Abstract
Coronary heart disease is a major cause of death in the western world. Although essential for successful recovery, reperfusion of ischemic myocardium is inevitably associated with reperfusion injury. To investigate a potential protective role of ADAMTS13, a protease cleaving von Willebrand factor multimers, during myocardial ischemia/reperfusion, we used a mouse model of acute myocardial infarction. We found that Adamts13−/− mice developed larger myocardial infarctions than wild-type control mice, whereas treatment of wild-type mice with recombinant human ADAMTS13 (rhADAMTS13) led to smaller infarctions. The protective effect of ADAMTS13 was further confirmed by a significant reduction of cardiac troponin-I release and less myocardial apoptosis in mice that received rhADAMTS13 compared with controls. Platelets adherent to the blood vessel wall were observed in few areas in the heart samples from mice treated with vehicle and were not detected in samples from mice treated with rhADAMTS13. However, we observed a 9-fold reduction in number of neutrophils infiltrating ischemic myocardium in mice that were treated with rhADAMTS13, suggesting a potent anti-inflammatory effect of ADAMTS13 during heart injury. Our data show that ADAMTS13 reduces myocardial ischemia/reperfusion injury in mice and indicate that rhADAMTS13 could be of therapeutic value to limit myocardial ischemia/reperfusion injury.
Introduction
Coronary heart disease is the leading cause of death in the western world with approximately 1 million myocardial infarctions (MIs) each year just in the United States.1,2 Acute myocardial infarction (AMI) is caused by thrombotic occlusion of a coronary artery. Although rapid restoration of the coronary circulation is critical for successful treatment, reperfusion itself exacerbates injury of previously ischemic myocardium.1 The exact mechanisms of myocardial ischemia/reperfusion (MI/R) injury are not fully understood.1 Given that cardiac ischemia is either unpredictable (MI) or inevitable (in patients undergoing cardioplegic arrest), there is great interest in developing strategies to minimize injury. von Willebrand factor (VWF) and its cleaving protease ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type-1 motif, member 13) play a pivotal role in platelet adhesion and thrombus formation. By specifically cleaving the VWF A2 domain, ADAMTS13 digests the most thrombogenic ultra-large VWF multimers (UL-VWF) into smaller, less hemostatically active VWF molecules. In addition, ADAMTS13's action on VWF down-regulates inflammatory responses. As a result, using experimental mouse models, ADAMTS13 was shown to reduce both thrombosis and inflammation, including atherosclerosis.3-5
An increasing amount of clinical evidence points to the possibility that VWF and ADAMTS13 are involved in MI pathogenesis.6 To test this experimentally, we used a mouse model of acute myocardial infarction. Using mice deficient in ADAMTS13 and treating wild-type mice with human rhADAMTS13, we show that ADAMTS13 indeed attenuates MI/R and that rhADAMTS13 might have therapeutic value in the management of AMI.
Methods
Mice
The Adamts13−/− mice7 were on C57BL/6J background. Wild-type (WT) C57Bl/6J mice were purchased from The Jackson Laboratory. All animals used in this work were males, 8 to 10 weeks old. All experimental procedures were approved by the Animal Care and Use Committee of the Immune Disease Institute.
rhADAMTS13
Preparation of rhADAMTS13 expressed by a stably transfected CHO (Chinese hamster ovary) cell line was previously described by Zhao et al.8 However, the terminal half-life of the research grade rhADAMTS13 was only roughly estimated at that time. Mouse VWF is readily cleaved by this rhADAMTS13 in vitro and in vivo, as shown in supplemental Figures 1 and 2, respectively (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Induction of myocardial ischemia
Mice were anesthetized using 2% isoflurane and placed in supine position on a heated operating table. An endotracheal tube (PE90) was inserted into the trachea and mice were mechanically ventilated using a small animal ventilator (Harvard Apparatus) with 2% isoflurane. After a midline skin incision, a median sternotomy was performed via a lateral incision along the left side of the sternum (cutting the second, third, and fourth rib) to expose the heart and allow visualization of the left anterior descending branch (LAD) of the left coronary artery. An 8-0 polyamide suture (Aros Surgical) was passed underneath the LAD 1 to 3 mm from the tip of the normally positioned left auricle. Both ends of the suture were passed through a 1-mm piece of PE-10 tubing (BD) and then through the chest wall. After implantation of the occluding device, the chest was closed and the animal was allowed to recover for 1 week. After 1 week, the mouse was reanesthetized with 2% isoflurane and a 3-lead ECG system (Powerlab 4/30 with Labchart 7 software, AD Instruments) was attached to monitor the mouse electrocardiogram. The midline skin incision was reopened and the implanted 8-0 silk suture was pulled to acutely occlude the LAD, which was verified by visual inspection of the coronary bed as well as distinguishing specific changes in the electrocardiographic tracing. After 1 hour of occlusion, blood flow was restored by releasing the 8-0 silk suture. Reperfusion was verified by ECG monitoring. After occlusion and reperfusion, the skin was again closed and the animal was allowed to recover. Throughout the implantation of the occluding device as well as during the 1 hour of ischemia, the body temperature was continuously monitored and tightly controlled (36.5 ± 1°C) using a rectal probe connected to a temperature controller (Fine Science Tools). Sham-operated animals underwent all the steps except for pulling the suture to occlude the LAD. Mice treated with rhADAMTS13 were injected intravenously into the retro-orbital sinus with 3500 U/kg rhADAMTS13 (or saline as vehicle) 1 hour before reperfusion.
