Abstract
Abstract  4207
4207
We retrospectively analyzed outcomes of 90 consecutive patients with hematologic malignancies (median age: 34, range: 3–68 years) who relapsed after first allogeneic hematopoietic cell transplantation (HCT) and underwent second or subsequent allografts. Diagnoses included acute myeloid leukemia (AML, n=45), acute lymphocytic leukemia (n=22), myelodysplastic syndrome (MDS, n=11), chronic myeloid leukemia (n=4), non-Hodgkin lymphoma (n=3), chronic lymphocytic leukemia (n=2), Hodgkin's lymphoma (n=2) and myelofibrosis (n=1). Eighty-seven patients had recurrence of their original hematologic malignancy, while in three patients, AML (n=2) or MDS (n=1) arose from the donor cells.
Donors for first allogeneic HCT were HLA-matched related (n=45), HLA-matched unrelated (n=26) or HLA-mismatched (n=19, including 5 HLA-haploidentical and 6 double cord blood unit) donors. Conditioning regimens for first HCT included myeloablative regimens with or without ATG (cyclophosphamide-total body irradiation, TBI, n=49; busulfan-cyclophosphamide, n=24); low-dose TBI (2 Gy) with or without fludarabine (n=10) and other regimens (n=7).
The median interval between first and second allogeneic HCT was 24 (range: 2–137) months. Donors for second allogeneic HCT were HLA-matched related (n=24), HLA-matched unrelated (n=42) or HLA-mismatched (n=24; including 5 HLA-haploidentical and 8 double cord blood unit) donors. In 15 cases the original donors were used for second HCT, while in 75 cases different donors were identified. Conditioning for second HCT included low-dose TBI (2–4 Gy) with or without fludarabine, with or without cyclophosphamide (n=49), treosulfan and fludarabine containing regimens with or without low-dose (2 Gy) TBI (n=17), high-dose TBI (n=16), or busulfan (n=8) based regimens.
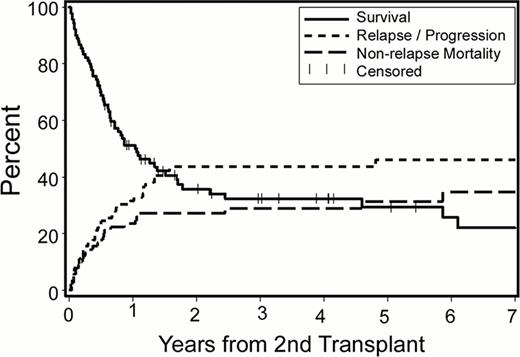
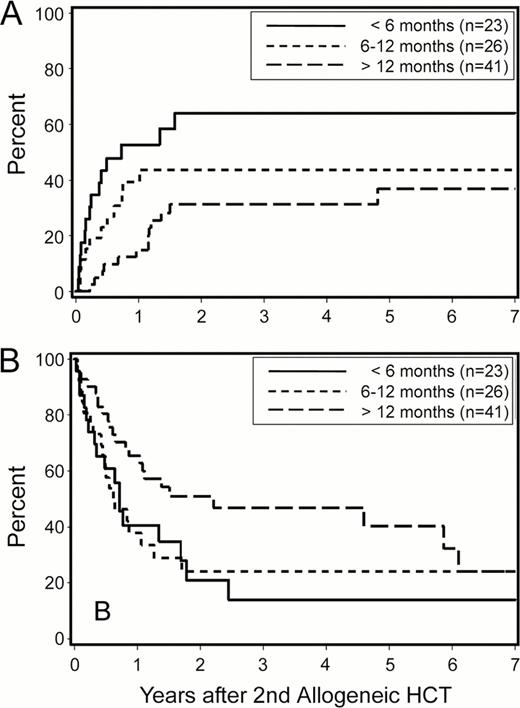
With a median follow-up of 38 (range, 7 to 154) months among surviving patients, the 2-year Kaplan-Meier overall survival (OS), relapse rate and non-relapse mortality (NRM) estimates were 36%, 44% and 27%, respectively (Figure 1). When the analysis was restricted to patients with AML, OS, relapse rate and NRM were 29%, 45% and 30%, respectively. For the whole cohort, cumulative incidences of grades 2–4 acute, grades 3–4 acute and extensive chronic graft-versus-host disease (GVHD) at 2 years were 67%, 13% and 31%, respectively. Relapse was the leading cause of treatment failure. Patients with early relapse after their first allogeneic HCT (within 6 months vs. 6–12 months vs. more than 12 months) had significantly higher 2-year relapse rates after second transplants (64%, 44%, 31%, respectively; p=0.01; Figure 2A). This translated into overall survival differences with borderline statistical significance (21%, 24% and 51%, respectively, p=0.08; Figure 2B).
Patient age, donor type (related, unrelated, HLA-mismatched), same vs. different donors for second HCT, conditioning intensity and HCT comorbidity scores did not appear to affect outcomes in univariate analyses in this group of patients.
Based on this retrospective analysis, we conclude that post-transplant relapse can be treated with second allogeneic HCT. Limitations of this study include the retrospective nature of the analysis and heterogeneity of conditioning regimens; nonetheless, rates of acute and chronic GVHD, relapse and NRM appear similar to those reported after 1st allogeneic HCT. Therefore, second allogeneic HCT should be considered for post-transplant relapse, especially in patients who relapse more than 12 months after their first allografts.
Overall survival, relapse/progression rate and non-relapse mortality of 90 patients undergoing 2nd allogeneic HCT for relapse after first allografts.
Overall survival, relapse/progression rate and non-relapse mortality of 90 patients undergoing 2nd allogeneic HCT for relapse after first allografts.
Relapse (A) and overall survival (B) after second allogeneic HCT according to timing of relapse following first allogeneic HCT.
Relapse (A) and overall survival (B) after second allogeneic HCT according to timing of relapse following first allogeneic HCT.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract