Key Points
GlycoPEGylated FVIII (N8-GP) demonstrates the same efficacy and prolonged effect in animal models as native FVIII.
Circulatory half-life of glycoPEGylated FVIII (N8-GP) is prolonged by approximately twofold in several species.
Abstract
Frequent infusions of intravenous factor VIII (FVIII) are required to prevent bleeding associated with hemophilia A. To reduce the treatment burden, recombinant FVIII with a longer half-life was developed without changing the protein structure. FVIII–polyethylene glycol (PEG) conjugates were prepared using an enzymatic process coupling PEG (ranging from 10 to 80 kDa) selectively to a unique O-linked glycan in the FVIII B-domain. Binding to von Willebrand factor (VWF) was maintained for all conjugates. Upon cleavage by thrombin, the B-domain and the associated PEG were released, generating activated FVIII (FVIIIa) with the same primary structure and specific activity as native FVIIIa. In both FVIII- and VWF-deficient mice, the half-life was found to increase with the size of PEG. In vivo potency and efficacy of FVIII conjugated with a 40-kDa PEG (N8-GP) and unmodified FVIII were not different. N8-GP had a longer duration of effect in FVIII-deficient mouse models, approximately a twofold prolonged half-life in mice, rabbits, and cynomolgus monkeys; however, the prolongation was less pronounced in rats. Binding capacity of N8-GP on human monocyte-derived dendritic cells was reduced compared with unmodified FVIII, resulting in several-fold reduced cellular uptake. In conclusion, N8-GP has the potential to offer efficacious prevention and treatment of bleeds in hemophilia A at reduced dosing frequency.
Introduction
Hemophilia A is an inherited bleeding disorder caused by deficiency or dysfunction of coagulation factor VIII (FVIII). Hemophilia A is treated by intravenous infusions of FVIII purified from human plasma or produced using recombinant DNA technology. Based on clinical experience and results from prospective controlled trials, prophylactic treatment is recommended in order to prevent frequent bleeding episodes that could otherwise lead to pain, irreversible joint damage, and life-threatening hemorrhages.1-3 With a circulatory half-life of 12 to 14 hours for current FVIII products, prophylaxis for hemophilia A typically requires injections 3 times per week or every other day to maintain a sufficient circulating level of FVIII. Various technologies have been described for increasing the duration of effect of therapeutic drugs. In particular, conjugation of therapeutic proteins with water-soluble polymers such as polyethylene glycol (PEG) has been investigated.4,5 Several PEGylated therapeutic proteins have been approved for use across a variety of indications, including chronic diseases like rheumatoid arthritis.6 However, conventional PEGylation of large proteins like FVIII is difficult to control and may result in a heterogeneous mixture of molecular forms with different characteristics and activity levels.4,7 Recently, chemical PEGylation of FVIII has been described where the selectivity of PEGylation was obtained by introduction of mutations in the light chain of a B-domain–deleted FVIII.8 Other approaches to increase the functional half-life of drugs include production of recombinant fusion proteins where the pharmacokinetic properties are modified through the fusion to albumin9 or an Fc-domain of an antibody.10,11 Common to these technologies are that the resulting FVIII products have been modified at the peptide level and that the modifications remain part of the activated FVIII (FVIIIa) mutant or fusion protein following activation by thrombin. This may potentially impact molecular interactions with various binding partners, and the FVIII mutant or fusion protein may have altered immunogenicity.
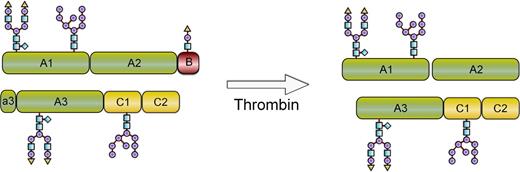
This report describes the development of a recombinant FVIII conjugate with improved pharmacokinetic properties and maintained generation of native FVIIIa at the site of vessel injury (Figure 1). To achieve this, site-specific modification of O-linked glycans using enzymatic glycoconjugation technology12,13 was developed and adapted to FVIII (turoctocog alfa). This allows for selective modification of the unique O-linked glycan in the B-domain of turoctocog alfa that results from the fusion of the 10 amino acids (aa) from the amino-terminal with the 11 aa from the carboxyl-terminal of the FVIII B-domain sequence.14 Turoctocog alfa was well tolerated in clinical studies and had pharmacokinetic properties comparable with a marketed FVIII,15 and it was therefore considered well suited for further modification. A series of O-glycoPEGylated FVIII conjugates with different sizes of PEG were produced, and pharmacokinetics and in vitro activity were compared. The FVIII glycoPEGylated with a single branched 40-kDa PEG (N8-GP) was selected for detailed in vitro and in vivo evaluation comparing potency, efficacy, and duration of effect with that of native FVIII. Formation of neutralizing antibodies (inhibitors) toward FVIII is a serious complication to FVIII therapy. The uptake and processing of proteins by antigen-presenting cells (APCs) are the initial steps in the immune response.16 Binding and uptake of N8-GP in human monocyte-derived dendritic cells were therefore compared with that of unmodified FVIII.