Determination of area at risk
To verify that there were no anatomical differences between WT and Adamts13−/− mouse strains that could lead to a bias in infarct size, we determined the area at risk of both genotypes. The area at risk was defined as the proportion of the left ventricle that is not perfused after occlusion of the LAD. To determine the area at risk, hearts were harvested 1 week after implantation of the occluding device as previously described. The ascending aorta was cannulated with a 22-gauge tubing adaptor and the LAD was tied off, after which 1% Evans Blue dye was perfused into the aorta and coronary arteries. As a result, all heart tissue stained blue, except for the territory that is normally perfused by the LAD, which remained white. Using the same approach as described for determination of infarct sizes, the area at risk was determined for each heart. Experiments were performed by an experimenter blinded to genotype and treatment.
Assessment of infarct size
After 23 hours of reperfusion, the mouse was killed and the heart was harvested. The heart was then sectioned transversely into 4 pieces with 1 piece including the site of LAD ligation. Each ventricular section was incubated with 1.0% 2,3,5-triphenyltetrazolium chloride (TTC, Sigma-Aldrich) for 15 minutes at 37°C. Viable myocardium appears red and infarcted tissue appears white after TTC staining. The right ventricle was then excised and the left ventricle was weighed. Both basal and apical sides were photographed and the area of infarction was determined by planimetry using ImageJ 1.43u software (National Institutes of Health; http://rsb.info.nih.gov/ij/index.html). Using the average area of infarction and the weight of each piece, the amount of infarcted tissue per piece was calculated. The total weight of infarcted tissue of the 4 pieces divided by the total amount of left ventricle tissue gave the percentage of infarction over the left ventricle for each heart. Analysis was performed by an experimenter blinded to treatment group.
Histology and immunologic assays
Mouse hearts were harvested after the 23 hour reperfusion period and fixed overnight in zinc-fixative at 4°C. Hearts were embedded in paraffin and the whole apical part of the heart, starting from the site of LAD ligation, was sectioned into 10-μm sections. Four sections per heart, evenly distributed throughout the sectioned tissue, were selected for analysis. To determine the origin of cells infiltrating myocardium, anti–mouse Gr-1 (Ly-6G and Ly-6C, marker for granulocytes/monocytes) antibody (dilution 1:500, clone RB6-8C5; BD Pharmingen) was used for immunofluorescent and immunohistochemical analyses. Sections were stained with TUNEL for apoptosis using an in situ cell death detection kit (Roche Diagnostics) according to the manufacturer's instructions. Double immunofluorescent staining against Gr-1 and TUNEL labeling was also performed. Anti–Gr-1 antibody was applied overnight followed by incubation with Alexa Fluor 555 goat anti–rat IgG (1:2000 dilution; Invitrogen) for 1 hour at room temperature and subsequently TUNEL staining was applied. Fluorescent images were acquired by a Zeiss Axiovert 200 inverted fluorescence microscope connected to a monochrome camera (AxioCam MRm) using Axiovision Version 4.6 software.
For immunohistochemical analysis, an anti–mouse CD41 (integrin αIIb) antibody (dilution 1:250, clone MWReg30; BD Pharmingen) and an anti–Gr-1 antibody were used as primary antibodies. Histofine simple stain MAX PO for rat (414311F; Nichirei Corporation) was applied as secondary antibody. Diaminobenzidine (DAB) substrate kit (415192F, Nichirei Corporation) was used for visualization of staining. Finally, sections were counterstained with hematoxylin. Control stainings did not contain primary antibodies.
