Abstract
Eltrombopag (EP) is a small-molecule, nonpeptide thrombopoietin receptor (TPO-R) agonist that has been approved recently for the treatment of thrombocytopenia in patients with chronic immune thrombocytopenic purpura. Prior studies have shown that EP stimulates megakaryopoiesis in BM cells from patients with acute myeloid leukemia and myelodysplastic syndrome, and the results also suggested that it may inhibit leukemia cell growth. In the present study, we studied the effects of EP on leukemia cell proliferation and the mechanism of its antiproliferative effects. We found that EP leads to a decreased cell division rate, a block in G1 phase of cell cycle, and increased differentiation in human and murine leukemia cells. Because EP is species specific in that it can only bind TPO-R in human and primate cells, these findings further suggested that the antileukemic effect is independent of TPO-R. We found that treatment with EP leads to a reduction in free intracellular iron in leukemic cells in a dose-dependent manner. Experimental increase of intracellular iron abrogated the antiproliferative and differentiation-inducing effects of EP, demonstrating that its antileukemic effects are mediated through modulation of intracellular iron content. Finally, determination of EP's antileukemic activity in vivo demonstrated its ability to prolong survival in 2 mouse models of leukemia.
Introduction
Survival in acute myeloid leukemia (AML) and high-risk myelodysplastic syndrome (MDS) has remained poor despite recent efforts to treat patients with novel therapeutic regimens.1-4 In addition, complications secondary to thrombocytopenia and bleeding occur frequently in AML and MDS, leading to significant morbidity and mortality.5,6 Given that the median age of patients with AML is close to 70 years, novel antileukemia agents with limited BM toxicity are needed to improve outcomes.
Eltrombopag (EP) is an oral, nonpeptide, small-molecule thrombopoietin receptor (TPO-R) agonist that has proven efficacy in treating chronic immune thrombocytopenic purpura (ITP) and hepatitis C–related thrombocytopenia.7,8 Despite concerns that some leukemia blast cells express TPO-R, we and others have reported previously that EP does not stimulate leukemia or MDS cell growth, but may rather lead to a modest inhibition while continuing to stimulate normal megakaryopoiesis in BM samples of patients with AML or MDS.9,10 One study using a close chemical derivative of EP found a toxic effect on myeloid leukemia cells, suggesting that the entire substance class, including EP itself, may possess antileukemic activity.11 Studies using cell lines further suggested that the growth-inhibitory effect of EP is not related to expression levels of TPO-R.12 However, this hypothesis has not yet been formally tested, and the mechanism through which EP exerts its potential antileukemic effect is not known. The concentrations at which EP inhibits leukemia cell proliferation in vitro are clinically achievable with few side effects and have led to desirable increases in circulating platelet counts in healthy volunteers.13
In the present study, we examined the cellular and molecular mechanisms by which EP exerts its antileukemic effect using both in vitro and in vivo models of AML. We show that EP has a profound leukemia-inhibitory effect that is independent of TPO-R in vitro and in vivo, that EP leads to an induction of myeloid differentiation, and that these effects are mediated by a reduction of intracellular iron levels by EP.
Methods
Reagents
EP (SB-497115) was dissolved as a 1 mg/mL stock solution in distilled water and stored light protected at room temperature for up to 2 weeks. Salicylaldehyde isonicotinyl hydrazine (SIH) was a generous gift of Dr Katherine Franz (Duke University, Durham, NC). Deferoxamine mesylate (DFO), ferrous ammonium citrate, and N-acetyl-L-cysteine were from Sigma-Aldrich. Recombinant human and mouse TPO were from Invitrogen.
Cell culture
HL60, U937, and HS5 cells were cultured in RPMI medium (CellGro) with 10% FBS (Gemini; at 37°C. Upstream regulatory element (URE) murine AML cells were obtained from PU.1-knockdown mice with targeted disruption of the distal enhancer (URE) −14 kb upstream of the PU.1 gene.14,15 URE cells were maintained in M5300 medium (StemCell Technologies) supplemented with 10% heat-inactivated FBS, 15% supernatant of WEHI-3B culture medium, 15% supernatant of baby hamster kidney culture medium, and penicillin/streptomycin.
Cell-proliferation assays
For 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assays, cells were plated into 96-well plates with 100 μL of culture medium. After 72 hours, cells were incubated with 10 μL of MTS reagent (CellTiter 96 AQueous One Solution Cell Proliferation Assay kit; Promega), OD490 and OD650 were detected by a microplate reader (VersaMAX; Molecular Devices). The MTS proliferative index was calculated by subtracting the background from raw values, defined as: (OD490 − OD650 of a well with cells) − (OD490 − OD650 of a well with medium only). Manual cell counts were performed culturing 2 × 105 cells/mL in 12-well plates. Viable cells were counted using trypan blue exclusion. Doxorubicin and cytarabine were from Hospira.
Intracellular iron-detection assays
HL60, URE, and U937 cells were harvested and resuspended in RPMI medium supplemented with 10% FBS and 250nM calcein-AM (Invitrogen) and incubated for 5 minutes at 37°C. Cells were washed and resuspended in prewarmed culture medium. Cells were then left untreated or were treated with EP, TPO, SIH, or DFO for 1 or 4 hours. Intracellular calcein fluorescence was measured by flow cytometry on a FACSAria II (BD Biosciences) and mean fluorescence was calculated by Diva Version 6.1.3 software (BD Biosciences). Transferrin receptor expression was measured by FACS analysis using Abs against human CD71 (11071973; eBiosciences) and murine CD71 (557416; BD Pharmingen).
Cell-cycle assays
The Click-iT EdU Flow Cytometry Assay system (Invitrogen) was used following the manufacturer's instructions. Briefly, after culture of cells with 5-ethynyl-2′-deoxyuridine (10μM) for 2 hours, cells were fixed by 4% paraformaldehyde, treated with saponin-containing buffer, and then incubated with Alexa Fluor 647 dye azide. 4′,6-diamidino-2-phenylindole was added immediately before flow cytometric analysis.
