Abstract
Abstract 886
The diagnosis acute myeloid leukemia (AML) describes a heterogeneous group of myeloid stem cell disorders. Based on current concepts of disease development, the combination of at least two mutations is necessary for transformation, typically affecting transcription factors (e.g. RUNX1) blocking normal differentiation and growth promoting genes, e.g. receptor tyrosine kinases like FLT3. This model has been challenged by more recent results of genome-wide mutational analysis, which revealed a typical load of 8–12 mutations affecting several additional pathways, e.g. epigenetic regulation (DNMT3A, TET2 or IDH1). However, the sequence of acquisition and the individual impact of these mutations are largely unknown because these aspects are difficult to study. Here we describe a unique case of a donor cell leukemia giving unexpected insights into the development of AML in man.
In May 2004, a 51-year old male (P1) with an 8-year history of B-CLL received G-CSF mobilized peripheral blood stem cells after dose reduced conditioning from his HLA-identical sister, because he had relapsed after several lines of conventional therapy. He rapidly engrafted and showed complete donor chimerism (DC). In February 2012, he was admitted to the hospital with elevated WBC counts and circulating blasts. Bone marrow (BM) aspiration and morphology revealed an infiltration of the BM with 94% myeloid blasts (FAB M1). Cytogenetic and standard molecular assessment showed a normal female karyotype and NPM1 and FLT3-ITD mutations. STR-based analysis also revealed a persistent, 100% DC, thus the diagnosis of a donor cell AML was made, which developed almost 8 years after SCT. Interestingly, his sister, the donor (P2) had been also diagnosed with AML (FAB M2, cytogenetics: 47, XX,+8; NPM1 and FLT3-ITD neg.) only 3 months before her brother in Nov. 2011. Currently, P1 is in CR after re-SCT from an unrelated donor, whereas P2 relapsed and is scheduled for SCT after reinduction. Since DNA material of both individuals was available and due to this unique constellation, we performed next generation sequencing of whole exome enriched material using an Illumina HiSEQ 2000 platform after obtaining informed consent to compare both AMLs. Identified mutations were then confirmed using conventional Sanger sequencing and traced back by 454-based amplicon deep sequencing in a pre-SCT sample of the donor/P2 as well as several post SCT samples collected from P1 for the documentation of chimerism.
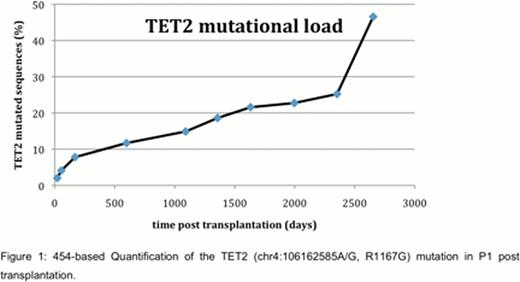
Comparison of the two AML-samples with a pre-SCT donor sample and a sample taken after 1.SCT as well as the HG19 and dbSNP135 releases revealed more than 100 unknown SNPs. Confirmation focused on cancer related changes or genes in critical pathways. In P1, in addition to the known NPM1 and FLT3-ITD mutations, we found somatic changes in CLCA1, PKHD1 and TET2, whereas in P2, we identified and confirmed somatic mutations in CDCA2, CBL, IDH1, NEK9 and PHF6. In addition, a typical DNMT3A R882C mutation was found in both leukemias. Interestingly, this mutation was also detectable by conventional Sanger sequencing in the pre-SCT sample of P2, but not in P2-germline DNA derived from buccal swaps. As shown in the figure, the 454-amplicon sequencing revealed a gradual increase of the TET2 (R1167G) mutational load over time in P1, and showed also that this mutation was present at low levels (4%) already in the pre-SCT sample of P2, but not in her final AML. FLT3-ITD and NPM1 mutations were detectable only in the AML-sample of P1, but not at any prior time points or in P2.
These data indicate that mutations like the DNMT3A R882C can be present in normal appearing hematopoiesis at high levels years before the development of AML. The presence of the mutation in the absence of overt leukemia or MDS indicates that these mutations might not have a direct effect on the development of the disease, but favor the development of aberrant clones which then acquire additional changes in a latency phase. Other mutations (e.g. TET2) might give a small clonal advantage, but only the final acquisition of abnormalities like NPM1 and FLT3-ITD might transform this latency phase into a rapidly proliferating status, consistent with the “driver” status of these aberrations. The divergent mutational pattern found in the two AMLs emerging from the same DNMT3A-starting clone points to the high clonal diversity which might develop even within a single individual.
Thiede:AgenDix GmbH: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.