Key Points
Markedly additive antitumor activity with the combination of a selective survivin suppressant (YM155) and alemtuzumab in adult T-cell leukemia.
Abstract
Adult T-cell leukemia (ATL) is an aggressive malignancy of CD4+CD25+ lymphocytes caused by human T-cell lymphotropic virus type 1. Currently, there is no accepted curative therapy for ATL. In gene expression profiling, the antiapoptotic protein survivin (BIRC5) demonstrated a striking increase in ATL, and its expression was increased in patient ATL cells resistant to the anti-CD52 monoclonal antibody alemtuzumab (Campath-1H). In this study, we investigated the antitumor activity of a small-molecule survivin suppressant YM155 alone and in combination with alemtuzumab in a murine model of human ATL (MET-1). Both YM155 alone and its combination with alemtuzumab demonstrated therapeutic efficacy by lowering serum soluble IL-2Rα (sIL-2Rα) levels (P < .001) and prolonged the survival of tumor-bearing mice (P < .0001). Moreover, the combination of YM155 with alemtuzumab demonstrated markedly additive antitumor activity by significantly lowering serum sIL-2Rα levels and improving the survival of leukemia-bearing mice compared with monotherapy with either YM155 (P < .001) or alemtuzumab (P < .05). More significantly, all mice that received the combination therapy survived and were tumor free >6 months after treatment. Our data support a clinical trial of the combination of YM155 with alemtuzumab in ATL. This trial was registered at www.clinicaltrials.gov as #NCT00061048.
Introduction
Adult T-cell leukemia (ATL) is an aggressive T-cell malignancy characterized by the clonal expansion of CD4+CD25+ T lymphocytes.1 The etiologic agent of ATL is human T-cell lymphotropic virus type 1 (HTLV-1). HTLV-I is a type C retrovirus that is endemic in Central and Southern Africa, southern Japan, the Caribbean basin, and northern South America.2 Less than 5% of individuals infected with HTLV-1 develop either ATL or a chronic inflammatory disease of the central nervous system, HTLV-1 associated myelopathy/tropical spastic paraparesis. The course of ATL is variable, and 4 clinical subtypes have been described: smoldering, chronic, lymphomatous, and acute.
The treatment of the aggressive forms of ATL has been a challenge. The aggressive ATL subtypes are characterized by hypercalcemia, a large tumor burden with multiorgan failure, multidrug resistance, and frequent infectious complications due to a profound T-cell immunodeficiency. Leukemic cells from patients with ATL are often resistant to conventional chemotherapeutic agents as a result of the overexpression of the MDR gene and mutations of the p53 gene.3,4 The overall survival of ATL patients is poor, with a median survival ranging from 5 to 13 months. Single agents such as the nucleoside analogs fludarabine,5 pentostatin,6 and cladribine7 yield low response rates. Several combination chemotherapy regimens have been investigated, but none have demonstrated a survival advantage compared with standard cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy.8-11 Significant treatment-related toxicities and relapses are common. To improve the treatment outcomes of patients with ATL, both autologous and allogeneic hematopoietic stem cell transplantation (HSCT) have been studied. Although the number of cases is limited, autologous HSCT does not appear to be effective.12 Allogeneic HSCT has the potential of inducing a graft-versus-leukemia effect and appears to cure approximately half of the patients who undergo this therapy.12,13 The selected nature of the populations studied, the need for HLA-matched donors, and the expense of the procedure in developing nations limit the applicability of this approach. The antiretroviral agent zidovudine combined with interferon α showed promising results for patients with ATL with a median survival of 6 to 18 months.14 However, a retrospective meta-analysis showed that patients with lymphomatous ATL and those with mutant p53 or with elevated interferon regulatory factor 4 or c-Rel did not benefit from this treatment.15-17
We initiated a number of trials studying receptor-directed monoclonal antibody therapy for ATL. ATL cells express CD2, CD4, CC chemokine receptor 4 (CCR4), CD52,18 and high levels of CD25 (IL-2Rα) that can be targeted by various monoclonal antibodies.19 Using our MET-1 murine model of human ATL, we demonstrated efficacy using anti-CD25 (daclizumab), anti-CD30 (HeFi), anti-CD2 (siplizumab), and anti-CD52 (alemtuzumab). This was paralleled by efficacy with these same antibodies in clinical trials in patients with ATL. Daclizumab, a monoclonal antibody that recognizes CD25, demonstrated responses in 6 out of 19 ATL patients.20 Siplizumab, an anti-CD2 monoclonal antibody, also demonstrated promising activity in a phase 1 trial in patients with ATL; however, the development of Epstein-Barr virus–related lymphoproliferative disease prevented its further use as a single agent.21 An anti-CCR4 antibody, KW-0761, achieved objective responses in 13 out of 26 patients with CCR4-positive relapsed ATL including 8 complete responses.22 Following completion of our study of alemtuzumab in the MET-1 model of ATL where it showed considerable efficacy, we conducted a phase 2 trial in patients that showed comparable efficacy. Alemtuzumab treatment in patients yielded a 52% response rate in 29 ATL patients studied (data not shown). Although the results of monoclonal antibody therapy are promising, new approaches and targets are clearly needed to improve the overall survival of patients with aggressive forms of ATL. One of the approaches to improve the therapeutic efficacy of single monoclonal antibody therapy is to combine it with chemotherapeutic reagents that function via different mechanisms that may result in synergistic cytotoxicity leading to enhanced tumor cell death. In support of this view, we demonstrated that the combination of romidepsin,23 bortezomib,24 and flavopiridol25 with daclizumab provided synergistic antitumor activity in a mouse model of ATL.
In a study attempting to identify disease-related genes in ATL using global gene expression profiling, survivin (BIRC5) showed a striking increase in 32 ATL patients examined.26 Transcription factor 4 (TCF4) regulates survivin gene expression in primary ATL leukemic cells.26 TCF4 was overexpressed on average more than 10-fold in ATL leukemic cells. Interaction of TCF4 with the β-catenin, which was also overexpressed in ATL cells, results in import of the heterodimer to the nucleus to regulate gene expression. Tansfection of dominant negative TCF4 in ATL primary cells inhibited survivin expression and decreased cell viability.26
Survivin is a member of the inhibitor of apoptosis protein family that acts as a suppressor of apoptosis and plays a central role in cell division.27 Survivin is upregulated in many human tumors and plays a key role in cancer progression and treatment resistance.28 Currently, survivin is being studied as a potential therapeutic target in a variety of tumors.
In this work, we evaluated the antitumor activity of a small-molecule survivin suppressant, YM155, alone and in combination with alemtuzumab in a mouse model of ATL (MET-1). YM155 demonstrated therapeutic efficacy by decreasing serum soluble IL-2Rα (sIL-2Rα) levels and prolonging the survival of leukemia-bearing mice. Furthermore, the combination of YM155 with alemtuzumab demonstrated markedly additive antitumor activity by additionally lowering sIL-2Rα levels and prolonging the survival of MET-1–bearing mice. Most significantly, all the mice that received the combination therapy survived >6 months and were tumor free. Our data support the development of a clinical trial of the combination of YM155 with alemtuzumab in the treatment of patients with ATL.
Materials and methods
Drug and antibody
The survivin suppressant YM155 was obtained from Astellas Pharma Inc (Deerfield, IL). Alemtuzumab (Campath-1H) was acquired from Genzyme Corporation (Cambridge, MA).
Clinical samples
Peripheral blood mononuclear cells (PBMCs) were obtained by leukapheresis from ATL patients treated on an institutional review board approved phase 2 trial of alemtuzumab in accordance with the Declaration of Helsinki (www.clinicaltrials.gov, #NCT00061048). Briefly, standard escalating daily doses of alemtuzumab of 3, 10, and 30 mg were administered over 3 consecutive days and continued at a dose of 30 mg 3 times weekly for up to 3 months. PBMCs were collected before and after treatment. Flow cytometry was used to define the time point for posttherapy sample collection based on a shift in the mean florescence intensity of CD52 staining, which suggests saturation of alemtuzumab on patients’ cells (supplemental Figure 1; see the Blood Web site).
MTT proliferation assay
ATL cell lines ATL-43b and ATL-55(+) were maintained in RPMI 1640 containing 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin, and 100 µg/mL streptomycin at 37°C with 5% CO2. ATL-55(+) cells were maintained in medium containing 100 U/mL recombinant human Interleukin 2. Aliquots of 2 × 104 cells were seeded in 96-well plates and incubated with medium alone or with serial dilutions of YM155. After 72 hours, 3-(4,5-dimethylthiazol-2-yl)-2,5-dimethyltetrazolium bromide (MTT) dye was added to the culture (Promega, Madison, WI). After 4 hours of incubation, Solubilization Solution/Stop Mix (Promega, Madison, WI) was added, and the absorbance at 570 nm was recorded using a 96-well plate reader.
Apoptosis assay
Quantification of apoptosis was performed by using the Vybrant FAM caspase 3 and 7 assay kit to detect active caspase 3 and 7 with flow cytometry (Invitrogen, Grand Island, NY) and by immunostaining of cleaved poly ADP-ribose polymerase (PARP) (BD Pharmingen, San Diego, CA). Cells were treated with medium alone or 1 nM, 10 nM, 100 nM, or 1000 nM YM155 for 48 hours and then were analyzed following the assay protocol.
Real-time reverse transcription–polymerase chain reaction
PBMCs from ATL patients were analyzed for survivin, TCF4, and β-catenin messenger (mRNA) levels before and after alemtuzumab treatment. Total RNA was extracted using RNeasy Mini Plus kit (Qiagen, Valencia, CA). Reverse transcription reactions were carried out for each sample (250 ng) using the Superscript First-Strand synthesis System (Invitrogen). The Taqman Universal PCR Master Mix, the human survivin, TCF4 and β-catenin primer/probe, and the HPRT1 primer/probe sets were purchased from Applied Biosystems (Carlsbad, CA). The detection of human survivin and HPRT1 was performed using an ABI Prism 7500 sequence detection system (Applied Biosystems) according to the manufacturer’s instructions. The copy numbers of surviving mRNA were normalized to the copy numbers of the HPRT1 gene and were expressed as fold increase vs normalized copy numbers of survivin mRNA in CD4 T cells isolated from normal donors.