Cell-division assays
The Cell Tracker Orange Assay (Invitrogen) was used following the manufacturer's instructions. Briefly, cells were labeled with cell tracker at a concentration of 10μM via incubation at 37°C for 30 minutes. Cells were washed, maintained in culture, and analyzed by FACS every 24 hours.
Differentiation assays
Cell differentiation was assessed 72 hours after treatment. Morphology was examined after Diff Quik (IMEB) staining and FACS analysis using Abs directed against CD11b (555389; BD Pharmingen) and CD14 (MHCD1406; Invitrogen) for human cell lines, and CD11b (12011282; eBiosciences) for murine cell lines. Morphology was evaluated and documented using an Axiovert 200M microscope with an AxioCam color camera at 37°C (Zeiss).
Intracellular ROS assays
FACS analysis was used to determine the level of intracellular reactive oxygen species (ROS) in response to various treatments. Briefly, HL60, URE, and U937 cells were loaded with 5μM H2DCFDA (Invitrogen) for 30 minutes, washed, and evaluated by flow cytometry.
In vivo leukemia models
All animal experiments were performed in compliance with institutional guidelines and approved by the Animal Institute Committee of the Albert Einstein College of Medicine (protocol number 20110102).
NOD-SCID IL-2Rγ–null (NSG) mice were sublethally irradiated (200 rad) and intravenously inoculated with 1 × 106 URE cells. At day +3, mice were divided into 3 groups: group 1 received untreated drinking water, group 2 received drinking water with 0.4 mg/mL of EP, and group 3 received 1.0 mg/mL of EP in the drinking water. The untreated and treated water was changed every 3 days. Mice were monitored for signs of leukemia and their BM and spleens were harvested at death. For experiments with human leukemia cells, NSG mice were sublethally irradiated (200 rad) and intravenously inoculated with 1 × 107 HL60 cells. At day +3, mice were divided into 3 groups: group 1 received untreated drinking water, group 2 received drinking water with 1.0 mg/mL of EP, and group 3 received 3.0 mg/mL of EP. The untreated and treated water was changed twice every week. Mice were monitored, assessed for engraftment, and their BM and spleens were harvested at death. Engraftment was assessed by FACS analysis using Abs directed against murine CD45.1 (12045383; eBiosciences) and human CD15 (11015973; eBiosciences).
Microarray experiments and analysis
RNA was extracted from HL60 cells after 12 and 36 hours of treatment with EP, or without treatment using TRIzol reagent (Invitrogen). After evaluation of the quality of RNA with an Agilent 2100 Bioanalyzer, RNA was labeled with the GeneChip WT terminal labeling kit (Affymetrix) and labeled cRNA of each individual sample was hybridized to Affymetrix Humane Gene 1.0ST microarrays (Affymetrix), stained, and scanned by the GeneChip Scanner 3000 7G system (Affymetrix) according to standard protocols. The complete array data are deposited in the Gene Expression Omnibus as accession number GSE37580.
Statistical analysis
Results are reported as the means ± SD. The Student t test was used to determine statistical significance in the in vitro experiments. P < .05 was considered statistically significant. For in vivo experiments, the log-rank test was used to determine statistical significance of observed differences in overall survival.
Results
EP leads to a decreased cell division rate and a block in the G1 phase of the cell cycle in human and murine leukemia cells
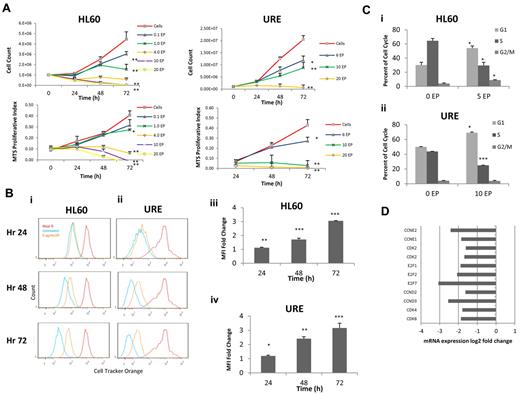
We evaluated the dose of EP required to inhibit HL60 cell (human) and URE cell (murine) proliferation. A murine cell line was evaluated in all experiments because EP is highly species specific and does not activate the TPO-R pathway in murine cells.16 HL60 cells and URE cells were incubated with increasing concentrations of EP for 72 hours. Manual cell counts and MTS assays were performed every 24 hours. EP inhibits HL60 cells and URE cells (Figure 1A) in a dose-dependent manner at in vitro concentrations that are achievable in vivo.13,17 To evaluate the effect of EP on the rate of cell division, HL60 cells and URE cells were labeled with Cell Tracker Orange (Invitrogen) and cells were analyzed by FACS every 24 hours for 3 days. EP slowed the cell-division rate by 48 hours of treatment in both human HL60 cells and murine URE cells (Figure 1Bi-iv).
EP inhibits cell cycling and leads to a block in the G1 phase. (A) HL60 and URE cells were treated with increasing concentrations of EP. Cell viability was measured by cell counts with trypan blue exclusion (top panel), and MTS assays (bottom panel) were performed every 24 hours for 72 hours. Data represent the means ± SD of viable cells performed in triplicate and MTS proliferative index. *P < .05; **P < .01. (B) HL60 cells (i) and URE cells (ii) were incubated in 10μM Cell Tracker Orange for 30 minutes, washed, and analyzed by FACS (red line = hour 0). Cells were treated without EP (blue line) or with 5 μg/mL of EP (orange line) and FACS analysis was performed every 24 hours for 3 days. Lower mean fluorescence indicates increased cell division. The fold change of HL60 cells (iii) and URE cells (iv) of FACS mean fluorescence intensity (MFI) ± SD (n = 3) of Cell Tracker Orange–labeled HL60 cells treated with 5 μg/mL of EP relative to untreated cells is shown. *P < .05; **P < .01; ***P < .001. EP slows cell division in HL60 and URE cells because higher MFI represents slower cell division. (C) Cell-cycle analysis of HL60 cells (i) and URE cells (ii) with or without 5 or 10 μg/mL of EP for 48 hours. EP induces a cell-cycle block in G1 phase with a subsequent decrease in the S phase. (D) The fold change variation of gene expression by microarray in HL60 cells treated with 3 μg/mL of EP for 36 hours relative to untreated cells. EP down-regulated genes necessary for the transition from the G1 to the S phase.
EP inhibits cell cycling and leads to a block in the G1 phase. (A) HL60 and URE cells were treated with increasing concentrations of EP. Cell viability was measured by cell counts with trypan blue exclusion (top panel), and MTS assays (bottom panel) were performed every 24 hours for 72 hours. Data represent the means ± SD of viable cells performed in triplicate and MTS proliferative index. *P < .05; **P < .01. (B) HL60 cells (i) and URE cells (ii) were incubated in 10μM Cell Tracker Orange for 30 minutes, washed, and analyzed by FACS (red line = hour 0). Cells were treated without EP (blue line) or with 5 μg/mL of EP (orange line) and FACS analysis was performed every 24 hours for 3 days. Lower mean fluorescence indicates increased cell division. The fold change of HL60 cells (iii) and URE cells (iv) of FACS mean fluorescence intensity (MFI) ± SD (n = 3) of Cell Tracker Orange–labeled HL60 cells treated with 5 μg/mL of EP relative to untreated cells is shown. *P < .05; **P < .01; ***P < .001. EP slows cell division in HL60 and URE cells because higher MFI represents slower cell division. (C) Cell-cycle analysis of HL60 cells (i) and URE cells (ii) with or without 5 or 10 μg/mL of EP for 48 hours. EP induces a cell-cycle block in G1 phase with a subsequent decrease in the S phase. (D) The fold change variation of gene expression by microarray in HL60 cells treated with 3 μg/mL of EP for 36 hours relative to untreated cells. EP down-regulated genes necessary for the transition from the G1 to the S phase.
Given the effect seen on cell division, we next investigated whether EP-induced changes in the cell cycle that could contribute to slowed cell division. After 48 hours, EP treatment led to a block in the G1 phase (increase from 30.1%-54.0%; P = .02) with a subsequent decrease in the S phase (64.5% for untreated vs 29.5% for EP-treated; P = .02) in both HL60 cells and URE cells (increase of cells in G1 from 50.0%-69.1%, P = .01; decrease of cells in S phase from 43.7%-25.0%, P < .001; Figure 1Ci-ii). Consistent with these findings, gene-expression analysis by microarray of HL60 cells performed 36 hours after EP treatment showed the down-regulation of genes important for the transition from the G1 to the S phase compared with untreated cells (Figure 1D and supplemental Figure 1A-B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). To confirm that the effect of EP on cell division and cell cycling was consistent in other human cell lines, EP was tested in U937 cells. Supporting our findings, EP slowed cell division and led to a block in the cell cycle in the G1 phase in U937 cells (supplemental Figure 2).
EP induces differentiation of leukemia cell lines
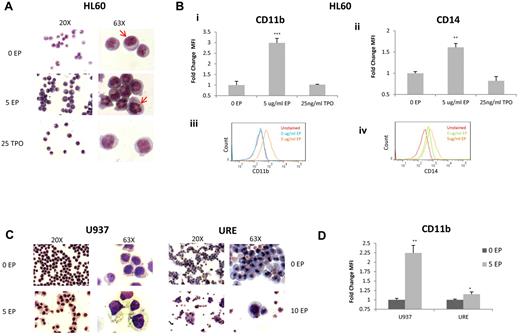
We also investigated the effect of EP on differentiation. HL60 cells were incubated without EP, with 5 μg/mL of EP, or with TPO for 72 hours and cytospins were performed followed by Diff Quik staining. HL60 cells treated with EP showed clear signs of differentiation, significant changes in the organization of the nuclear contents, and an increase in the cytoplasm/nucleus ratio, whereas TPO did not induce significant morphologic changes (Figure 2A). FACS analysis confirmed that EP differentiates HL60 cells with significant increases in the mature myeloid markers CD11b (2.98-fold; P < .001) and CD14 (1.61-fold; P = .003; Figure 2Bi-iv).
EP induces differentiation of leukemia cell lines. (A) Representative morphology of HL60 cells treated for 72 hours without EP, with EP, or with TPO shown at 20× (left panel) and 63× (right panel). Cells treated with EP portray reorganization of the nuclear contents compared with untreated cells (red arrows) and cells treated with TPO. (B) The fold change of FACS MFI ± SD (n = 3) of CD11B expression (i) and CD14 (ii) in HL60 cells treated with the above conditions relative to untreated cells for 72 hours. *P < .05; **P < .01; ***P < .001. FACS analysis of CD11B expression (iii) in untreated HL60 cells (blue line) versus cells treated with 5 μg/mL of EP (orange line) for 72 hours. FACS analysis of CD14 expression (iv) in untreated HL60 cells (green line) versus cells treated with 5 μg/mL of EP (orange line) for 72 hours. CD11B and CD14 are overexpressed with EP treatment. (C) Representative morphology of U937 cells and URE cells treated for 72 hours under the above conditions. U937 cells treated with EP demonstrate increased size with increased vacuoles in the cytoplasm. (D) The fold change of FACS MFI ± SD (n = 3) of CD11B expression in U937 cells and URE cells treated with or without EP for 72 hours. *P < .05; **P < .01.
EP induces differentiation of leukemia cell lines. (A) Representative morphology of HL60 cells treated for 72 hours without EP, with EP, or with TPO shown at 20× (left panel) and 63× (right panel). Cells treated with EP portray reorganization of the nuclear contents compared with untreated cells (red arrows) and cells treated with TPO. (B) The fold change of FACS MFI ± SD (n = 3) of CD11B expression (i) and CD14 (ii) in HL60 cells treated with the above conditions relative to untreated cells for 72 hours. *P < .05; **P < .01; ***P < .001. FACS analysis of CD11B expression (iii) in untreated HL60 cells (blue line) versus cells treated with 5 μg/mL of EP (orange line) for 72 hours. FACS analysis of CD14 expression (iv) in untreated HL60 cells (green line) versus cells treated with 5 μg/mL of EP (orange line) for 72 hours. CD11B and CD14 are overexpressed with EP treatment. (C) Representative morphology of U937 cells and URE cells treated for 72 hours under the above conditions. U937 cells treated with EP demonstrate increased size with increased vacuoles in the cytoplasm. (D) The fold change of FACS MFI ± SD (n = 3) of CD11B expression in U937 cells and URE cells treated with or without EP for 72 hours. *P < .05; **P < .01.
