxicity associated with bortezomib, which is more in line with the more recent practice of weekly administration.23
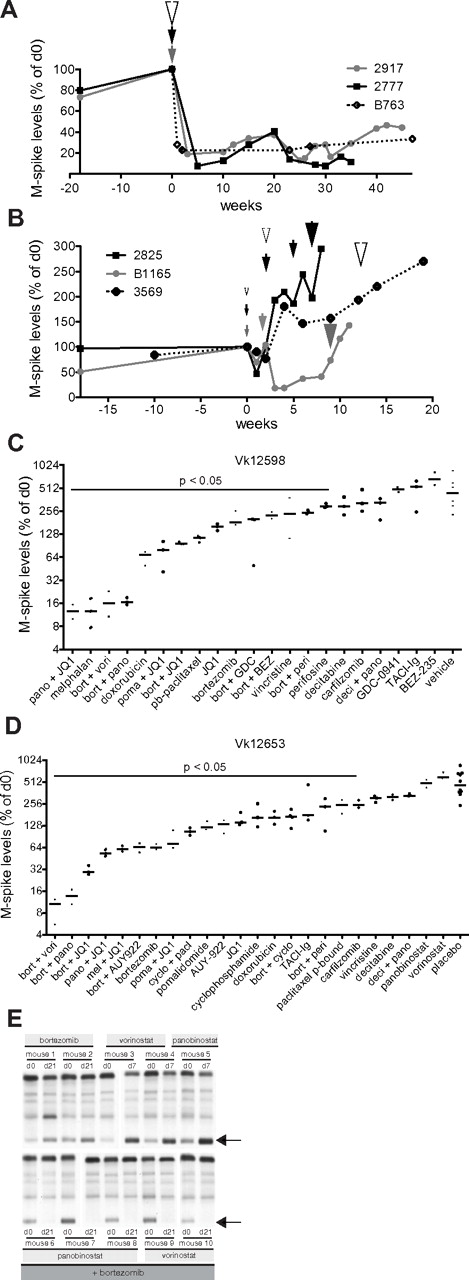
Establishment and characterization of bortezomib-resistant Vk*MYC tumors. (A) M-spike levels (normalized to day 0) of 2 mice (2917 and 2777) treated with bortezomib at 0.5 mg/kg (gray and black lines) or 1 mouse (B763) at 1 mg/kg (dotted line) for 2 weeks (1 cycle). Arrowheads indicate the time of administration of 1 cycle of therapy, their color refers to the treated mouse and their size is proportional to the drug dosage (medium = 0.5 mg/kg, large = 1 mg/kg). (B) M-spike levels (normalized to day 0) of 3 mice (2825, B1165, and 3569) treated with continuous escalating doses of bortezomib. Arrowheads and their size indicate treatment: small = 0.16 mg/kg, medium = 0.25 mg/kg, large = 0.5 mg/kg, and their color refers to the treated mouse. (C-D) Response to single or combination treatment of 2 independent bortezomib-resistant Vk*MYC tumors (Vk12598 and Vk12653) propagated by serial transplantation into congenic wild-type (wt) mice. M-spike levels at day 14 normalized to day 0 are shown in log2 scale. Each dot represents a mouse. Bars show median day 14 M-spike levels normalized to day 0. The P value of active drugs compared with vehicle is shown. (E) SPEP performed on the Vk12598 line of bortezomib-resistant mice shows effective bortezomib + HDACi combination treatment but ineffective single-agent activity of these 2 drug. Arrows point to M-spikes.
Establishment and characterization of bortezomib-resistant Vk*MYC tumors. (A) M-spike levels (normalized to day 0) of 2 mice (2917 and 2777) treated with bortezomib at 0.5 mg/kg (gray and black lines) or 1 mouse (B763) at 1 mg/kg (dotted line) for 2 weeks (1 cycle). Arrowheads indicate the time of administration of 1 cycle of therapy, their color refers to the treated mouse and their size is proportional to the drug dosage (medium = 0.5 mg/kg, large = 1 mg/kg). (B) M-spike levels (normalized to day 0) of 3 mice (2825, B1165, and 3569) treated with continuous escalating doses of bortezomib. Arrowheads and their size indicate treatment: small = 0.16 mg/kg, medium = 0.25 mg/kg, large = 0.5 mg/kg, and their color refers to the treated mouse. (C-D) Response to single or combination treatment of 2 independent bortezomib-resistant Vk*MYC tumors (Vk12598 and Vk12653) propagated by serial transplantation into congenic wild-type (wt) mice. M-spike levels at day 14 normalized to day 0 are shown in log2 scale. Each dot represents a mouse. Bars show median day 14 M-spike levels normalized to day 0. The P value of active drugs compared with vehicle is shown. (E) SPEP performed on the Vk12598 line of bortezomib-resistant mice shows effective bortezomib + HDACi combination treatment but ineffective single-agent activity of these 2 drug. Arrows point to M-spikes.