Schematic depiction of N8-GP before and after thrombin activation. N8-GP corresponds to FVIII (turoctocog alfa) PEGylated with a 40-kDa PEG on the O-linked glycan in the 21-aa B-domain. After cleavage with thrombin, the activated molecule has the same primary structure as native FVIIIa.
Schematic depiction of N8-GP before and after thrombin activation. N8-GP corresponds to FVIII (turoctocog alfa) PEGylated with a 40-kDa PEG on the O-linked glycan in the 21-aa B-domain. After cleavage with thrombin, the activated molecule has the same primary structure as native FVIIIa.
Materials and methods
Materials
Porcine ST3GalI was produced in-house as His-ST3GalI using CHOK1SV cells and the pEE14.4 expression vector system containing the glutamine synthetase selection marker (Lonza Biologics, Basel, Switzerland). Rat ST3GalIII was produced as an MBP-SBD-ST3GalIII fusion in Escherichia coli.13 Arthrobacter ureafaciens sialidase was produced as a His6-tagged truncated form in a BL21-derived E. coli strain.17 Tick anticoagulant protein18,19 and turoctocog alfa (previously named N8)14 were produced as described. Advate was from Baxter, Westlake Village, CA, and ReFacto from Wyeth, Philadelphia, PA.
Animals
C57BL/6, von Willebrand factor (VWF) exon 4+5 knockout mice (B6.129S2-VWFtm1Wgr/J),20 and FVIII exon 16 knockout mice (B6.129S4-F8tm1Kaz/J)21 were bred at Charles River, Wilmington, MA or Taconic, Hudson, NY. Male Spraque Dawley rats (∼280 g) and female New Zealand white rabbits (∼2.5 kg) were obtained from Charles River, and male cynomolgus monkeys (∼3.8 kg) were from Bioculture, Senneville, Mauritius. All animal studies were performed according to guidelines from and approved by the Danish Animal Experiments Council, the Danish Ministry of Justice.
GlycoPEGylation of O-glycosylated FVIII
GlycoPEGylation of FVIII was performed by incubating turoctocog alfa [5 mg/mL in 50 mM 2-(N-morpholino)ethanesulfonic acid, 50 mM CaCl2, 150 mM NaCl, 20% glycerol, 0.5 mM antipain, pH 6.0] with sialidase (160 mU/mL), and fivefold molar excess cytidine-5′-monophosphate(CMP)-sialic acid(SA)-PEG reagent with PEG ranging from 10 to 80 kDa13,22 and ST3GalI (540 mU) at room temperature for 16 to 20 hours. The sample was diluted with 25 mM tris(hydroxymethyl)aminomethane, 5 mM CaCl2, 20 mM NaCl, 20% glycerol, pH 7.5, and loaded on a Source 15Q column. Bound material was eluted with 1 M NaCl in the buffer. To block remaining free galactose residues on the N-glycans following sialidase treatment, the glycoPEGylated FVIII product (1.0 mg/mL) was mixed with 2000-fold molar excess CMP-SA and ST3GalIII13 (400 mU/mL) in 25 mM tris(hydroxymethyl)aminomethane, 5 mM CaCl2, 20 mM NaCl, 20% glycerol, pH 7.5, and incubated at room temperature for 16 hours. The resulting capped glycoPEgylated FVIII was separated from CMP-SA and ST3GalIII by gel filtration on a Superdex 200 column equilibrated with 50 mM 2-(N-morpholino)ethanesulfonic acid, 50 mM CaCl2, 150 mM NaCl, 10% glycerol, pH 6.0.