Assessment of apoptosis
In 3 fields of view of the ischemic area of each section, total number of cells (blue nuclei) and number of apoptotic cells (overlapping blue and green nuclei) were counted. The apoptotic index was defined as the total number of apoptotic cells divided by total number of cells. All steps in the analysis were done by an experimenter blinded to treatment group.
Assessment of Gr-1–positive cells
Five consecutive pictures of the ischemic area were taken from each section and the number of Gr-1–expressing cells (brown color) was quantified by an experimenter blinded to treatment group. The number of Gr-1–positive cells was expressed as number per 1 mm2 of infarcted area.
Plasma collection
For blood collection, animals were anesthetized using 2.0% isoflurane. Blood was collected by retro-orbital puncture on 3.8% trisodium citrate (1 volume to 6 volumes of blood) for VWF and ADAMTS13 assays and on 0.5M EDTA (1 volume to 100 volumes of blood) for cardiac troponin-I determination. Platelet poor plasma was prepared immediately after blood collection by centrifugation at 6800g for 5 minutes at room temperature (RT). Plasma was immediately stored at −80°C until analysis.
VWF antigen assay
Plasma levels of VWF were performed as described.9 Briefly, microtiter plates were coated overnight at 4°C with a polyclonal anti–VWF-Ig-solution (Dako). The plates were blocked for 2 hours at RT with 3% milk powder solution. Then, samples and a plasma pool were applied and incubated for 1.5 hours at 37°C. Anti–VWF-Ig-HRP (Dako) was added and incubated for 1 hour at RT. Visualization was obtained with 3,3′,5,5′-tetramethylbenzidine (Sigma-Aldrich) and the coloring reaction was stopped with 0.5M H2SO4. The absorbance was determined at 450 nm. After each incubation step, the plates were washed with PBS containing 0.1% Tween-20, 3 times after coating and blocking steps and 9 times elsewhere.
ADAMTS13 activity assay
ADAMTS13 activity in plasma was performed using the FRETS-VWF73 assay essentially as described.10 Reactions were performed in reaction buffer (50mM HEPES, 150mM NaCl, 5mM CaCl2, 1μM ZnCl2, pH 7.4) using 6 μL of plasma and a final concentration of 4μM FRETS-VWF73 substrate (Peptides International). Fluorescence intensities were measured every 5 minutes for 1.5 hours with a Fluoroskan Ascent microplate fluorometer (Thermo Scientific) using excitation at 355 nm and emission at 460 nm at 37°C.
Determination of plasma troponin-I levels
Plasma levels of troponin-I were determined using a mouse cardiac troponin-I ELISA kit (Life Diagnostics) as recommended by the manufacturer.
Statistical analysis
Data are expressed as mean ± SEM. For statistical analysis PrismGraph 4.0 software was used (GraphPad Software). Comparisons were performed using 1-way ANOVA followed by a Bonferroni multiple comparison test or by the unpaired, 2-tailed Student t test. P values less than .05 were considered statistically significant.
Results
Pharmacokinetic and toxicologic profile of rhADAMTS13
To establish the pharmacokinetics of the CHO cell-derived rhADAMTS13 used in this study, a nominal dose of 400 U/kg rhADAMTS13 was injected intravenously into Adamts13−/− mice. A cmax of 4.49 U/mL was observed, with an in vivo recovery for rhADAMTS13 of 65.3% (data not shown). The terminal half-life of rhADAMTS13 was 24.3 hours and its mean residence time 26.7 hours. To test for potential acute effects of high doses of rhADAMTS13, Adamts13−/− mice were intravenously injected with 4000 (n = 4), 16 000 (n = 4), or 32 000 U/kg rhADAMTS13 (n = 8), or saline as a negative control (n = 4). No test-item-related mortality was observed and the general clinical condition of the animals remained unchanged throughout the observation period. One day after rhADAMTS13 administration, hematocrit, platelet count, prothrombin time (PT), and activated partial thromboplastin time (aPTT) were measured and all mice were subjected to a macroscopic and microscopic pathologic investigation. The administration of rhADAMTS13 did not affect the hematologic parameters tested and did not reveal macroscopic or microscopic pathologic findings. Together, a “no observed adverse effect level” of 32 000 U/kg could be established under the conditions of this study. Based on these results, we considered a dose of 3500 U/kg as safe and appropriate to provide sufficient rhADAMTS13 levels over 24 hours.