Novel combinations overcome bortezomib resistance
We sought to use bortezomib-resistant tumors that were induced in vivo in the Vk*MYC mice to identify drug treatments that would be effective in a bortezomib refractory setting. Bortezomib-resistant tumors generated in the Vk*MYC mice were harvested and passaged serially in mice. Two lines of tumor were generated (Vk12598 and Vk12653) and used for drug testing. These tumors were very aggressive with a TTE of 4 weeks after transplantation, although they were unable to propagate in vitro. With the exception of a minor response to doxorubicin, and CR to melphalan in one line (Vk12598, Figure 5C), there was no other single-agent activity detected. Inducing CR in Vk12598 therefore represents a remarkably stringent model for single-agent activity with a PPV of 100%. In both lines, however, dramatic CRs were observed after treatment for only 2 weeks with a combination of bortezomib and HDACi (Figure 5D-E) that was sustained for 20-70 weeks (data not shown). Similarly, remarkable activity was seen with combinations of the bromodomain inhibitor JQ1 with bortezomib and JQ1 with panobinostat, but not with any other bortezomib or panobinostat combination examined (Figure 5C-E). Several drugs and combinations did not induce response, but showed a statistically significant smaller increase in the M-spike compared with vehicle/placebo-treated mice. Presumably, this could translate into regimens that cause a prolongation of PFS without an increase in response rate (eg, pegylated liposomal doxorubicin24 ).
Discussion
The Vk*MYC model of untreated MM reproduces several key features of the human disease: a high level of Ig secretion, localization within the native tumor (BM) microenvironment and intact host immune system, low proliferation, and clinical features (anemia, renal disease, bone disease). As inevitably seen in the clinic, relapsed refractory MM is more proliferative7 and more frequently exhibits extramedullary spread.25,26 By congenic transplantation, the tVk*MYC model reproduces the transformation to a more aggressive disease phenotype. GEMMs have been proposed to be important preclinical tools for the identification of active anticancer drugs.27 However, a rigorous validation of their ability to predict clinical response using drugs with both clearly demonstrated activity or inactivity in human disease has not been conducted. In this regard, MM provides an ideal disease model because there are a large number of agents with clear activity/inactivity. We therefore validated the Vk*MYC GEMM using both types of drugs and showed that active anti-MM drugs are effective in the Vk*MYC model, but more importantly, inactive drugs are not, despite extensive evidence of preclinical activity in other MM models. We postulate that the mechanism underlying this therapeutic fidelity is the use of the end point of response of an endogenous tumor in its native microenvironment, as opposed to the end point of survival of a mouse harboring a disseminated and highly proliferative cell line. Different experimental designs and/or animal models will be required to address other aspects of therapy (eg, to determine the effect of drugs on cancer stem cells, or particular genetic subtypes like del17p).
A distinctive feature of this model is that it allows one to evaluate the activity of drugs at different stages of MM. For instance, agents such as doxorubicin and vincristine initially showed activity in patients with relapsed refractory MM28 and were subsequently moved into the front-line setting where a conclusive role has not been demonstrated. We show in Vk*MYC mice that the limited activity of these agents is confined to the more proliferative tVk*MYC model where they confer disease stabilization, suggesting that they are indeed more appropriate for the relapsed refractory patient. In contrast, the HDACi and TACI-Ig showed remarkable single-agent activity in Vk*MYC mice, while they were inactive in bortezomib-resistant tVk*MYC mice, consistent with their lack of activity in phase 1/2 clinical trials in relapsed refractory MM.18,29,30 This patient population has a high frequency of activating mutations of the NF-kB pathway that would be predicted to confer resistance to TACI-Ig,31 and based on the responses in the Vk*MYC model, we predict that it will be an active agent if used earlier in the disease course before the acquisition of NF-kB–activating mutations. Historically, agents without single-agent activity in patients have not contributed significant activity when used in drug combinations and we found consistently that agents inactive in Vk*MYC mice were ineffective when used in combinations in the tVk*MYC mice (eg, decitabine and panobinostat, BEZ-235, and bortezomib). However, as drugs are being initially evaluated in more advanced, relapsed refractory patients, promising single-agent activity may be missed. We propose that novel drugs with single-agent activity in Vk*MYC mice (eg, HDACi, bromodomain inhibitors) should not be discarded if they fail to induce response in end-stage patients, but should be examined in combinations that are most active in the tVk*MYC model. The Vk*MYC model appears to overestimate the clinical response to single-agent HDACi, and perhaps as a result the tVk*MYC model overestimates the response of the combination with bortezomib. Possibly, patients who share critical features with Vk*MYC MM (eg, MYC rearrangements as one possible biomarker) may be the ones who benefit most from such therapies.