FVIII:C
The FVIII activity (FVIII:C) was evaluated in a chromogenic FVIII assay using Coatest SP reagents (Chromogenix) as described.23 The FVIII one-stage clot assay was performed as described24 using Dade Actin FSL (Siemens), SynthASil (HemosIL, Instrumentation Laboratory), TriniCLOT aPTT HS (Trinity Biotech), and STA-PPT (Stago) as activated partial thromboplastin time (aPTT) reagents. An FVIII standard (turoctocog alfa) calibrated against the 7th International FVIII Standard National Institute for Biological Standards and Control (NIBSC) was used as standard. The specific activity was calculated by dividing the activity of the samples with the protein concentration determined by high-performance liquid chromatography as described.14,24
Binding to VWF and lipoprotein-receptor–related protein (LRP)
Effect of O-glycan PEGylation on cofactor activity and rate of FVIII activation
Cofactor activity of thrombin-activated N8-GP or FVIII (Advate) was analyzed as described.24 Using the FVIIIa activity assay (factor IXa [FIXa]–cofactor activity assay), reciprocal titrations of FIXa and FVIIIa against a fixed concentration (0.1 nM) of FVIIIa or FIXa, respectively, were performed to obtain apparent affinity of FIXa for FVIIIa (K1/2,FIXa) and functional FVIIIa concentration. The Michaelis constant (Km) and turnover number (kcat) of factor X (FX) activation were obtained from titrations of FX against a fixed concentration of FIXa-FVIIIa complex. The apparent rate (kapp) of activation of N8-GP and Advate was determined by measuring FVIIIa cofactor activity after a 1- to 3-minute incubation of 0.7 nM FVIII with 0.05 nM human α-thrombin. Formation of FVIIIa was linear in time. The rate of FVIIIa activation was expressed as moles FVIIIa formed per minute per mole of FVIII initially present (v/[FVIII]0).
In vitro plasma stability
Hirudin (Enzyme Research) and tick anticoagulant protein were added to citrate-stabilized FVIII-deficient plasma (George King Bio-Medical) to 5.7 µg/mL and 1.3 µg/mL, respectively, before addition of imidazole to 0.1 M to stabilize pH at 7.4. The plasma was recalcified by addition of CaCl2 to 20 mM. N8-GP or FVIII (turoctocog alfa, 1 U/mL) was added to 9 volumes of the plasma at 37°C at various time points prior to analysis of activity as described previously. The t1/2 was calculated by linear regression using GraphPad Prism 5.01 (GraphPad Software).
Cellular uptake
White blood cell–containing buffy coats were obtained from the Danish Blood Bank. Monocytes were isolated by density centrifugation on Ficoll-Paque gradient (GE Healthcare) followed by CD14 microbeads (Miltenyi Biotech) according to the manufacturer’s instructions. The monocytes were cultured in Iscove modified Dulbecco medium (GIBCO) supplemented with 1% penicillin/streptomycin, 10% fetal bovine serum, 40 ng/mL granulocyte macrophage–colony-stimulating factor (R&D Systems), and 40 ng/mL interleukin 4 for 5 days. The differentiation into dendritic cells was assessed by expression of CD86, CD209, and CD83 using antibodies and control IgG from BD Biosciences and an LSRII Fortessa flow cytometer (BD Biosciences). N8-GP and FVIII (turoctocog alfa) were 125I-labeled using the lactoperoxidase method. The amount of free iodine was <3%, and both labeled proteins had similar specific activity as the unlabeled protein. Cells were washed in 10 mM N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid, 150 mM NaCl, 4 mM KCl, 11 mM glucose, 5 mM CaCl2, 1 mg/mL BSA, pH 7.4, and incubated for 24 hours at 4°C and at a density of 1 × 106 cells per mL with 125I–N8-GP or 125I-FVIII at 1 to 250 nM. Nonspecific binding was assessed by adding 4 µM unlabeled N8-GP or FVIII. Cell-bound radioactivity was quantified in a γ-counter (Cobra). Concentrations of 125I–N8-GP and 125I-FVIII at Kd (70 and 110 nM, respectively) were applied in internalization studies at 37°C. Surface-bound material was removed by treating the cells with trypsin (10 µg/mL), proteinase K (50 ng/mL), and EDTA (10 mM) in phosphate-buffered saline for 30 minutes on ice.