We also evaluated whether EP induces differentiation of another human cell line (U937 cells) and murine URE leukemia cells. At 72 hours after EP treatment, both cell lines showed significant differentiation-associated morphologic changes with increased cell size and the formation of vacuoles within the cytoplasm of U937 cells (Figure 2C). EP caused an increase in CD11b (2.25-fold; P = .007), which was consistent with a premacrophage state in U937 cells, and also caused an increase in CD11b in URE cells (1.15-fold; P = .04; Figure 2D).
EP reduces intracellular iron in leukemia cells
After treatment with EP, gene-expression arrays of HL60 cells showed an overexpression of the transferrin receptor (CD71), which is required for iron transport. This increase in CD71 expression was validated by FACS (Figure 3D). Given the overexpression of CD71 and the knowledge that EP shares structural similarities to well-described metal chelators, we hypothesized that some of the observed cell biologic effects of EP could be mediated by reduction of intracellular iron (supplemental Figure 3). To address this hypothesis, we evaluated the effect of EP on intracellular iron levels.18,19 HL60 cells were loaded with the intracellular iron-binding compound calcein-AM (Invitrogen). When bound to Fe2+, the fluorescent signal of calcein-AM is quenched; however, on release from Fe with addition of an iron chelator, its fluorescent signal intensifies. After calcein-AM loading, HL60 cells were treated with various concentrations of EP, TPO (as a negative control), SIH (a highly potent iron chelator), or a high concentration of DFO (as another positive control). After 1 hour of treatment, 5 and 10 μg/mL of EP significantly increased calcein-AM fluorescence by 4.48% (P = .03) and 8.03% (P = .004), respectively, indicating reductions of free intracellular iron in cells treated with EP (Figure 3A). After 4 hours of treatment, concentrations as low as 1 μg/mL of EP increased the labile intracellular iron pool by 5.12% ± 0.79% (Figure 3A). Interestingly, treatment with 10 μg/mL of EP (a pharmacologically achievable concentration) resulted in even greater intracellular iron reduction (23.5%; P = .003) than the highly potent chelator SIH (Figure 3A). To determine whether reduction of intracellular iron is a consistent feature of EP across multiple leukemia cell lines, 5 additional cell lines were tested (4 human and 1 murine). All cell lines displayed significant intracellular iron reduction after treatment with 5 μg/mL of EP (Figure 3B).
EP depletes intracellular iron in leukemia cell lines. (A) HL60 cells were labeled with a 0.25μM concentration of the intracellular iron-chelating dye calcein-AM for 5 minutes. Cells were washed then treated with 0 μg/mL of EP, 5 μg/mL of EP, 10 μg/mL of EP, 25 ng/mL of TPO (− control), 100μM SIH (+ control), or 100μM DFO (+ control) for 1 hour (left panel) or 4 hours (right panel) at 37°C and cells were analyzed by FACS. Data represent the change in the MFI ± SD (n = 3) compared with untreated HL60 cells *P < .05; **P < .01; ***P < .001. (B) Five leukemia/lymphoma cell lines were labeled with calcein-AM and then treated with 5 μg/mL of EP for 4 hours. Cells were analyzed by FACS. Data represent the change in the MFI± SD (n = 3) compared with untreated cells. (C) The fold change variation of gene expression in HL60 cells treated with 3 μg/mL of EP or 100 ng/mL of TPO for 12 hours (left panel) or 36 hours (right panel) relative to untreated cells. EP up-regulated the transferrin receptor (TFRC) and down-regulated the ferritin light chain (FTL) and heavy chain (FTH1). (D) FACS analysis of CD71 expression in untreated HL60 cells (blue line) versus cells treated with 5 μg/mL of EP (orange line) for 24 hours (left panel). FACS analysis of CD71 expression in HL60 cells treated with 5 μg/mL of EP (orange line) versus cells preloaded with 500 μg/mL of ferric ammonium citrate (FAC) for 24 hours and then treated with 5 μg/mL of EP (blue line) for 24 hours (middle panel). FACS MFI ± SD (n = 3) of CD71 expression in HL60 cells and URE cells untreated, treated with 5 μg/mL of EP, or preloaded with 500 μg/mL of ferric ammonium citrate followed by treatment with 5 μg/mL of EP for 24 hours (right panel). P value (by t test) represents the difference in MFI between treated and untreated cells *P < .05; **P < .01; ***P < .001. CD71 is overexpressed in response to EP treatment and expression is decreased when cells are preloaded with iron.