Western blot analysis
Cells were solubilized at 4°C in lysis buffer. Cell lysates (50 µg) were resolved by electrophoresis on sodium dodecyl sulfate–polyacrylamide (4% to 12%) gels and transferred to polyvinylidine difluoride membranes. After blocking, the blots were incubated with polyclonal survivin antibody (SC-10811; Santa Cruz Biotechnology, Santa Cruz, CA). The blots were then washed and incubated with horseradish peroxidase–conjugated mouse anti-rabbit antibody (SC-2357; Santa Cruz Biotechnology). The protein bands recognized by the antibodies were visualized with an enhanced chemiluminescence western blotting detection system (Amersham, Piscataway, NJ).
Mouse model of ATL
MET-1 ATL cells were established from the peripheral blood of a patient with acute-type ATL, and the cells were maintained by serial transfer in NOD/SCID mice (Jackson Laboratories, Bar Harbor, ME). MET-1 cells have a distinct phenotype elucidated by fluorescence-activated cell sorting analysis: CD3dim, CD4+/−, CD7–, CD20–, CD25+, and CD52+ (supplemental Figure 2). The leukemic mouse model was established by intraperitoneal injection of 1.5 × 107 MET-1 cells into NOD/SCID mice as described previously.29 The therapeutic experiments were performed on these mice when serum sIL-2Rα levels were >1000 pg/mL, which occurs 10 to 14 days after tumor inoculation. All animal experiments were performed in accordance with National Institutes of Health Animal Care and Use Committee guidelines.
Therapeutic studies
Therapeutic studies were performed in MET-1 leukemia-bearing mice with serum surrogate tumor marker sIL-2Rα values of 1000 to 10 000 pg/mL. There were 4 groups: group 1, the YM155 treatment alone, received 2 mg/kg body weight per day by pump continuous infusion for a week. YM155 was administered as a 7-day continuous infusion using micro-osmotic pump (Alzet Model 2001; Durect Corporation, Cupertino, CA); group 2, alemtuzumab alone, was given intravenous injections of 100 µg alemtuzumab on days 0, 7, 14, and 21; group 3, the combination therapy group, received YM155 and alemtuzumab (dose and dosing schedule as in group 1 plus group 2); and group 4 received 200 µL of phosphate-buffered saline (PBS) weekly for 4 weeks and served as a control. There were 8 mice per group. The groups were randomly assigned and had comparable average levels of sIL-2Rα at the beginning of the experiments (6000 pg/mL). Two mice in the combination therapy group were euthanized 3 months after therapy due to weight loss. No serum sIL-2Rα was detected in the mice, and no tumor was found at necropsy. These 2 mice were excluded from the analysis.
Monitoring of tumor growth
Measurements of serum sIL-2Rα were performed using enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN). The enzyme-linked immunosorbent assays were performed following the manufacturer’s recommendation.
Statistical analysis
Serum levels of human sIL-2Rα were analyzed at different time points among different treatment groups using the Student t test for unpaired data. The statistical significance of differences in survival of mice in different groups was determined by the log-rank test using the StatView program (Abacus Concepts, Berkeley, CA).
Results
Expression of survivin in the primary ATL cells
Survivin (BIRC5) is an antiapoptosis protein that belongs to the IAP gene family.27 In a study of ATL-related gene expression using global gene expression profiling, a striking increase of survivin was observed.26 We examined the expression of survivin in the PBMCs of our ATL patients. Western blot analysis demonstrated that survivin was highly expressed in the PBMCs from 5 out of 7 ATL patients examined (Figure 1A). Furthermore, survivin was also expressed in the HTLV-1–infected cell lines Hut102, MT-2, and CaGT1, as well as in MET-1 ATL tumor cells (Figure 1B).
Increased survivin expression in ATL patients’ PBMCs, MET-1 ATL tumor cells, and HTLV-1–infected cell lines. (A) Western blot analysis of survivin protein levels in PBMCs of ATL patients. (B) Western blot analysis of survivin protein levels in MET-1 ATL tumor cells and in HTLV-1–infected cell lines MT-2, Hut102, and CaGT1.
Increased survivin expression in ATL patients’ PBMCs, MET-1 ATL tumor cells, and HTLV-1–infected cell lines. (A) Western blot analysis of survivin protein levels in PBMCs of ATL patients. (B) Western blot analysis of survivin protein levels in MET-1 ATL tumor cells and in HTLV-1–infected cell lines MT-2, Hut102, and CaGT1.