Pharmacokinetics in mice
Mice received FVIII (turoctocog alfa or ReFacto) and O-glycoPEGylated FVIII (280 U/kg based on activity in chromogenic assay) as a single intravenous injection in a tail vein. This dose allowed detection of FVIII until ∼90% was cleared. Blood samples were collected in a sparse sample schedule with 3 samples per mouse and 2, 3, or 4 samples at each time point between 0 and 64 hours (FVIII-deficient and C57BL/6 mice) and 0 and 48 hours (VWF-deficient mice) and processed as described.25 Plasma from VWF-deficient mice and C57BL/6 mice was analyzed by enzyme-linked immunosorbent assay (ELISA) (Asserachrom FVIII:CAg; Diagnostica Stago) and plasma from FVIII-deficient mice by chromogenic activity assay23,25 using a plasma standard (HemosIL Calibration Plasma; Instrumentation Laboratory). Pharmacokinetic analysis was carried out on mean FVIII values using noncompartmental methods (WinNonlin Pro version 4.1 software; Pharsight). The following pharmacokinetic parameters were estimated: terminal half-life (t1/2), clearance (CL), volume of distribution at steady state, and mean residence time (MRT).
Pharmacokinetics in rats, rabbits, and cynomolgus monkeys
The pharmacokinetics of N8-GP and FVIII (turoctocog alfa or ReFacto) was further studied in rats, rabbits, and cynomolgus monkeys. The rats received a single intravenous injection of 200 U/kg FVIII compound in a tail vein. Blood was sampled in a sparse sample schedule between 0 and 30 hours after dosing with 6 samples from each animal and 3 samples per time point. Blood was processed as described previously and analyzed by ELISA. The rabbits received a single intravenous injection of 200 U/kg FVIII in an ear vein, and blood was sampled in full profiles from an ear artery between 0 and 55 hours (n = 4). Cynomolgus monkeys were administered 250 U/kg N8-GP or 1000 U/kg FVIII (turoctocog alfa) in a saphenous vein, and blood was sampled in full profiles from a femoral vein between 0 and 48 hours (n = 3). Rabbit and cynomolgus monkey blood was stabilized in sodium citrate, and plasma was analyzed by ELISA (rabbit, cynomolgus monkey dosed with N8-GP) or activity assay (cynomolgus monkey dosed with FVIII). Pharmacokinetic calculations were performed on mean values (rats) or individual animals (rabbit and cynomolgus monkey) of FVIII antigen data as described for the mice studies, except for FVIII in cynomolgus monkeys, which was evaluated in Kinetica version 4.2.1 for EP Series 2 (Thermo Electron Corp) without subtraction of background FVIII activity.
Acute and prolonged effect in tail bleeding model in FVIII-deficient mice
Studies of acute as well as prolonged effect in the tail bleeding model were performed essentially as described.25 For determination of acute effect, FVIII-deficient mice were anesthetized with isoflurane/N2O/O2 and dosed intravenously with 0 to 200 U/kg N8-GP or FVIII (Advate). Five minutes after dosing, 4 mm of the tip of the tail was cut. The tail was placed in 37°C saline, and blood collected over a 30-minute period. Total visual bleeding time was recorded, and blood loss determined by quantifying the amount of hemoglobin bled into the saline. Dose-response curves were fitted by use of nonlinear regression. The duration of effect was determined by analyzing bleeding at 5 minutes, 24 hours, 48 hours, and 72 hours after intravenous dosing of 200 U/kg N8-GP or FVIII. Groups were compared using the Kruskal-Wallis test including Dunn’s posttest.
Duration of effect in FeCl3-induced injury model in FVIII-deficient mice
Duration of effect of N8-GP vs FVIII (Advate) was investigated in an FeCl3-induced injury model as described.26 The carotid artery was exposed, and an ultrasound flow-probe (0.5 PSB Nanoprobe) placed around the artery. The injury was made 5 minutes, 24 hours, 48 hours, 60 hours, and 72 hours after dosing of 280 U/kg N8-GP, FVIII, or vehicle by applying a filter paper (2 × 5 mm) soaked in 10% FeCl3 around the exposed artery for 3 minutes. The artery was washed with 0.9% NaCl. Blood flow was recorded for 25 minutes, and the time to occlusion was determined by measuring the time from removal of the filter paper until cessation of blood flow. If occlusion did not occur, the occlusion time was reported as 25 minutes.
Duration of effect in joint injury model in FVIII-deficient mice
FVIII-deficient mice were dosed with N8-GP, Advate (280 U/kg), or vehicle. Hairs were plucked from the knee, and the diameter measured with a digital caliper (Mitutoyu). Bleeding was induced by introduction of a 30G needle into the joint cavity using an anterior approach at 5 minutes, 24 hours, 36 hours, 48 hours, 66 hours, 72 hours, and 88 hours postadministration of test substances.23 The joint bleeding was induced in the right knee, whereas the left knee served as control. Twenty-four hours following injury, mice were euthanized, joint diameters measured, and the knee bleeding graded according to a visual bleeding score (VBS), ranked from 0 to 3.23 Prior to and during all procedures, mice were anesthetized with isoflurane/N2O/O2.