Although a rearrangement of the MYC locus is one of the most frequent genetic lesions in human MM,10 there is considerable molecular heterogeneity with different Ig gene translocations, hyperdiploidy, and mutations of RAS, the NFkB pathway, and p53. Outside of the MYC rearrangements, the relationship of the Vk*MYC MM to these other molecular subtypes of human MM is unclear, and worthy of further investigation. Importantly, lack of response in this model could miss targeted agents that may be effective in a particular molecular subtype of MM not represented by Vk*MYC MM (eg, FGFR3 inhibitors for t(4;14) MM). This model might be expected to be particularly adept at identifying drugs that target critical pathways required by all malignant PCs (eg, IL6, NFkB, ER-stress, hypoxia), and perhaps also by normal PCs. Finally, as the tumors cells develop orthotopically in the BM of an immunocompetent mouse, it may also prove useful to identify drugs that act in part or exclusively through modulating the microenvironment (eg, inhibitors of angiogenesis, immune modulators) that may have been overlooked in the past because they lack activity against cell lines in vitro or xenograft models.
In summary, the Vk*MYC GEMM is a faithful preclinical model that predicts the clinical activity of drugs in untreated and relapsed MM, and should provide a useful filter to prioritize a huge number of novel agents for evaluation in clinical trials in humans.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr James Bradner for supplying JQ1 and for critical review of the manuscript. They thank Dr Carl Walkley and Megan Russell for help with micro-CT scans, and David Faulkner for help with SPEP studies.
This work was supported by National Institutes of Health grant AG20686, National Cancer Institute grant CA136671 (P.L.B.), and by the Multiple Myeloma Research Foundation (M.C.). R.W.J. is a Principal Research Fellow of the National Health and Medical Research Council of Australia (NHMRC) and is supported by NHMRC Program and Project grants, the Susan G. Komen Breast Cancer Foundation, the Prostate Cancer Foundation of Australia, Cancer Council Victoria, The Victorian Cancer Agency, The Leukemia Foundation of Australia, Victorian Breast Cancer Research Consortium, and the Australian Rotary Health Foundation. G.M.M. is supported by an Australian Biomedical Fellowship (Peter Doherty) from the NHMRC. J.S. is supported by the Leukemia Foundation of Australia and the Cooperative Research Center for Biomedical Imaging Development. M.L. is supported by the Leukemia Foundation of Australia. The laboratory of R.W.J. receives research funding from Novartis for studies involving panobinostat.
National Institutes of Health
Authorship
Contribution: M.C. designed and conducted or supervised the experiments and wrote the manuscript; G.M.M., V.M.G., S.E.P., J.S., and M.L. managed the mouse colony, administered drugs, and performed transplantation experiments, necropsy, and flow cytometric analysis; G.M.M. and J.S. contributed to the writing of the manuscript; G.M.M., J.S., M.L., and A.K.S. provided critical review of the manuscript; and R.W.J. and P.L.B. conceived the experiments, provided critical input, and wrote the manuscript.
Conflict-of-interest disclosure: P.L.B. and M.C. hold intellectual property in Vk*MYC mice. The laboratory of R.W.J. is supported by a research grant from Novartis. The remaining authors declare no competing financial interests.
Correspondence: P. Leif Bergsagel, Comprehensive Cancer Center, Mayo Clinic, 13400 E Shea Blvd, Scottsdale, AZ, 85259; e-mail: bergsagel.leif@mayo.edu.