Abstract
Abstract 2706
Adult T-cell leukemia/lymphoma (ATLL) is a mature T-cell malignancy associated with human T-cell lymphotropic virus -1 (HTLV-1) infection. Four clinical subtypes are recognized including acute/lymphomatous (aggressive) or chronic/smouldering (indolent). For the aggressive subtypes the median overall survival is 6 months despite aggressive combination chemotherapy. Novel therapeutic targets against CD25, CD52, and NF μB have shown preliminary activity in ATLL. Recently, the chemokine receptor 4 (CCR4) antibody (KW-0761) was approved in Japan based on a phase II overall response rate of 50% and median overall survival of 13.7 months in 28 patients with relapsed/refractory ATLL. CD30 (Ki-1) is a marker of immune activation expressed on lymphocytes, and is an emerging target in lymphoma. The novel antibody-drug conjugate brentuximab vedotin targets CD30 and has shown efficacy in Hodgkin lymphoma and anaplastic large cell lymphoma (ALCL). While CD30 positive ATLL is infrequent in Japanese ATLL patients, ranging from 12.1% to 19.4%, the prevalence of CD30 positive ATLL outside of Japan has not been extensively studied.
To investigate the prevalence of CD30 expression and its importance in ATLL outside of Japan we conducted a retrospective review of all patients who underwent treatment or consultation for ATLL at our institution between 1998 and 2012. Individual chart review was performed to report the clinical presentation, pathological features, and outcomes. Pathology review of CD30 expression status was conducted if specimens were available. The CD30 (Ber-H2) antibody from Ventana was used on automated platforms per manufacturer's instructions. Specimens were determined to be positive if the CD30 expression was >20% by an expert hematopathologist (AC).
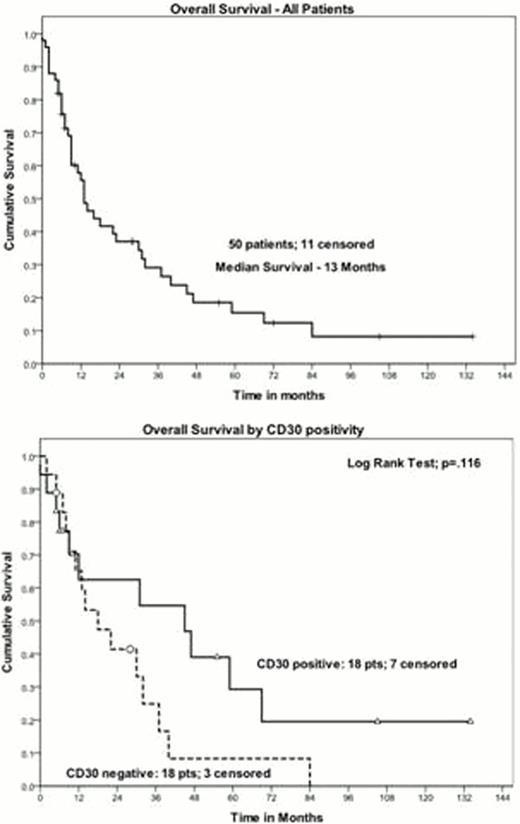
We identified 50 patients with ATLL. Patient characteristics were: median age 54 (range 30–82); male:female 19:31; Caribbean-39, American-6, Western African-2, Peruvian-2, and Greek-1. Subtype of disease at diagnosis was: Acute/Lymphomatous—41 and Chronic/Smouldering—9. Thirty-six of 50 (72%) patients' samples had been tested at least once for CD30. Eighteen patients (50%) were reported positive. Of all cases reviewed (N=50), eight had pathology available for confirmatory review. Of those, five patients remained positive, 1 patient was reclassified as positive after initially being reported as negative, and two remained negative. The median overall survival of all patients was 13 months. In the subset who had CD30 status assessed the median overall survival for CD30 negative and positive ATLL was 18 and 45 months respectively (p=0.116).
CD30 expression on normal lymphocytes is rare. Our retrospective data suggests that ATLL patients presenting outside of Japan may demonstrate CD30 positivity at a higher prevalence compared to Japanese reports. In pre-clinical studies of CD30 positive ATLL, SGN-35 has been shown in vitro and in vivo to demonstrate similar cytotoxicity compared to ALCL (Maeda et al. Cancer Sci 2010). Novel strategies are clearly needed to improve the outcome of ATLL despite recent advances. Therefore, CD30 testing may be considered in ATLL as a novel target for future research strategies.
Matasar:GSK: Research Funding; Genentech: Consultancy. Straus:Seattle Genetics: Consultancy. Zelenetz:GSK: Consultancy, Research Funding; Genentech/Roche: Consultancy, Research Funding; Gilead: Consultancy; Sanofi Aventis: Consultancy; Cephalon: Consultancy; Celgene: Consultancy; Seattle Genetics: Consultancy; Amgen: Research Funding. Horwitz:Seattle Genetics: Consultancy, Research Funding; Allos: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy; Genzyme: Consultancy; Kyowa Hakko Kirin Pharma: Consultancy; Johnson & Johnson: Consultancy; Infinity Pharmaceuticals, Inc.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.