Results
Selective O-glycoPEGylation of FVIII
Turoctocog alfa (previously named N8) contains 4 N-glycosylation sites, of which 2 are complex biantennary glycans and 2 are high-mannose structures (Figure 1).14 Although these N-glycans are well suited for selective enzymatic glycoPEGylation technology, the unique O-glycan in the B-domain of turoctocog alfa14 was selected for glycoPEGylation as the B-domain is released upon thrombin activation to produce FVIIIa with the same primary structure as endogenous FVIIIa (Figure 1). The initial step in the modification process is removal of all sialic acids from the glycans. The process then utilizes the ability of the ST3GalI sialic acid transferase to selectively transfer a PEG-modified sialic acid onto the O-glycan with PEG size of 10, 20, 40, and 80 kDa. The glycoPEGylation itself is conducted as a single-step reaction as sialidase does not remove PEG-sialic acid from the O-glycan. The resulting product is isolated by a single ion exchange chromatography step, which allows for separation of PEGylated and non-PEGylated material, as well as removal of enzymes and excess PEG–sialic acid–CMP. Capping of nonsialydated glycans is performed by addition of excess CMP-SA and ST3GalIII. The processing enzymes are subsequently removed.
VWF binding and activity of glycoPEGylated FVIII conjugates
The interaction between the glycoPEGylated FVIII conjugates and VWF was analyzed using immobilized human VWF (Table 1). All glycoPEGylated FVIII conjugates displayed high-affinity binding for human VWF represented by apparent Kd values in the sub-nanomolar range similar to native FVIII.
The specific activity of the O-glycoPEGylated FVIII conjugates was similar to the activity of native FVIII when measured in a chromogenic assay (Table 1). However, the specific activity of the conjugates measured in a one-stage clot assay varied depending on the aPTT reagent used. For FVIII conjugated with 40-kDa PEG (N8-GP), a specific activity of 9303 ± 428 U/mg (n = 4) was obtained using an ellagic acid containing aPTT reagent Actin FSL, which is similar to the activity measured with chromogenic assay. With colloidal silica (SynthasIL), a slightly lower specific activity of 7124 ± 375 U/mg was obtained, whereas specific activities of 4467 ± 492 U/mg and 3001 ± 430 U/mg were obtained with silica-based aPTT reagents (TriniCLOT and STA-PTT, respectively).
Relationship between prolongation of in vivo half-life and PEG size
The pharmacokinetics of glycoPEGylated FVIII conjugates was studied after intravenous administration to FVIII- and VWF-deficient mice aimed at identifying the optimal size of the PEG moiety (Table 2). In general, increased half-life was obtained by increasing the PEG size. The effect was most pronounced in the VWF-deficient mice with PEG moieties up to 40 kDa. For the FVIII conjugate with 40-kDa PEG attached, the t1/2 and MRT in VWF-knockout mice was increased 21- and 26-fold, respectively, whereas clearance was reduced 20-fold. Modification with 80-kDa PEG had no or only minor additional effect on any of the parameters. In FVIII-deficient mice, the pharmacokinetic improvements were less pronounced; that is, approximately twofold increased t1/2 and MRT and twofold reduced clearance were observed when attaching a 20-kDa or larger PEG to FVIII. A more extensive characterization was conducted on FVIII conjugated with 40-kDa PEG (N8-GP). The pharmacokinetic profile of N8-GP in FVIII-deficient mice is shown in supplemental Figure 1 (see the Blood Web site). Infused N8-GP bound VWF in vivo (supplemental Table 1). The prolonged half-life of N8-GP did not affect the level of endogenous VWF; that is, the murine VWF levels were not different between mice treated with FVIII or N8-GP.
O-glycoPEGylation did not affect the protein structure or N-linked glycans of N8-GP
Analysis of N8-GP by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (supplemental Figure 2) and mass spectrometry revealed the expected shift in molecular weight. Amino-terminal sequencing showed full comparability among N8-GP and the FVIII starting material. Thrombin cleavage of N8-GP released the glycoPEGylated B-domain peptide and resulted in a comparable fragment pattern to that of the FVIII starting material (supplemental Figure 2). Peptide maps with sequence coverage >80% were obtained for N8-GP and FVIII (turoctocog alfa) within 10-ppm precision (supplemental Figure 3). The maps were nearly identical. The major difference could be assigned to the absence of the O-linked glycopeptide from the N8-GP peptide map and the presence of a late eluting peak expected to be the PEGylated peptide. Comparable signals from sulfated tyrosine residues were observed in both N8-GP and FVIII peptide maps.
