Abstract
Abstract 221
In vivo T cell depletion with either Antithymocyte Globulin (ATG) or Alemtuzumab (Alem) for GVHD prophylaxis is frequently included in reduced intensity conditioning (RIC). We recently reported the results of two Phase II studies performed by GITMO in high risk patients undergoing allogeneic stem cell transplantation (HSCT) using an unrelated donor (UD) after conditioning with either an ATG or Alem based RIC (Rambaldi et al.: Leukemia 2012). Since both these programs proved to be well tolerated and reasonably effective we compared them in a prospective randomized Phase II clinical trial (ClinicalTrial.gov Identifier: NCT00354120).
The composite primary end point was the efficacy (overall and event free survival) and the safety (infectious complications and GVHD) of the two conditioning regimens. In addition, the outcome of patients receiving an UD transplant was compared (by a time dependent analysis, correcting for time to transplant) to patients who were not randomized for the lack of a donor or any other reasons. From March 2005 to September 2008, 245 patients were registered into this study when the UD search was activated. Of these patients, 121 were randomized and 112 (AML n=27, NHL n=27, HD n=26, MDS n=14, B-CLL n=11, ALL n= 4, CML n= 2, and MF n= 1) underwent HSCT (median age 58 years, range 17–67). The 2 randomized groups were well balanced. All these patients were unfit for conventional transplants due to age (between 55 and 65 years for acute leukemias, myelodysplastic syndromes, chronic myeloid leukemia and myelofibrosis) or advanced disease (for Hodgkin and non-Hodgkin Lymphomas). A previous autologous transplant was performed in 49% of patients while 26% had received more than 3 courses of chemotherapy. Patients were randomized to the following RIC programs: program A, (Melphalan 30 mg/m2, Fludarabine 90 mg/m2, TBI 200cGy and Alem 80 mg) (n= 58) or program B, (Thiotepa 10 mg/Kg, Cyclophosphamide 100 mg/Kg, Melphalan 30 mg/m2 and ATG 7,5 mg/Kg) (n= 54). At transplant, 52 patients were in complete remission (CR), 38 patients were in partial remission (PR) while the remaining 22 patients had active disease. Of the 124 patients who were not randomized, 58 stopped the UD search because of death before randomization. Of the remaining 66 patients, 31 were transplanted with an alternative program and 35 were unable to proceed to HSCT.
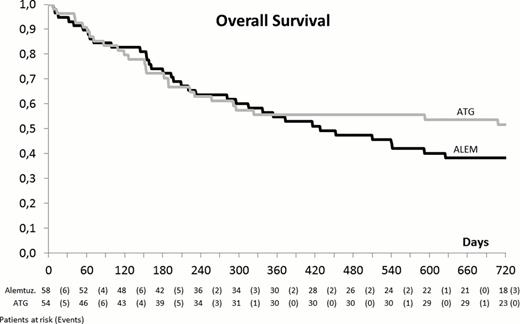
The median time of neutrophil and platelet engraftment was similar in the 2 study arms (18 vs. 16 days and 18 vs. 18 days, respectively) but graft failure and graft rejection occurred most likely in arm A (9 vs. 1, p 0.036) and a better full donor chimerism in peripheral blood CD4+ cells was observed in arm B (93% vs. 68%, p 0.052). Of the 24 patients who had to be treated with DLI, 15 belonged to arm A and 9 to arm B (p 0.476). At day +100 the incidence of acute GVHD (> grade II) was slightly higher in the ATG arm (37% vs. 26%, p 0.224) but a higher incidence of late occurring acute GVHD occurred in the Alem arm, most likely because of DLI (5/6 patients, all with GVHD > grade III). The incidence of chronic GVHD was similar (40% and 41%) in both treatment arms. The immunologic reconstitution (time to CD4+ cells > 100/mcl) was slower in arm A (174 vs. 113 days, p 0.058) but no significant difference was observed in the incidence of viral, bacterial or fungal infections. A similar incidence of CMV disease (10% vs. 6% in arm A and B, respectively) and PTLD (5% in A vs. 11% in B) was observed. With a median follow up of 15 months (range 1–39), at 2 years, the non-relapse mortality (NRM) (36% vs. 35%) and the relapse rate (34% vs. 25%, p 0.294) do not differ in the 2 arms. Similarly, despite a trend for a better outcome for patients randomized to arm B, no significant difference was observed in terms of EFS (29% vs. 41%, p 0.295) and OS (36% vs. 52%, p 0,206) (Figure 1). By a multivariable model for the prediction of OS, a significant decrease of the risk of death was associated with the incidence of acute (p= 0.02) and chronic GVHD (p <0.001). At 3 years, the OS of randomized and non-randomized patients is similar (43% vs. 34%, p= ns).
A better engraftment and immune reconstitution as well as a trend for a lower relapse rate was observed in patients receiving an ATG based RIC. GVHD confirms its protective role on the risk of death confirming that an accurate dosing of in vivo T cell depleting agents is crucial for the long term clinical outcome after HSCT.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.