EP depletes intracellular iron in leukemia cell lines. (A) HL60 cells were labeled with a 0.25μM concentration of the intracellular iron-chelating dye calcein-AM for 5 minutes. Cells were washed then treated with 0 μg/mL of EP, 5 μg/mL of EP, 10 μg/mL of EP, 25 ng/mL of TPO (− control), 100μM SIH (+ control), or 100μM DFO (+ control) for 1 hour (left panel) or 4 hours (right panel) at 37°C and cells were analyzed by FACS. Data represent the change in the MFI ± SD (n = 3) compared with untreated HL60 cells *P < .05; **P < .01; ***P < .001. (B) Five leukemia/lymphoma cell lines were labeled with calcein-AM and then treated with 5 μg/mL of EP for 4 hours. Cells were analyzed by FACS. Data represent the change in the MFI± SD (n = 3) compared with untreated cells. (C) The fold change variation of gene expression in HL60 cells treated with 3 μg/mL of EP or 100 ng/mL of TPO for 12 hours (left panel) or 36 hours (right panel) relative to untreated cells. EP up-regulated the transferrin receptor (TFRC) and down-regulated the ferritin light chain (FTL) and heavy chain (FTH1). (D) FACS analysis of CD71 expression in untreated HL60 cells (blue line) versus cells treated with 5 μg/mL of EP (orange line) for 24 hours (left panel). FACS analysis of CD71 expression in HL60 cells treated with 5 μg/mL of EP (orange line) versus cells preloaded with 500 μg/mL of ferric ammonium citrate (FAC) for 24 hours and then treated with 5 μg/mL of EP (blue line) for 24 hours (middle panel). FACS MFI ± SD (n = 3) of CD71 expression in HL60 cells and URE cells untreated, treated with 5 μg/mL of EP, or preloaded with 500 μg/mL of ferric ammonium citrate followed by treatment with 5 μg/mL of EP for 24 hours (right panel). P value (by t test) represents the difference in MFI between treated and untreated cells *P < .05; **P < .01; ***P < .001. CD71 is overexpressed in response to EP treatment and expression is decreased when cells are preloaded with iron.
Consistent with the finding that EP chelates intracellular iron, gene-expression analysis at 12 and 36 hours after EP treatment showed that the cells were responding to iron depletion by up-regulating the transferrin receptor (CD71) and down-regulating the ferritin light and heavy chains (Figure 3C). The transferrin receptor transports iron intracellularly, whereas ferritin stores iron when it is in excess. In addition, we found CD71 surface antigen in both HL60 and URE cells to be overexpressed as early as 24 hours after treatment with EP (Figure 3D). To show that CD71 expression is increased in response to EP-induced iron deprivation, HL60 cells were preloaded with iron using ferrous ammonium citrate and subsequently treated with EP. Consistent with our hypothesis, cells loaded with iron and treated with EP showed decreased expression of CD71 compared with cells treated only with EP (Figure 3D). To assess whether iron depletion is characteristic of other TPO agonists, we assessed the ability of a peptidyl TPO agonist to deplete intracellular iron. Four hours after treatment with high-dose Romiplostim, no significant reduction in iron was observed (supplemental Figure 4).
Inhibition of leukemia cell proliferation and induction of differentiation by EP is mediated by a reduction of intracellular iron
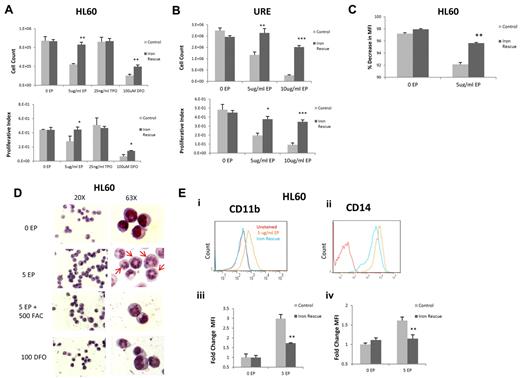
The mechanism by which EP inhibits leukemia cell growth has not been well characterized. Given that EP leads to a reduction of intracellular iron and that iron chelators have been shown to have antiproliferative effects in myeloid leukemia cells, we investigated whether the antileukemic effect of EP is secondary to its ability to deplete intracellular iron. We performed iron-rescue experiments by preloading HL60 cells with 500 μg/mL of ferric ammonium citrate for 24 hours, which resulted in a 9.76% ± 2.10% increase in intracellular iron. HL60 cells were subsequently left untreated (control) or were treated with EP, TPO (negative control), or DFO (positive control). Preloading cells with iron resulted in a rescue from the antiproliferative effects of EP as determined by cell count (95% growth restoration; P < .01) and MTS assay after 72 hours (Figure 4A). We made very similar observations in murine leukemia cells: iron-loaded URE cells were also rescued from the antiproliferative effects of EP (84% growth restoration; P < .01; Figure 4B).
EP-induced cell death is iron dependent. (A) HL60 cells were untreated or treated with 5 μg/mL of TPO, 25 ng/mL of EP, or 100u DFO for 72 hours preloaded or not with 500 μg/mL of ferric ammonium citrate (FAC) for 24 hours. Cell viability was measured by cell counts with trypan blue exclusion (top panel) and MTS assays (bottom panel) performed at 72 hours. Data represent the means ± SD of viable cells performed in triplicate. P values represent the difference between iron-loaded and noniron-loaded cells. *P < .05; **P < .01. (B) URE−/− cells were untreated or treated with 5 or 10 μg/mL of EP for 72 hours preloaded or not with 500 μg/mL of ferric ammonium citrate for 24 hours. Cell viability was measured by cell counts with trypan blue exclusion (top panel) and MTS assays (bottom panel) performed at 72 hours. Data represent the means ± SD of viable cells performed in triplicate. P values represent the difference between iron-loaded and noniron-loaded cells. *P < .05; **P < .01; ***P < .001. (C) HL60 Cells were incubated in 10μM Cell Tracker Orange for 30 minutes, washed, and analyzed by FACS. Cells were treated without EP, with 5 μg/mL of EP, or were preloaded with 500 μg/mL of ferric ammonium citrate and then treated with or without 5 μg/mL of EP. FACS analysis was performed at 72 hours. The percent change in FACS MFI ± SD (n = 3) of Cell Tracker Orange labeled HL60 cells treated with 5 μg/mL of EP with or without iron preload relative to untreated cells, corrected for hours 0 MFI. The P value represents the difference between the MFI of the iron-preloaded cells treated with EP and the cells treated with EP without iron preload. *P < .05; **P < .01. EP slows cell division in HL60 cells and cell division is rescued by preloading cells with iron. (D) Representative morphology of HL60 cells treated for 72 hours under the above conditions shown at 20× (left panel) and 63× (right panel). HL60 cells preloaded with iron and subsequently treated with EP display less segmented nuclei than EP-treated cells without iron (cells with increased nuclear segmentation are indicated by arrows). (E) FACS analysis of CD11b expression (i) in HL60 cells treated with 5 μg/mL of EP (orange line) versus cells preloaded with 500 μg/mL of ferric ammonium citrate (FAC) for 24 hours and then treated with 5 μg/mL of EP (blue line) for 72 hours. FACS analysis of CD14 expression (ii) in HL60 cells treated with 5 μg/mL of EP (orange line) versus cells preloaded with 500 μg/mL of FAC for 24 hours and then treated with 5 μg/mL of EP (blue line) for 72 hours. CD11b and CD14 are overexpressed with EP treatment and rescued by preloading cells with iron. The fold change of FACS MFI ± SD (n = 3) of CD11b expression (iii) and CD14 (iv) in HL60 cells preloaded with 500 μg/mL of FAC and then treated with 5 μg/mL of EP compared with cells treated with 5 μg/mL of EP without iron load is shown. P values represent the difference between iron-loaded and noniron-loaded cells. *P < .05; **P < .01.