ADAMTS13 reduces myocardial infarct size
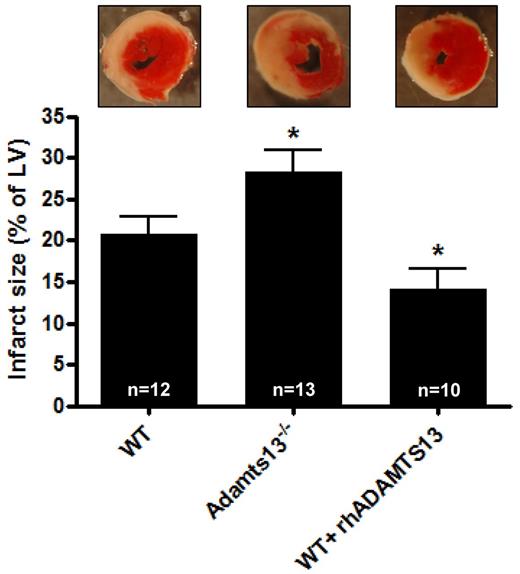
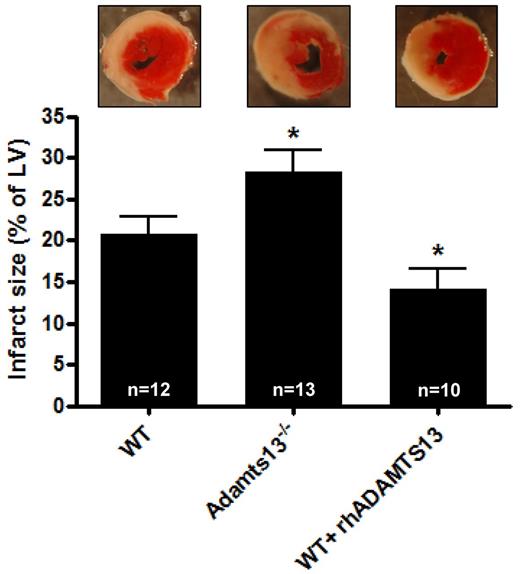
To assess the role of ADAMTS13 in MI/R injury we subjected WT and Adamts13−/− mice to transient LAD occlusion. WT and Adamts13−/− mice did not have differences in the area at risk (the proportion of cardiac tissue that is not perfused on LAD occlusion, supplemental Figure 3). After 1 hour occlusion of the LAD, followed by 23 hours of LAD reperfusion, hearts were harvested to analyze myocardial infarct size. We found that Adamts13−/− mice developed significantly larger infarctions compared with WT animals (28.2 ± 2.7% vs 20.8 ± 2.0%, respectively; P < .05; Figure 1). Because deficiency of ADAMTS13 leads to bigger infarctions, we hypothesized that myocardial damage could be reduced in the presence of elevated levels of ADAMTS13. To test this, we treated mice with rhADAMTS13 (3500 U/kg). Residual plasma ADAMTS13 activity after 23 hours of reperfusion was 5.2 ± 0.2 U/mL (n = 14). Interestingly, the ADAMTS13-treated mice indeed developed significantly smaller infarcts compared with control WT mice (14.2 ± 2.4%; P < .05; Figure 1). Together, these data point toward a protective role of ADAMTS13 in MI/R injury.
Infarct volumes after transient LAD occlusion in WT mice, Adamts13−/− mice and WT mice treated with rhADATMS13. Top: Representative TTC stains of transverse sections of the left ventricle (LV) of the 3 groups after 1 hour of LAD occlusion followed by 23 hours of reperfusion. Infarctions appear as white. Bottom: Myocardial infarctions (expressed as percentage of the LV) of the 3 groups as measured by planimetry at day 1 after LAD occlusion (*P < .05 compared with the WT group).
Infarct volumes after transient LAD occlusion in WT mice, Adamts13−/− mice and WT mice treated with rhADATMS13. Top: Representative TTC stains of transverse sections of the left ventricle (LV) of the 3 groups after 1 hour of LAD occlusion followed by 23 hours of reperfusion. Infarctions appear as white. Bottom: Myocardial infarctions (expressed as percentage of the LV) of the 3 groups as measured by planimetry at day 1 after LAD occlusion (*P < .05 compared with the WT group).