Glycosylations on N8-GP and turoctocog alfa were compared (supplemental Figure 4). The glycoprofiles show a consistent ratio among high-mannose structures and complex-type glycans before and after the glycoPEGylation process. Minor variations were observed, that is, a minor increase in the number of charged complex-type glycans and a more than fourfold reduction of N-glycolylneuraminic acid consistent with the ability of the A. ureafaciens sialidase to remove this specific glycan form.27 The glycoprofile revealed that the N-linked glycans were fully recovered after the desialylation steps in the glycoPEGylation process and that the complex-type glycans have been capped to the same extent as the glycans present on the FVIII starting material. The data thus showed that although there were minor quantitative differences between the glycans in N8-GP and the FVIII starting material, there were no qualitative differences. Thus, protein characterization confirmed that the primary amino acid sequence and the posttranslational modifications of N8-GP were conserved and were comparable to that of the FVIII starting material.
Prolonged half-life of N8-GP in several species
The pharmacokinetics of N8-GP was further explored in C57BL/6 mice, rats, rabbits, and cynomolgus monkeys. An approximately twofold increase in the t1/2 of N8-GP compared with FVIII was observed in normal mice, rabbits, and cynomolgus monkeys, whereas a 1.4-fold increase was observed in rats. The effects on clearance and MRT were generally greater than twofold with clearance in rabbits being slightly lower (Table 3).
Reduced LRP binding and cellular uptake of N8-GP
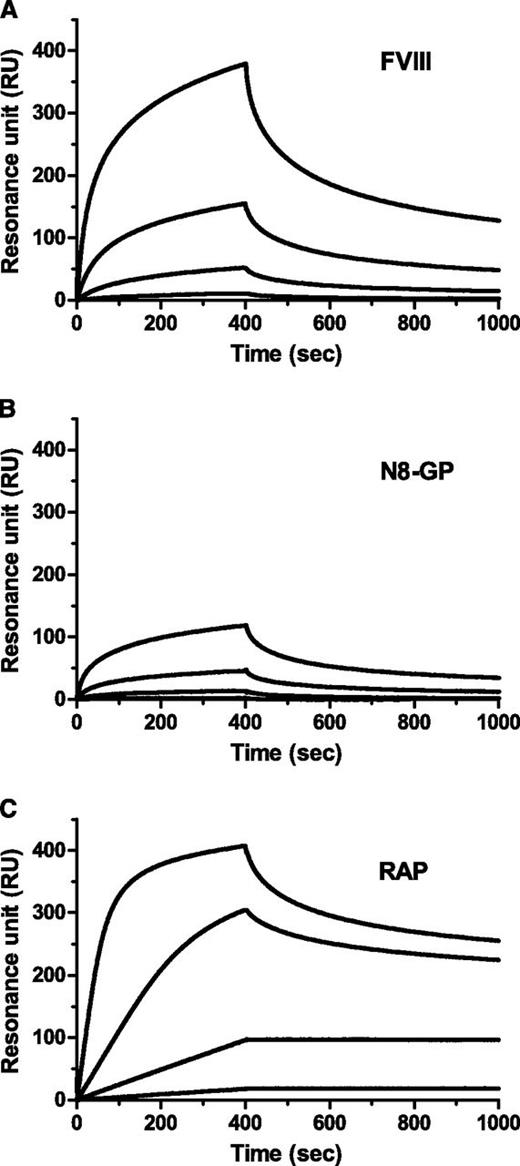
LRP has been implicated in clearance of FVIII.28-30 Binding to immobilized LRP was reduced for N8-GP (Figure 2). The data did not fit a 1:1 binding model; however, when data were assumed to fit this model, Kd values of 4.1 nM and 13 nM were calculated for the FVIII starting material and N8-GP, respectively (Table 4). The reduced binding of N8-GP was primarily a result of slower on-rate, whereas the off-rates were largely unaffected.
Reduced LRP binding of N8-GP. LRP was immobilized to a CM5 chip in a Biacore T100 instrument to 4000 to 5000 resonance units (RUs), and (A) FVIII (turoctocog alfa), (B) N8-GP, or (C) receptor-associated protein (RAP) at 12.5 nM, 3.13 nM, 0.78 nM, and 0.2 nM allowed to associate for 400 seconds and dissociate for 600 seconds using a flow of 10 µL/min.
Reduced LRP binding of N8-GP. LRP was immobilized to a CM5 chip in a Biacore T100 instrument to 4000 to 5000 resonance units (RUs), and (A) FVIII (turoctocog alfa), (B) N8-GP, or (C) receptor-associated protein (RAP) at 12.5 nM, 3.13 nM, 0.78 nM, and 0.2 nM allowed to associate for 400 seconds and dissociate for 600 seconds using a flow of 10 µL/min.
Initial experiments with an LRP-expressing cell line U87MG suggested fivefold lower cell binding of N8-GP as compared with FVIII (turoctocog alfa, supplemental Figure 5), but once bound, the same proportion of N8-GP and FVIII was internalized. The LRP antagonist receptor–associated protein inhibited internalization of both N8-GP and FVIII, suggesting that once bound to the cell surface internalization via LRP was similar for N8-GP and FVIII.
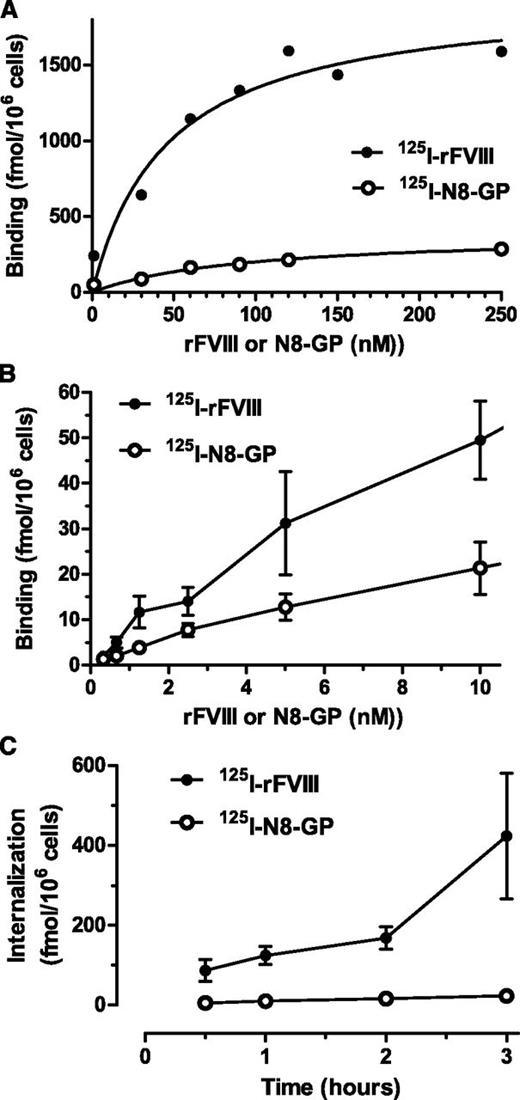
Cellular uptake was further studied in human monocyte-derived dendritic cells.31 Both proteins showed a dose-dependent specific binding (Figure 3A-B) with similar Kd values (ie, 104 ± 52 nM for N8-GP and 73 ± 28 nM for FVIII). The maximal binding capacity of the dendritic cells was sevenfold lower for N8-GP (302 ± 91 fmol/106 cells) than for FVIII (2187 ± 441 fmol/106 cells). Internalization of N8-GP and FVIII at 37°C increased over time (Figure 3C). At 3 hours, the specific internalization of N8-GP was 22.7 ± 1.8 fmol/106 cells, whereas that of FVIII was 20-fold higher (ie, 423 ± 158 fmol/106 cells).
Binding and uptake of N8-GP in human monocyte-derived dendritic cells. (A-B) Monocytes were differentiated into dendritic cells by 5 days of culturing with granulocyte macrophage–colony-stimulating factor and interleukin 4 before incubating with 125I–N8-GP or 125I-FVIII for 24 hours at 4°C. Unspecific binding determined by adding 4 µM unlabeled N8-GP or FVIII was subtracted. (C) Internalization was assessed at 37°C for up to 3 hours using concentrations at Kd (ie, 110 nM 125I–N8-GP and 70 nM 125I-FVIII). Data are mean and standard deviation from 4 (A) or 3 (B-C) experiments.
Binding and uptake of N8-GP in human monocyte-derived dendritic cells. (A-B) Monocytes were differentiated into dendritic cells by 5 days of culturing with granulocyte macrophage–colony-stimulating factor and interleukin 4 before incubating with 125I–N8-GP or 125I-FVIII for 24 hours at 4°C. Unspecific binding determined by adding 4 µM unlabeled N8-GP or FVIII was subtracted. (C) Internalization was assessed at 37°C for up to 3 hours using concentrations at Kd (ie, 110 nM 125I–N8-GP and 70 nM 125I-FVIII). Data are mean and standard deviation from 4 (A) or 3 (B-C) experiments.