EP-induced cell death is iron dependent. (A) HL60 cells were untreated or treated with 5 μg/mL of TPO, 25 ng/mL of EP, or 100u DFO for 72 hours preloaded or not with 500 μg/mL of ferric ammonium citrate (FAC) for 24 hours. Cell viability was measured by cell counts with trypan blue exclusion (top panel) and MTS assays (bottom panel) performed at 72 hours. Data represent the means ± SD of viable cells performed in triplicate. P values represent the difference between iron-loaded and noniron-loaded cells. *P < .05; **P < .01. (B) URE−/− cells were untreated or treated with 5 or 10 μg/mL of EP for 72 hours preloaded or not with 500 μg/mL of ferric ammonium citrate for 24 hours. Cell viability was measured by cell counts with trypan blue exclusion (top panel) and MTS assays (bottom panel) performed at 72 hours. Data represent the means ± SD of viable cells performed in triplicate. P values represent the difference between iron-loaded and noniron-loaded cells. *P < .05; **P < .01; ***P < .001. (C) HL60 Cells were incubated in 10μM Cell Tracker Orange for 30 minutes, washed, and analyzed by FACS. Cells were treated without EP, with 5 μg/mL of EP, or were preloaded with 500 μg/mL of ferric ammonium citrate and then treated with or without 5 μg/mL of EP. FACS analysis was performed at 72 hours. The percent change in FACS MFI ± SD (n = 3) of Cell Tracker Orange labeled HL60 cells treated with 5 μg/mL of EP with or without iron preload relative to untreated cells, corrected for hours 0 MFI. The P value represents the difference between the MFI of the iron-preloaded cells treated with EP and the cells treated with EP without iron preload. *P < .05; **P < .01. EP slows cell division in HL60 cells and cell division is rescued by preloading cells with iron. (D) Representative morphology of HL60 cells treated for 72 hours under the above conditions shown at 20× (left panel) and 63× (right panel). HL60 cells preloaded with iron and subsequently treated with EP display less segmented nuclei than EP-treated cells without iron (cells with increased nuclear segmentation are indicated by arrows). (E) FACS analysis of CD11b expression (i) in HL60 cells treated with 5 μg/mL of EP (orange line) versus cells preloaded with 500 μg/mL of ferric ammonium citrate (FAC) for 24 hours and then treated with 5 μg/mL of EP (blue line) for 72 hours. FACS analysis of CD14 expression (ii) in HL60 cells treated with 5 μg/mL of EP (orange line) versus cells preloaded with 500 μg/mL of FAC for 24 hours and then treated with 5 μg/mL of EP (blue line) for 72 hours. CD11b and CD14 are overexpressed with EP treatment and rescued by preloading cells with iron. The fold change of FACS MFI ± SD (n = 3) of CD11b expression (iii) and CD14 (iv) in HL60 cells preloaded with 500 μg/mL of FAC and then treated with 5 μg/mL of EP compared with cells treated with 5 μg/mL of EP without iron load is shown. P values represent the difference between iron-loaded and noniron-loaded cells. *P < .05; **P < .01.
To assess whether the inhibition of the cell-division rate by EP could also be rescued by increasing the intracellular iron content of HL60 cells, ferric ammonium citrate was preloaded into cells for 24 hours, followed by Cell Tracker labeling of HL60 cells. At 48 hours after EP treatment, cell division was significantly greater in cells preloaded with iron and treated with EP compared with cells only treated with EP (Figure 4C). Similarly, the ability of EP to differentiate HL60 cells was reduced by preloading the cells with iron, as shown by morphologic changes (Figure 4D) and cell-surface expression of CD11b and CD14 (Figure 4E). The morphologic changes induced by EP in HL60 cells were similar to changes induced by treatment with DFO. These findings show that the leukemia-inhibitory and differentiation-inducing effects of EP are mediated by a reduction of intracellular iron.
Knowing that ROS levels vary with intracellular iron levels and that iron chelators have been shown to induce ROS formation, we investigated whether EP also induces ROS.20 We used H2DCFDA (Invitrogen) with flow cytometry to assess intracellular ROS levels. HL60, URE, and U937 cells were treated with 5 μg/mL of EP for 1 hour and then incubated with 5μM H2DCFDA. All cell lines showed significant increases in ROS formation after only 1 hour of treatment (supplemental Figure 5A-B). To determine whether ROS generation was involved in EP-induced differentiation of HL60 cells, we incubated the cells with the antioxidant N-acetyl-L-cysteine during EP treatment, which resulted in a 92% rescue of ROS activity (supplemental Figure 5C). CD11b and CD14 expression (−22.1%, P = .045 and −23.6%, P = .01, respectively) both decreased in antioxidant-treated cells treated with EP compared with cells treated with EP alone (supplemental Figure 5D).