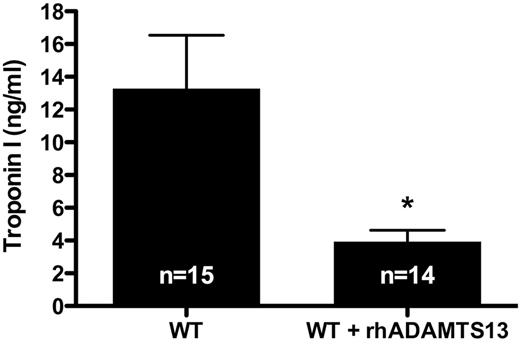
Troponin-I levels are lower in mice treated with rhADAMTS13
Cardiac troponin-I is a specific marker used to determine the extent of myocardial damage. Troponin-I plays a role in cardiac muscle contraction, is released into the circulation on heart injury and remains elevated for several days. To further establish the protective role of ADAMTS13 in MI/R injury, we determined the levels of troponin-I in plasma of WT mice that received rhADAMTS13 and control mice after 1 hour of LAD occlusion followed by 23 hours of reperfusion. Troponin-I levels were more than 3 times lower in mice treated with rhADAMTS13 than in control animals (3.95 ± 0.68 ng/mL vs 13.29 ± 3.24 ng/mL, respectively; P < .05; Figure 2). These results corroborated the reduced infarct size observed in mice treated with rhADAMTS13 (Figure 1) and further established the protective role of rhADAMTS13 in MI/R injury.
Troponin-I levels after transient LAD occlusion are decreased in mice treated with rhADAMTS13. After 1 hour of LAD occlusion followed by 23 hours of reperfusion, plasma was collected from WT mice and WT mice treated with rhADAMTS13. In these plasma samples, troponin-I levels were determined as measure for cardiac injury (*P < .05).
Troponin-I levels after transient LAD occlusion are decreased in mice treated with rhADAMTS13. After 1 hour of LAD occlusion followed by 23 hours of reperfusion, plasma was collected from WT mice and WT mice treated with rhADAMTS13. In these plasma samples, troponin-I levels were determined as measure for cardiac injury (*P < .05).
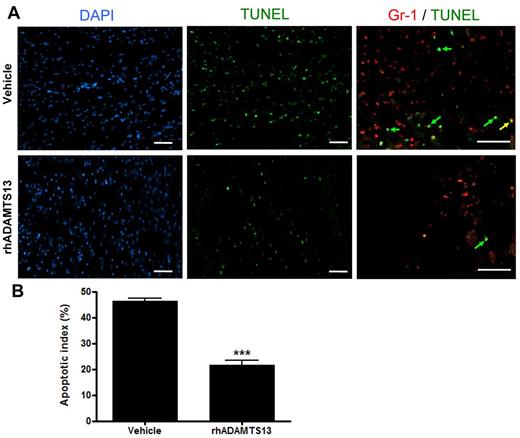
rhADAMTS13 limits MI/R-induced apoptosis
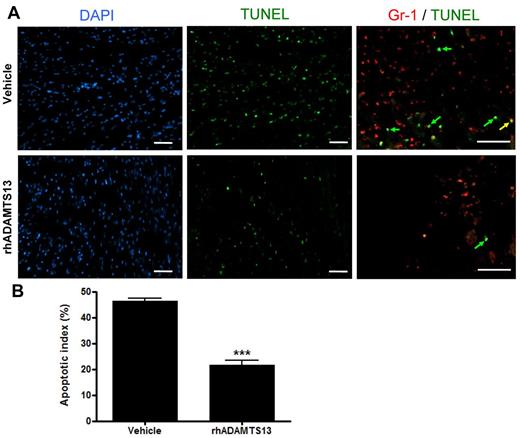
To understand the beneficial effect of rhADAMTS13 infusion on MI/R, we assessed the degree of apoptosis in the myocardial LAD territory via TUNEL staining. After 1 hour LAD occlusion and 23 hours of reperfusion, hearts of mice treated with rhADATMS13 or vehicle were collected, sectioned and stained with TUNEL (Figure 3A). The ratio of apoptotic cell number over total cell number was determined as expressed in percent (apoptotic index). We found a significantly lower apoptotic index in mice treated with rhADAMTS13 in comparison to control animals (21.6 ± 1.9% vs 46.3 ± 1.2%, respectively; P < .001; Figure 3B). On double staining for Gr-1 and TUNEL, we found that the main population of TUNEL-positive cells (Figure 3A green arrows) were not expressing Gr-1, and only a very limited fraction of Gr-1 positive cells were apoptotic (Figure 3A yellow arrow). Thus, these results suggest that rhADAMTS13 reduces the amount of myocardial apoptosis in the LAD territory, which is in agreement with the smaller infarct size (Figure 1) and reduced plasma troponin-I levels (Figure 2) in mice that received rhADAMTS13.
rhADAMTS13 reduces myocardial apoptosis after transient LAD occlusion. (A) Representative immunofluorescent DAPI (blue), TUNEL (green), and Gr-1 (red) stainings of sections from the ischemic area in the hearts of mice that received rADAMTS13 or vehicle. Apoptosis was absent in nonischemic areas (not shown). The higher magnification of the double stain (Gr-1/TUNEL) shows that majority of TUNEL-positive cells are not Gr-1–positive, that is, they are green rather than yellow (arrows). (B) The apoptotic index of both groups was determined by calculating the percentage of apoptotic cells (measured in 3 different view fields per section, 4 sections per mouse with 3 mice per group, ***P < .001). Scale bar = 50 μm.
rhADAMTS13 reduces myocardial apoptosis after transient LAD occlusion. (A) Representative immunofluorescent DAPI (blue), TUNEL (green), and Gr-1 (red) stainings of sections from the ischemic area in the hearts of mice that received rADAMTS13 or vehicle. Apoptosis was absent in nonischemic areas (not shown). The higher magnification of the double stain (Gr-1/TUNEL) shows that majority of TUNEL-positive cells are not Gr-1–positive, that is, they are green rather than yellow (arrows). (B) The apoptotic index of both groups was determined by calculating the percentage of apoptotic cells (measured in 3 different view fields per section, 4 sections per mouse with 3 mice per group, ***P < .001). Scale bar = 50 μm.
rhADAMTS13 reduces neutrophil infiltration in the ischemic myocardium
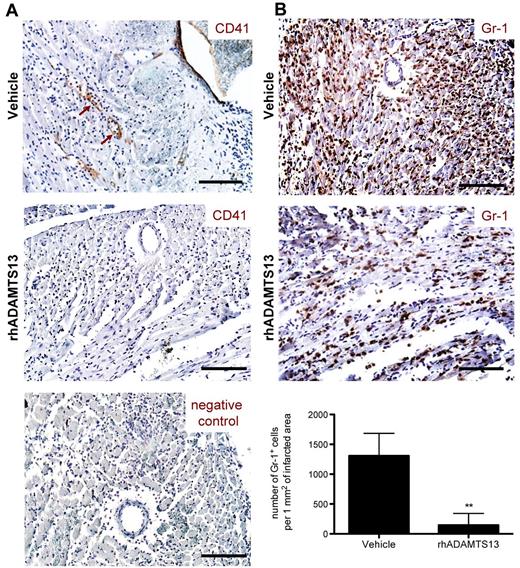
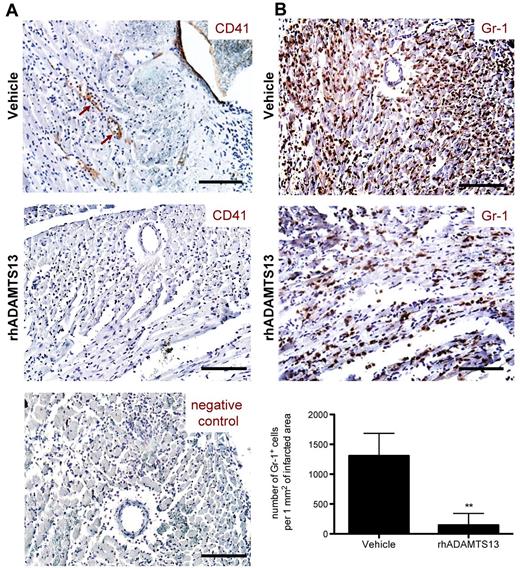
After having established the protective effect of rhADAMTS13 in MI/R injury, we searched to elucidate the potential mechanism of ADAMTS13 action. The only known enzymatic activity of ADAMTS13 is the cleavage of a specific bond in the VWF A2 domain, converting large VWF multimers into smaller ones.11 By doing so, ADAMTS13 has been shown to limit both thrombosis and inflammation.3,4 To investigate a potential antithrombotic effect, heart sections from rhADAMTS13 and vehicle-treated mice were stained using an antibody against CD41 (platelet specific integrin αIIb). Whereas no evidence of CD41-positive cells attached to the blood vessel wall was seen in mice treated with rhADAMTS13 (Figure 4A), adherent cells expressing CD41 (Figure 4A brown arrows) were detected in blood vessel lumen of reperfused LAD territory of vehicle-treated mice. However, CD41-positive events were too low to undeniably confirm an antithrombotic effect of rhADAMTS13 infusion.