Survivin expression in ATL patients treated with alemtuzumab
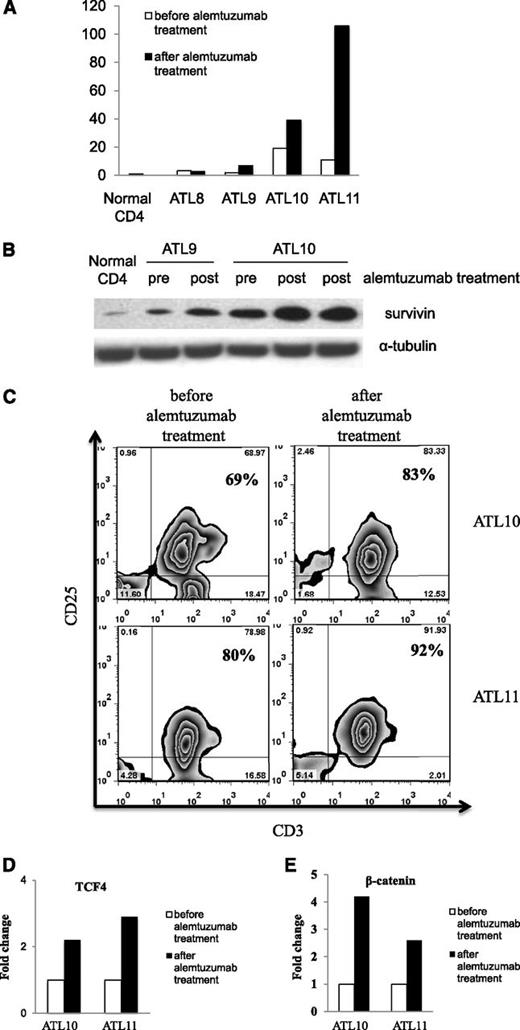
CD52, a glycosyl-phosphatidylinositol–linked protein, is expressed on normal and malignant leukocytes including ATL leukemic cells.30 We studied the efficacy of alemtuzumab in a phase 2 trial in patients with chronic, lymphoma, and acute-type ATL. Fifty-two percent of patients who received alemtuzumab achieved a partial or complete remission (data not shown). Although the overall response rate of alemtuzumab monotherapy is encouraging, the median duration of response was only 3.4 months. Because increased expression of survivin has been implied as a common mechanism for tumor cells to escape antitumor treatment,31-33 we analyzed the expression of survivin before and after alemtuzumab treatment in a number of our ATL patients. Among the 4 patients with samples available for analysis, we observed an increase of survivin mRNA levels after alemtuzumab treatment in patients 9 (ALT9), 10 (ALT10), and 11 (ALT11) (Figure 2A). The increased expression of survivin during alemtuzumab therapy in ALT10 was confirmed by western blot analysis (Figure 2B). Malignant cells obtained from ALT9 at relapse also demonstrated increased survivin expression by western analysis (Figure 2B).
Increased survivin expression in the PBMCs of ATL patients after alemtuzumab treatment. (A) The expression of survivin mRNA levels was measured by real-time reverse transcription–polymerase chain reaction in the ATL PBMCs before and after alemtuzumab treatment (patients ATL8 and ATL11, 1 week after treatment; patients ATL9 and ATL10, 5-6 months after treatment). The fold change was calculated based on the survivin mRNA levels in the normal CD4 cells. (B) Western blot analysis of survivin protein expression levels in patients ATL9 and ATL10 before and after alemtuzumab treatment. (C) Flow cytometry analysis of leukemic cell population (CD3+CD25+) in ATL patients before and after alemtuzumab treatment. (D) Expression of transcription factor TCF4 mRNA in ATL10 and ATL11. Fold change was calculated based on TCF4 mRNA expression before treatment. (E) Expression of β-catenin mRNA in ATL10 and ATL11. Fold change was calculated based on β-catenin mRNA expression before treatment.
Increased survivin expression in the PBMCs of ATL patients after alemtuzumab treatment. (A) The expression of survivin mRNA levels was measured by real-time reverse transcription–polymerase chain reaction in the ATL PBMCs before and after alemtuzumab treatment (patients ATL8 and ATL11, 1 week after treatment; patients ATL9 and ATL10, 5-6 months after treatment). The fold change was calculated based on the survivin mRNA levels in the normal CD4 cells. (B) Western blot analysis of survivin protein expression levels in patients ATL9 and ATL10 before and after alemtuzumab treatment. (C) Flow cytometry analysis of leukemic cell population (CD3+CD25+) in ATL patients before and after alemtuzumab treatment. (D) Expression of transcription factor TCF4 mRNA in ATL10 and ATL11. Fold change was calculated based on TCF4 mRNA expression before treatment. (E) Expression of β-catenin mRNA in ATL10 and ATL11. Fold change was calculated based on β-catenin mRNA expression before treatment.
We next examined the mechanisms of survivin upregulation after alemtuzumab treatment in ATL10 and ATL11. Although there was a drastic reduction of leukemic cell counts in the patients after alemtuzumab treatment (46 872 to 392 in ATL10; 49 107 to 1127 in ATL11) (Table 1), the leukemic cell percentage in the PBMCs was increased in the posttherapy samples (69% to 83% in ATL10; 80% to 92% in ATL11) (Figure 2C). Furthermore, the expression levels of transcription factor TCF4 and β-catenin that regulate survivin expression in ATL leukemic cells were increased twofold to fourfold in the posttherapy samples (Figure 2D-E). Viral transcription factor Tax remained negative in the patients after alemtuzumab treatment (data not shown). Therefore, the combination of increased leukemic cell percentage and increased expression of TCF4 and β-catenin may lead to increased survivin expression in the posttherapy samples. Moreover, alemtuzumab treatment of ATL leukemic cell lines did not induce higher expression of survivin (supplemental Figure 3) or TCF4/β-catenin (data not shown). Considering the major reduction of leukemic cell counts after alemtuzumab treatment, in vivo in the human study there may be a selection for preferential survival of cells that manifested a high survivin level due to their high TCF4/β-catenin expression to escape death induced by alemtuzumab.