EP prolongs survival in mouse models of leukemia
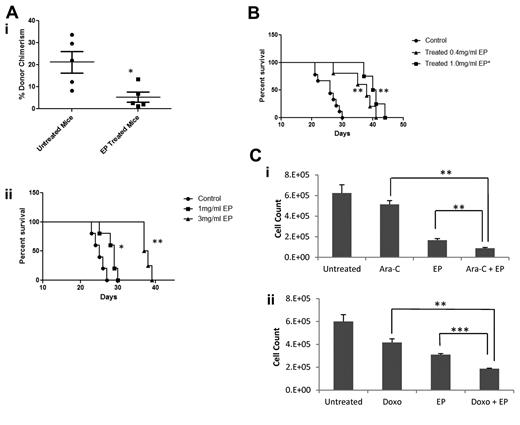
Given the in vitro antileukemic effect of EP, we next evaluated the effect of EP in murine transplantation models of leukemia. Ten million HL60 cells were IV injected into NSG mice via the tail vein and mice were treated starting at day +3 with 1.0 mg/mL of EP in the drinking water or with untreated drinking water. BM aspirations at day +21 showed that mice treated with EP had significantly lower donor cell chimerism (5.6% vs 21.1%; P = .03; Figure 5Ai). EP treatment significantly improved survival in these mice (P = .05; Figure 5Aii), and engrafted leukemia cells in EP-treated mice showed increased CD11b and CD14 expression (supplemental Figure 6), consistent with our observations in vitro. Given that high doses of EP have been well tolerated in humans, we assessed the effect of higher-dose EP in our HL60 leukemia mouse model. Mice treated with 3.0 mg/mL of EP in the drinking water showed significantly increased median survival compared with control mice and mice treated with 1.0 mg/mL of EP, respectively (P < .01; Figure 5Aii).
EP prolongs survival in mouse models of leukemia. (A) Ten million HL60 cells were transplanted into the tail veins of NSG mice (n = 10) 4 hours after sublethal irradiation. Mice were divided into 2 groups, one receiving untreated drinking water, and the other receiving 1 mg/mL of EP in the drinking water starting at day +3. BM aspirates were performed on day +21 and FACS analysis was performed assessing donor cell chimerism (CD15+Ly5.1−; i). Kaplan Meier survival curve of HL60-transplanted mice (ii) treated with 1 mg/mL or 3.0 mg/mL EP in the drinking water versus mice with untreated drinking water. *P < .05; **P < .01. (B) One million URE cells were transplanted into the tail veins of NSG mice 4 hours after sublethal irradiation. Mice were divided into 3 groups, with one group receiving untreated drinking water (n = 7), one group receiving 0.4 mg/mL of EP in the drinking water (n = 5), and the other receiving 1 mg/mL of EP in the drinking water (n = 4) starting at day +3. Kaplan-Meier survival curve of URE transplanted mice treated with 0.4 or 1.0 mg/mL of EP in the drinking water versus mice with untreated drinking water is shown. **P < .01. (C) HL60 cells were treated with or without 20nM cytarabine (i), with or without 24 ng/mL of doxorubicin (ii) ± 5 μg/mL of EP for 72 hours. Cell viability was measured by cell counts with trypan blue exclusion. Data represent the means ± SD of viable cells performed in triplicate. **P < .01; ***P < .001.
EP prolongs survival in mouse models of leukemia. (A) Ten million HL60 cells were transplanted into the tail veins of NSG mice (n = 10) 4 hours after sublethal irradiation. Mice were divided into 2 groups, one receiving untreated drinking water, and the other receiving 1 mg/mL of EP in the drinking water starting at day +3. BM aspirates were performed on day +21 and FACS analysis was performed assessing donor cell chimerism (CD15+Ly5.1−; i). Kaplan Meier survival curve of HL60-transplanted mice (ii) treated with 1 mg/mL or 3.0 mg/mL EP in the drinking water versus mice with untreated drinking water. *P < .05; **P < .01. (B) One million URE cells were transplanted into the tail veins of NSG mice 4 hours after sublethal irradiation. Mice were divided into 3 groups, with one group receiving untreated drinking water (n = 7), one group receiving 0.4 mg/mL of EP in the drinking water (n = 5), and the other receiving 1 mg/mL of EP in the drinking water (n = 4) starting at day +3. Kaplan-Meier survival curve of URE transplanted mice treated with 0.4 or 1.0 mg/mL of EP in the drinking water versus mice with untreated drinking water is shown. **P < .01. (C) HL60 cells were treated with or without 20nM cytarabine (i), with or without 24 ng/mL of doxorubicin (ii) ± 5 μg/mL of EP for 72 hours. Cell viability was measured by cell counts with trypan blue exclusion. Data represent the means ± SD of viable cells performed in triplicate. **P < .01; ***P < .001.
We next assessed the effect of EP in NSG mice transplanted with URE leukemia cells. One million URE cells were IV injected via the tail vein in NSG mice. Mice were then treated starting at day +3 with 0.4 or 1.0 mg/mL of EP in the drinking water or with untreated drinking water. We have previously shown that treatment with 0.3 mg/mL of EP in the drinking water results in EP serum levels greater than 2 μg/mL.10 EP showed a dose-dependent, statistically significant (0.4 mg/mL of EP, P = .005; 1.0 mg/mL of EP, P = .002) effect on the survival of mice with URE cell leukemia (Figure 5B), once again demonstrating the leukemia-inhibitory effect of EP in vivo.
We assessed the effect of EP given in combination with standard AML chemotherapeutic agents. HL60 cells were incubated with EP with or without cytarabine or doxorubicin. Manual cell counts at 72 hours showed the addition of EP significantly enhanced the antiproliferative effects of cytarabine and doxorubicin (P = .002 and P = .005, respectively; Figure 5Ci-ii).
Finally, given that stromal cells in multiply transfused patients are often iron overloaded and may mitigate the antileukemic effect of EP, we performed coincubation experiments in vitro. Human HS5 stromal cells were preloaded with iron or mock treated for 72 hours and then coincubated with HL60 cells with or without EP treatment. Neither untreated nor iron-overloaded stromal cells alleviated the inhibitory effect of EP on HL60 cell proliferation (supplemental Figure 7).