Treatment with rhADAMTS13 reduces neutrophil infiltration and affects microthrombi events during myocardial ischemia/ reperfusion injury. After 1 hour of LAD occlusion and 23 hours of reperfusion, hearts from mice treated with either vehicle or rhADAMTS13 were harvested and sectioned for immunohistochemical analysis. (A) CD41 staining of heart sections. Adherent platelets, positive for CD41 (brown arrows), were seen only in some vessels and were detected only in vehicle, but not in rhADAMTS13-treated mice. Bottom: negative control staining without a primary antibody. (B) Gr-1–expressing neutrophils (brown color) infiltrated ischemic myocardium of vehicle-treated mice but significantly less did so in rhADAMTS13-treated mice. Bottom: Gr-1–positive cells in the ischemic area were counted in 5 different fields of view per section and numbers were expressed as positive cells per 1 mm2 of infracted area (3 mice per group, **P < .01). Scale bar = 100 μm.
Treatment with rhADAMTS13 reduces neutrophil infiltration and affects microthrombi events during myocardial ischemia/ reperfusion injury. After 1 hour of LAD occlusion and 23 hours of reperfusion, hearts from mice treated with either vehicle or rhADAMTS13 were harvested and sectioned for immunohistochemical analysis. (A) CD41 staining of heart sections. Adherent platelets, positive for CD41 (brown arrows), were seen only in some vessels and were detected only in vehicle, but not in rhADAMTS13-treated mice. Bottom: negative control staining without a primary antibody. (B) Gr-1–expressing neutrophils (brown color) infiltrated ischemic myocardium of vehicle-treated mice but significantly less did so in rhADAMTS13-treated mice. Bottom: Gr-1–positive cells in the ischemic area were counted in 5 different fields of view per section and numbers were expressed as positive cells per 1 mm2 of infracted area (3 mice per group, **P < .01). Scale bar = 100 μm.
We next looked for a possible anti-inflammatory effect of rhADAMTS13 administration. Heart sections of both control and rhADAMTS13-treated mice were stained for Gr-1. In control mice, we observed a large amount of cells positive for Gr-1, which were recognized as neutrophils infiltrating the reperfused LAD territory (Figure 4B). Strikingly, in mice that received rhADAMTS13, neutrophil infiltration was dramatically reduced (Figure 4B). Quantification analysis revealed a 9-fold decrease of neutrophils per 1 mm2 of infarcted area in mice treated with rhADAMTS13 compared with control mice (145.3 ± 196.7 vs 1307.3 ± 376.1 cells per 1 mm2 of infarcted area; P < .01). These results suggest a strong anti-inflammatory effect of rhADAMTS13 in MI/R injury.
Discussion
The metalloprotease ADAMTS13 is an important regulator of thrombosis and hemostasis. By reducing the size of highly reactive UL-VWF multimers, ADAMTS13 prevents excessive accumulation of these prothrombotic molecules. Deficiency of ADAMTS13 is associated with the devastating disease thrombotic thrombocytopenic purpura (TTP), caused by VWF-mediated micro-vascular thrombosis. One of the typical signs of TTP is MI/R injury, suggesting a link between low ADAMTS13 activity and MI/R injury.12-16
Clinical studies revealed that low ADAMTS13 (and high VWF) levels are associated with an increased risk of MI.6,17-22 ADAMTS13 polymorphisms that decrease ADAMTS13 activity are also associated with an increased risk of death in patients with coronary artery disease.23 Moreover, VWF and ADAMTS13 were both identified in coronary thrombi isolated from patients with AMI, although UL-VWF has been detected in plasma from patients with AMI.20,22,24-26 Whereas it is difficult to directly compare the extent of acute injury in our experimental mouse model of AMI with the clinical risk of MI occurrence, these clinical data at least further indicate a role for ADAMTS13 in MI progression. One limitation of clinical case-control studies is the intricacy of determining whether low ADAMTS13 levels are cause or consequence of the cardiovascular event. To examine a potential consequential effect, we used our mouse model to measure VWF levels and ADAMTS13 activities in plasma of mice before and after MI/R. As observed in patients with MI, we measured an increase in VWF levels and a concomitant decrease in ADAMTS13 activity after MI/R. However, this was also the case in sham-operated animals, indicating that these changes were not a consequence of MI/R itself (supplemental Figure 4).