YM155 treatment downregulated survivin expression, inhibited cell proliferation, and induced cell apoptosis in ATL leukemic cell lines
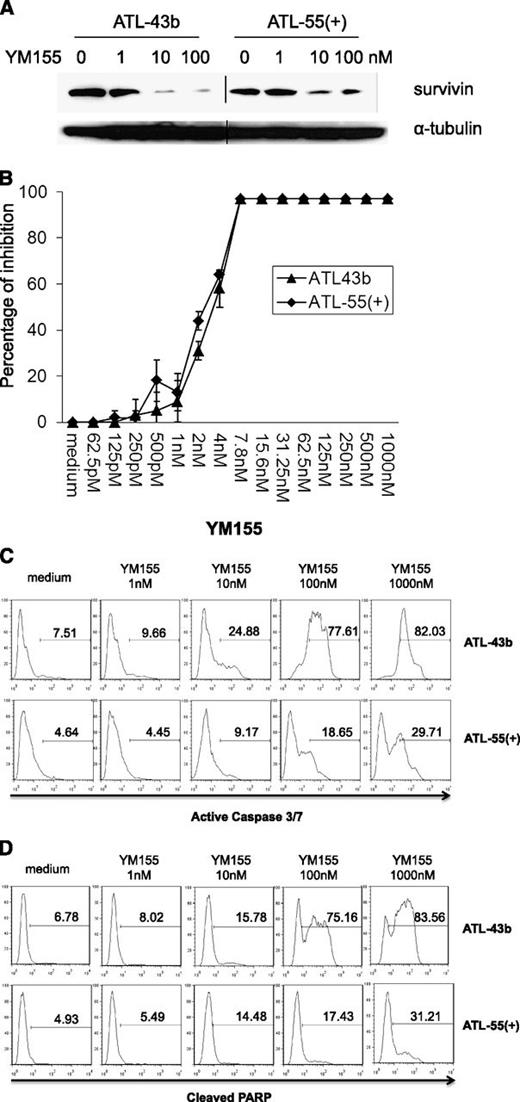
YM155, a small-molecule survivin suppressant, demonstrated activity in a broad spectrum of human tumor cell lines and induced tumor regression in lymphoma, prostate cancer, and non–small cell lung cancer xenografts.34 We determined whether YM155 could inhibit survivin expression in the ATL leukemic cell lines ATL-43b and ATL-55(+).35 As shown in Figure 3A, the addition of 10 nM or 100 nM YM155 to the culture (for 48 hours) significantly suppressed survivin expression in ATL-43b and ATL-55(+) cells. Furthermore, there was a dose-dependent inhibition of cell proliferation by YM155 in ATL-43b and ATL-55(+) cells (Figure 3B). The leukemic cell lines ATL-43b, ATL-55(+), SO4, and others (data not shown) were very sensitive to YM155 treatment with a 50% inhibition concentration of approximately 2 to 4 nM. To examine the cell apoptosis induced by YM155, we treated ATL-43b and ATL-55(+) cells with different concentrations of YM155 for 48 hours, after which the cells were either stained with Fluorochrome Inhibitor of Caspases (Invitrogen) to detect active caspase 3 and 7 or fixed and then stained with antibody against cleaved PARP (BD Pharmingen). As shown in Figure 3C-D, increasing concentrations of YM155 induced increasing numbers of apoptotic cells in ATL-43b and ATL-55(+) that had active caspase 3/7 (Figure 3C) or cleaved PARP (Figure 3D).
YM155 downregulated survivin expression, inhibited cell proliferation, and induced cell apoptosis in ATL leukemic cell lines ATL-43b and ATL-55(+). (A) ATL-43b and ATL-55(+) cells were cultured with medium alone or 1 nM, 10 nM, or 100 nM YM155 for 48 hours. Cells were harvested and analyzed for survivin expression by western blot analysis. (B) MTT cell proliferation assay of ATL-43b and ATL-55(+) cells treated with a serial dilution of concentrations of YM155 for 72 hours. (C) ATL-43b and ATL-55(+) cells were cultured with medium alone or 1 nM, 10 nM, 100 nM, or 1000 nM YM155 for 48 hours. The cells were then labeled with fluorochrome inhibitor of caspases reagent and analyzed by flow cytometry to detect cells with active caspase 3 and caspase 7. (D) ATL-43b and ATL-55(+) cells were cultured with medium alone or 1 nM, 10 nM, 100 nM, or 1000 nM YM155 for 48 hours. The cells were then fixed and labeled with antibody to cleaved PARP.
YM155 downregulated survivin expression, inhibited cell proliferation, and induced cell apoptosis in ATL leukemic cell lines ATL-43b and ATL-55(+). (A) ATL-43b and ATL-55(+) cells were cultured with medium alone or 1 nM, 10 nM, or 100 nM YM155 for 48 hours. Cells were harvested and analyzed for survivin expression by western blot analysis. (B) MTT cell proliferation assay of ATL-43b and ATL-55(+) cells treated with a serial dilution of concentrations of YM155 for 72 hours. (C) ATL-43b and ATL-55(+) cells were cultured with medium alone or 1 nM, 10 nM, 100 nM, or 1000 nM YM155 for 48 hours. The cells were then labeled with fluorochrome inhibitor of caspases reagent and analyzed by flow cytometry to detect cells with active caspase 3 and caspase 7. (D) ATL-43b and ATL-55(+) cells were cultured with medium alone or 1 nM, 10 nM, 100 nM, or 1000 nM YM155 for 48 hours. The cells were then fixed and labeled with antibody to cleaved PARP.
Definition of the maximum tolerated dose of YM155 in vivo
Survivin is a short-lived protein with a half-life of approximately 30 minutes.36 To achieve sustained inhibition of survivin expression, we chose an osmotic pump (Alzet; Durect Corporation) to continuously deliver constant amounts of YM155 to the mice. A 7-day continuous infusion of 0.5, 2, 5, or 10 mg/kg per day of YM155 was tested in the NOD/SCID mice to determine the maximum tolerated dose. All of the mice infused with 10 mg/kg per day of YM155 died at day 3 (n = 5), and 60% of mice in the group that received 5 mg/kg per day (n = 5) died at day 6 indicating unacceptable toxicity at these doses. Groups receiving 0.5 and 2 mg/kg per day survived for >6 months. Furthermore, PK analysis indicated that 2 mg/kg per day as a 7-day infusion in mice recapitulated clinical dosing and the steady-state serum concentrations in humans (data not shown). Based on this finding, we chose the dose of 2 mg/kg per day as a 7-day continuous infusion to deliver YM155 in our therapeutic trial.
Markedly additive antitumor activity with the combination of YM155 and alemtuzumab in MET-1 mouse model of ATL
Alemtuzumab has demonstrated activity in the MET-1 mouse model of human ATL37 and in patients with ATL.38,39 We previously demonstrated that in the MET-1 model, the antitumor activity of alemtuzumab was mediated through antibody-dependent cell cytotoxicity that required the expression of Fc receptors (FcγRs).37 As discussed earlier, primary ATL cells expressed high levels of survivin. Moreover, ATL cells that resist alemtuzumab treatment demonstrated increased survivin expression, which may be one molecular mechanism for the ATL cells to escape alemtuzumab treatment. On the other hand, the survivin suppressant YM155 demonstrated excellent antiproliferative and apoptosis-inducing activity in ATL leukemic cell lines in vitro. Based on these observations, we hypothesized that the combination of YM155 with alemtuzumab might be a rational new combination therapy for ATL patients. To test this, we conducted a therapeutic trial with YM155 alone, alemtuzumab alone, and the combination of YM155 with alemtuzumab in the MET-1 model of human ATL.
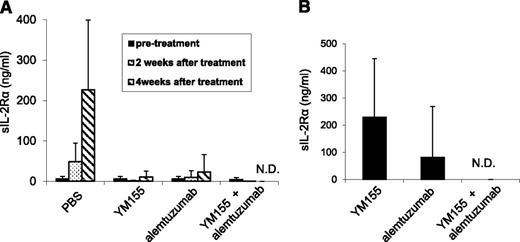
A 1-week course of treatment with YM155 (2 mg/kg per day as a 7-day infusion), a 4-week course of treatment with alemtuzumab (100 μg per mouse, weekly), and the combination of YM155 and alemtuzumab demonstrated therapeutic efficacy as assessed by demonstration of lower serum levels of human sIL-2Rα (Figure 4) and prolonged the survival of leukemia-bearing mice (Figure 5). The serum levels of sIL-2Ra were significantly reduced in the groups treated with YM155 alone (P < .01), alemtuzumab alone (P < .01), and the combination of YM155 with alemtuzumab (P < .001) 4 weeks posttherapy when compared with the levels in the PBS group. Furthermore, the sIL-2Ra levels were significantly lower in the combination group 8 weeks posttherapy when compared with that of the group that received YM155 alone (P < .01, Figure 4B). More significantly, the human sIL-2Rα levels were undetectable in the mice that received the combination of YM155 and alemtuzumab treatment when measured at 4 and 8 weeks posttherapy (Figure 4), as well as at 6 months posttherapy (Table 2). This suggested that the mice that received the combinational therapy were virtually tumor free.
YM155, alemtuzumab, and the combination of YM155 with alemtuzumab treatment inhibited the growth of MET-1 ATL tumor cells in the MET-1 mouse model of human ATL. (A) The mean concentrations of tumor surrogate marker, human sIL-2Rα levels in MET-1–bearing mice before and after YM155, alemtuzumab, and the combination of YM155 with alemtuzumab treatment (2 and 4 weeks posttherapy). The animals treated with YM155 alone, alemtuzumab alone, and the combination of YM155 with alemtuzumab had significantly decreased values of sIL-2Rα when compared with those of the PBS control group 4 weeks posttherapy (YM155, P < .01; alemtuzumab, P < .01; the combination, P < .001). Furthermore, there were no detectable levels of sIL-2Rα in the mice that received the combination therapy 4 weeks posttherapy. (B) The mean concentration of tumor surrogate marker, human sIL-2Rα levels in MET-1–bearing mice 8 weeks after treatment with YM155, alemtuzumab, and the combination of YM155 with alemtuzumab. The animals receiving the combination of YM155 with alemtuzumab had no detectable levels of sIL-2Rα 8 weeks posttherapy. N.D., not detected.
YM155, alemtuzumab, and the combination of YM155 with alemtuzumab treatment inhibited the growth of MET-1 ATL tumor cells in the MET-1 mouse model of human ATL. (A) The mean concentrations of tumor surrogate marker, human sIL-2Rα levels in MET-1–bearing mice before and after YM155, alemtuzumab, and the combination of YM155 with alemtuzumab treatment (2 and 4 weeks posttherapy). The animals treated with YM155 alone, alemtuzumab alone, and the combination of YM155 with alemtuzumab had significantly decreased values of sIL-2Rα when compared with those of the PBS control group 4 weeks posttherapy (YM155, P < .01; alemtuzumab, P < .01; the combination, P < .001). Furthermore, there were no detectable levels of sIL-2Rα in the mice that received the combination therapy 4 weeks posttherapy. (B) The mean concentration of tumor surrogate marker, human sIL-2Rα levels in MET-1–bearing mice 8 weeks after treatment with YM155, alemtuzumab, and the combination of YM155 with alemtuzumab. The animals receiving the combination of YM155 with alemtuzumab had no detectable levels of sIL-2Rα 8 weeks posttherapy. N.D., not detected.
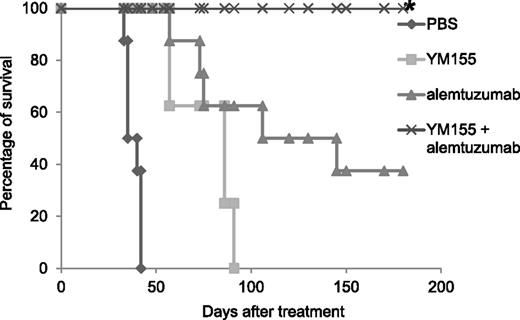
YM155 and its combination with alemtuzumab significantly prolonged the survival of MET-1 leukemia-bearing mice. The animals treated with YM155 alone, alemtuzumab alone, and the combination of YM155 with alemtuzumab had significantly prolonged survivals when compared with the PBS control group (P < .0001). The combination of YM155 with alemtuzumab significantly prolonged the survival of leukemia-bearing mice when compared with YM155 alone (P < .001) or alemtuzumab alone (P < .05). *Two tumor-unrelated deaths excluded from the analysis are described in the “Materials and methods.”
YM155 and its combination with alemtuzumab significantly prolonged the survival of MET-1 leukemia-bearing mice. The animals treated with YM155 alone, alemtuzumab alone, and the combination of YM155 with alemtuzumab had significantly prolonged survivals when compared with the PBS control group (P < .0001). The combination of YM155 with alemtuzumab significantly prolonged the survival of leukemia-bearing mice when compared with YM155 alone (P < .001) or alemtuzumab alone (P < .05). *Two tumor-unrelated deaths excluded from the analysis are described in the “Materials and methods.”
The mice in the PBS control group died between day 33 and day 42 with a median survival of 38 days. The YM155 treatment alone, the alemtuzumab treatment alone, and the combination significantly prolonged the survival of leukemia-bearing mice (Figure 5, P < .0001). The mice in the group that received YM155 treatment alone had a median survival of 86 days. The mice in the group that received alemtuzumab treatment alone had a median survival of 125 days. All the mice in the combination treatment group survived >6 months (Figure 5) and were tumor free when analyzed 6 months posttherapy (no sIL2Rα detected, Table 2). Three mice in the group that received alemtuzumab treatment alone also survived >6 months posttherapy. Among them, 1 mouse had detectable sIL-2Ra when analyzed 6 months posttherapy, indicating residual tumor (Table 2).
Discussion
Survivin overexpression has been reported in many human tumors and correlates with advanced disease, treatment resistance, and poor outcome.28 Survivin has been shown to inhibit cell apoptosis and promote cell proliferation and angiogenesis, all of which make survivin a potentially attractive target for therapy.28 Moreover, a growing numbers of studies suggest that survivin is also responsible for resistance to anticancer chemotherapy and radiation. High levels of survivin in tumor cells were found to confer resistance to a number of anticancer drugs; conversely, blocking or neutralization of survivin was found to enhance the response to multiple cancer therapies.40,41 This highlights the potential value of inhibiting survivin action. In ATL, survivin was found to be strikingly increased in malignant cells.26 We showed in this study that the survivin protein was highly expressed in the PBMCs of ATL patients (5 out of 7), MET-1 ATL tumor cells, and HTLV-1–infected cell lines (Figure 1). Moreover, the expression levels of survivin, both mRNA levels and protein levels, were significantly increased in ATL patients after alemtuzumab treatment (Figure 2B-C, ATL9, ATL10, and ATL11). We demonstrated previously that alemtuzumab treatment leads to tumor regression through antibody-dependent cell-mediated cytotoxicity that requires the expression of Fc receptors (FcγRs) in the MET-1 model of ATL.37 Because high levels of survivin could confer tumor cell resistance to anticancer drugs, we speculated that ATL cells with high levels of survivin expression might be able to escape the cytotoxicity induced by alemtuzumab.
Several strategies have been used to suppress survivin expression.42 One approach is to use antisense oligonucleotides to knock down survivin expression. Many studies suggested that survivin antisense oligonucleotides, delivered to the cells either as a chemically synthesized molecule or expressed by viral gene transfer vectors, induced specific inhibition of survivin mRNA and protein, cell growth arrest, and apoptosis in a broad range of tumor cell lines, including melanoma, lung, bladder, head and neck, thyroid cancers, sarcoma, and lymphoma.43,44 We tested in our MET-1 ATL model an RNA-based antisense oligonucleotide, SPC3042.45 Although SPC3042 induced downregulation of survivin expression and cell apoptosis in ATL cell lines in vitro (data not shown), administration of SPC3042 into the MET-1 ATL mouse model did not improve the survival of the mice (data not shown). Another approach to inhibit survivin expression is to use small-molecular antagonists. Several small-molecule survivin inhibitors are currently under development. One is terameprocol (EM1421), an inhibitor of transcriptional factor specificity protein 1, which inhibits the expression of both cell division control protein 2 and survivin.46 Terameprocol is currently in a phase 1 trial in patients with leukemia. We tested the efficacy of terameprocol treatment alone and in combination with alemtuzumab in our model of ATL. Terameprocol treatment alone did not significantly improve the survival of MET-1–bearing mice, and the combination of terameprocol with alemtuzumab did not have additive or synergistic antitumor activity in the MET-1 ATL model (data not shown). YM155 is a survivin suppressant identified in a high-throughput screen of compounds that selectively inhibited survivin promoter activity.34 In a phase 1 trial in patients with advanced solid malignancies or lymphoma, 1 complete and 2 partial responses were observed in 3 patients with non-Hodgkin lymphoma.47 No severe toxicity was observed associated with YM155 administration.47 A phase 2 trial of YM155 in patients with refractory diffuse large B-cell lymphoma showed limited single-agent activity, which suggested that combinational therapies may be needed to improve its therapeutic efficacy.48 Currently, the combination of YM155 with rituximab is under evaluation in non-Hodgkin lymphoma. Other than the antisense and small-molecule antagonist approaches discussed previously, antisurvivin vaccines are also being tested.49
In the present study, we evaluated the combination of a selective survivin suppressant (YM155) and alemtuzumab in the treatment of ATL. We chose this combination based on the observations that ATL cells express high levels of survivin; YM155 demonstrated antiproliferative activity and induced apoptosis in a number of human ATL cell lines; alemtuzumab demonstrated therapeutic efficacy in the MET-1 ATL model37 and in patients with ATL38,39 ; and ATL cells that have resistance to alemtuzumab treatment had increased expression of survivin, which may be the molecular mechanism for ATL cells to escape death induced by alemtuzumab. The addition of YM155 to alemtuzumab might demonstrate enhanced antitumor effects and induce tumor cell death by lowering survivin expression and sensitizing tumor cells to alemtuzumab. Indeed, we observed markedly additive antitumor efficacy of the combination of YM155 with alemtuzumab in the MET-1 ATL mouse model. The combination therapy significantly lowered the tumor surrogate marker sIL-2Rα levels (P < .001) and prolonged the survival of MET-1 mice (Figure 5) compared with no treatment (P < .0001), YM155 alone (P < .0001), and alemtuzumab treatment alone (P < .05). All the mice that received the combination of YM155 and alemtuzumab survived >6 months posttherapy compared with only 3 long-term survivors in the group that received alemtuzumab treatment and none in the group that received YM155 alone. Most significantly, all the mice that received the combination therapy had no detectable levels of serum sIL-2Rα at 4 weeks, 8 weeks, and even 6 months posttherapy, suggesting that they were tumor free.
In conclusion, we demonstrated markedly additive antitumor efficacy in a mouse model of human ATL with the combination of the survivin suppressant YM155 and the anti-CD52 monoclonal antibody alemtuzumab. Our data suggest that a clinical trial of such a combination is warranted in patients with ATL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by the intramural research program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
National Institutes of Health
Authorship
Contribution: J.C. designed and performed research, collected, analyzed, and interpreted data, and wrote the paper; C.A.P.M. performed research; J.H.S. performed statistical analysis; J.C.M. and K.C.C. provided patient care and collected patient samples; A.K. provided YM155 and facilitated the work; and J.E.J. and T.A.W. designed research.
Conflict-of-interest disclosure: A.K. works for Astellas Pharma.
Correspondence: Thomas A. Waldmann, Building 10, Room 4N115, 10 Center Drive, National Institutes of Health, Bethesda, MD 20892-1374; e-mail: tawald@helix.nih.gov.