Discussion
In the present study, the nonpeptide, small-molecule TPO-R agonist EP inhibits leukemia cell growth by depletion of intracellular iron. EP is currently being investigated in clinical trials as a treatment for thrombocytopenia in patients with MDS, and this new discovery that EP reduces intracellular iron provides support that EP may be a promising adjuvant agent for patients with AML and MDS for several reasons. First, the majority of patients with AML and MDS are over the age of 60 years and thus many patients are not able to tolerate intensive chemotherapy and transplant conditioning regimens.4-6 In addition, many patients with AML and MDS are transfusion dependent and suffer from iron overload. Finally, many patients endure significant morbidity and mortality related to thrombocytopenic bleeding in MDS. An agent such as EP that has an antileukemic effect and does not induce thrombocytopenia, but rather stimulates megakaryopoiesis and platelet production, has the potential to decrease clinically significant thrombocytopenic bleeding and thus improve survival.
Our in vitro studies demonstrate that EP inhibits leukemia cell growth through induction of differentiation, as well as slowed cell division mediated by inhibition of the cell-cycle transition from the G1 to the S phase. These observations are similar to and consistent with prior studies describing the antileukemic effects of known iron chelators and differentiating agents.20-26 Studies show that iron chelators have anticancer effects in a variety of malignancies, including breast cancer and melanoma, as shown in patients and solid tumor xenograft models.25,27,28 It is unclear which exact molecular pathways mediate the antileukemic effects of EP-induced reduction of intracellular iron. Our present data suggest that down-regulation of cell-cycle–promoting genes and/or an increase of ROS may play a role. However, nearly complete rescue of ROS with N-acetyl-L-cysteine resulted only in a partial rescue from the differentiating effects of EP, suggesting that ROS is not the sole pathway mediating differentiation. Further mechanistic studies will be required to understand the precise molecular and functional sequelae of EP treatment in leukemia cells. It will also be important to further understand how EP interacts with traditional chemotherapy agents, because it would likely be given clinically in conjunction with other cytotoxic therapies. Given the ability of EP to reduce intracellular iron and inhibit growth in leukemia cells, it also seems promising to evaluate the antiproliferative or differentiating effects of EP in other malignancies.
Previous studies have shown that TPO-R is present on a significant percentage of AML cells and that TPO may stimulate blast cells expressing TPO-R.29-31 Recent studies have postulated that the antileukemic effect of EP is independent of the TPO-R pathway by demonstrating that the effect of EP in leukemia and lymphoma cells lines was not related to the level of TPO-R expression.12 In addition, a peptibody that stimulates platelet production through TPO-R may stimulate blast production and the transformation from MDS to AML in patients with low-risk MDS.32 In the present study, we demonstrate that the ability of EP to reduce intracellular iron is not a mechanism shared by the peptibody TPO-R agonist (supplemental Figure 3), and show for the first time that the antileukemic effect of EP is not related to the TPO-R pathway. It has been shown previously that EP interacts with the transmembrane domain of TPO-R and that this interaction and consecutive TPO-R downstream signaling is highly species specific, occurring only in humans and primates and not in murine cells.16 Our present finding of growth inhibition and differentiation of murine leukemia by EP in vitro and in vivo demonstrates that these effects are TPO-R independent.
The design of small-molecule, nonpeptidyl TPO mimics was originally based on the premise that these molecules have 3 main features: a lipophilic end, an acidic end, and a chelator backbone (supplemental Figure 2).33,34 The structure of EP has these 3 elements and supports the ability of EP to reduce intracellular iron and potentially other polyvalent cations.35,36 Given that EP has a chelator backbone that is not iron specific, it is likely that EP chelates other polyvalent cations such as zinc and copper.36 These cations are required for the function of a variety of important regulatory proteins and have been shown to play a role in cancer growth. Further studies are warranted to determine their contribution to EP-induced inhibition of leukemia cells and potentially other malignancies.
A recent double-blind, placebo-controlled, randomized dose-escalation study showed that maximum serum concentrations of EP of more than 20 μg/mL are clinically achievable with minimal toxicity.13 These levels are equal to or higher than the levels at which EP reduced intracellular iron and exhibited potent antileukemia effects in our in vitro experiments and in vivo mouse models.10 Interestingly, BM suppression and anemia have not been reported in patients receiving EP,7,8,17 supporting the selective anticancer effect of iron chelators and the ability of normal hematopoietic cells to better tolerate iron depletion compared with malignant cells.10,37,38
The properties of EP may prove important as both an adjuvant anticancer therapy and as a supportive care agent, potentially improving platelet counts and platelet recovery in patients receiving BM-suppressive treatments and reducing excess iron in multiply transfused patients.39 The results of the present study provide the basis to further study EP in MDS and AML patients and to consider exploring its effectiveness in other cancers.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the staff of the Stem Cell FACS and Xenotransplantation Facility (supported by NYSTEM, C024172) and the Albert Einstein Genomics Core Facility.
This work was supported by an American Cancer Society/J. T. Tai & Company postdoctoral fellowship (to B.W.). U.S. is the Diane and Arthur B. Belfer Faculty Scholar in Cancer Research of the Albert Einstein College of Medicine and is the recipient of a Howard Temin Award of the National Cancer Institute (R00CA131503) and a Medical Research Award of the Gabrielle's Angel Foundation for Cancer Research.
National Institutes of Health
Authorship
Contribution: M.R. designed the experiments, performed most of the experiments, analyzed the data, and wrote the manuscript; B.W. contributed to the design of experiments, performed the experiments, analyzed the data, and reviewed the manuscript; G.S. and R.T. performed the experiments and analyzed the data; S.N. performed the experiments; L.B. designed the experiments and reviewed the manuscript; B.B. analyzed the data and wrote and reviewed the manuscript; C.S.M. and A.V. contributed to the design of experiments, analyzed the data, and reviewed the manuscript; and U.S. designed the research, supervised the study, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: A.V. and U.S. are the recipients of funding from GlaxoSmithKline for research different from the research reported in this manuscript. The remaining authors declare no competing financial interests.
Correspondence: Ulrich Steidl, Department of Cell Biology, Chanin Bldg No. 606, Albert Einstein College of Medicine, 1300 Morris Park Ave, Bronx, NY 10461; e-mail: ulrich.steidl@einstein.yu.edu.