Using an experimental mouse model of acute myocardial infarction, we found that ADAMTS13-deficient mice developed larger myocardial infarctions than WT mice (Figure 1). Treatment of WT mice with rhADAMTS13 resulted in a decrease of infarct size (Figure 1), troponin-I release (Figure 2), and myocardial apoptosis (Figure 3). These results provide compelling evidence for a protective role of ADAMTS13 in MI/R injury. Notably, WT mice (on a C57BL/6J background) have a hypomorphic Adamts13 allele, resulting in a truncated ADAMTS13 protein that lacks the C-terminal Tsp-1 and CUB domains. This C-terminally truncated form of ADAMTS13 was shown to have reduced activity in vivo.27 This, together with concentration differences, could in part explain why infarct sizes were even smaller in rhADAMTS13-treated animals than in WT mice (P < .05). Although a dose-response study would be needed to estimate the minimal-effective dose of rhADAMTS13 in this model, it is interesting to note that, even with the relatively high dose of rhADAMTS13 used, no adverse effects were observed in mice subjected to the MI/R procedure. A possible concern of ADAMTS13 over-reactivity could be induction of a bleeding diathesis, although this has never been documented so far. In our studies, none of the mice treated with rhADAMTS13 showed any signs of bleeding, neither systemically, nor in the ischemic heart. These data are consistent with previous findings where the same high dose of rhADAMTS13 was shown to be protective in a mouse model of ischemic stroke without increasing the bleeding risk.8 Interestingly, similar to tissue plasminogen activator, rhADAMTS13 was shown to have a thrombolytic effect on occlusive thrombi in venules, without the hemorrhage tendency seen with tissue plasminogen activator.28 Although no side effects were observed with 3500 U/kg rhADAMTS13, it will be valuable to determine whether lower concentrations of rhADAMTS13 are still effective.
Given the specific role of ADAMTS13 in intravascular homeostasis, our data lead to the hypothesis that, by cleaving VWF, ADAMTS13 prevents excessive VWF-mediated platelet and/or leukocyte recruitment in the ischemic myocardium. We observed only weak platelet adhesion to the blood vessel wall and this only in heart samples from vehicle-treated mice (Figure 4A). In contrast, MI/R injury caused a very prominent recruitment of Gr-1–positive cells to the infarcted area and neutrophil infiltration was 9 times lower in mice treated with rhADAMTS13 (Figure 4B). It is well known that neutrophils are the first leukocytes that infiltrate the damaged myocardial area.29 Infiltrating neutrophils mediate direct cardiomyocyte injury through the release of toxic products, such as reactive oxygen species and proteolytic enzymes.30,31 In accordance with our results, neutrophil depletion in animals undergoing reperfusion after myocardial infarction led to a marked decrease in infarct size.32,33 Interestingly, neutrophilia has also been related to worse prognosis in patients with AMI, predicting a larger infarct size and death during the subsequent months.34-36
In conclusion, we show that ADAMTS13 greatly reduces inflammatory responses in ischemic myocardium and consequently MI/R injury in mice. We and others previously established a critical role for VWF and ADAMTS13 in cerebral ischemia/reperfusion injury, indicating that ADAMTS13 could be beneficial to reduce I/R injury in other organs.8,37-40 We suggest that targeting VWF by rhADAMTS13 could become a new treatment strategy in the management of MI or in patients undergoing cardioplegic arrest for cardiac surgery.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank B. Plaimauer and H. Gritsch for carrying out the in vitro and in vivo cleavage of mouse VWF. S.F.D.M. was a postdoctoral fellow of the Research Foundation Flanders (Fonds voor Wetenschappelijk Onderzoek Vlaanderen).
This work was supported by National Heart, Lung, and Blood Institute of the National Institutes of Health grant R01 HL041002 (to D.D.W.) and a basic research grant from Baxter Biosciences to support animal housing.
National Institutes of Health
Authorship
Contribution: S.F.D.M. performed experiments, analyzed data, designed research, and wrote the paper; D.S. performed experiments; A.S.S. performed experiments and analyzed data; M.S.H. and M.C.C. provided essential expertise on the mouse MI model; A.S. and B.D. generated the pharmacokinetic and toxicologic data for rhADAMTS13; H.R. and F.S. provided rADAMTS13 and helpful discussions; and D.D.W. designed research, analyzed data, and cowrote the paper.
Conflict-of-interest disclosure: H.R., A.S., B.D., and F.S. are employees of Baxter Bioscience. The remaining authors declare no competing financial interests.
Correspondence: Denisa D. Wagner, Harvard Medical School, Immune Disease Institute, 3 Blackfan Cir, 3rd Fl, Boston, MA 02115; e-mail: wagner@idi.harvard.edu.