In vitro functional characteristics of N8-GP
In the in vivo efficacy evaluation of N8-GP, a marketed FVIII product (Advate) was used as a comparator. Therefore, Advate was also used as a comparator in most of the in vitro functional characterization of N8-GP (Table 4). The rate of N8-GP activation by thrombin was assessed by quantifying the rate of thrombin-catalyzed FVIII activation during the initial (0-3 minutes) formation of FVIIIa. In the absence of VWF, the rate of thrombin activation was identical for N8-GP and FVIII; however, with VWF present, the rate of activation of FVIII was twofold higher. The Kd of FVIIIa-FIXa interaction, as well as the kinetic parameters for FIXa-catalyzed activation of FX, was not different between N8-GP and FVIII. Furthermore, the in vitro stability of N8-GP and FVIII was within the same range.
Maintained in vivo efficacy and potency of N8-GP in FVIII-deficient mice
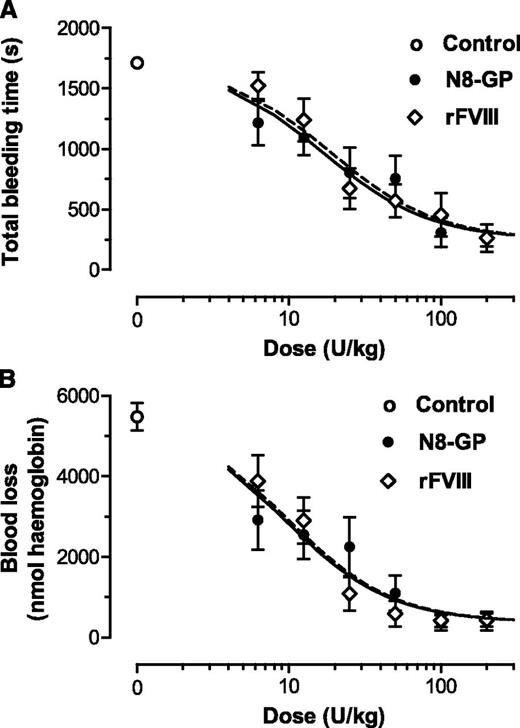
The in vivo effect of N8-GP was evaluated in the tail bleeding model in FVIII-deficient mice. The dose-response curves of N8-GP and FVIII were close to superimposable both with regard to total bleeding time (Figure 4A) and blood loss (Figure 4B). Both compounds reduced the bleeding time and blood loss to the same level as in normal mice. The doses giving half-maximal effect were 17 ± 6 U/kg for bleeding time and 19 ± 6 U/kg for N8-GP and FVIII (mean and standard error of the fits). For blood loss, 50% effective dose values of 9.3 ± 2.8 U/kg (N8-GP) and 9.8 ± 2.4 U/kg (FVIII) were found. Thus, no differences in potency and efficacy were found between N8-GP and FVIII.
Acute effect of N8-GP in a tail bleeding model in FVIII-deficient mice. The mice received N8-GP or FVIII (Advate) intravenously at the indicated doses, and tail bleeding was measured after clipping 4 mm of the tail tip 5 minutes after dosing. Dose-response curves of total bleeding time (A) and blood loss (B) are shown for N8-GP (closed circles, black line) and FVIII (open diamonds, dotted lines). A blood loss of 1000 nmol hemoglobin approximates 0.125 mL whole blood using a hemoglobin concentration of 8 mmol/L. Control animals receiving the vehicle only are shown with open circles. Data are mean ± standard error of the mean (SEM) of n = 12 mice per group.
Acute effect of N8-GP in a tail bleeding model in FVIII-deficient mice. The mice received N8-GP or FVIII (Advate) intravenously at the indicated doses, and tail bleeding was measured after clipping 4 mm of the tail tip 5 minutes after dosing. Dose-response curves of total bleeding time (A) and blood loss (B) are shown for N8-GP (closed circles, black line) and FVIII (open diamonds, dotted lines). A blood loss of 1000 nmol hemoglobin approximates 0.125 mL whole blood using a hemoglobin concentration of 8 mmol/L. Control animals receiving the vehicle only are shown with open circles. Data are mean ± standard error of the mean (SEM) of n = 12 mice per group.
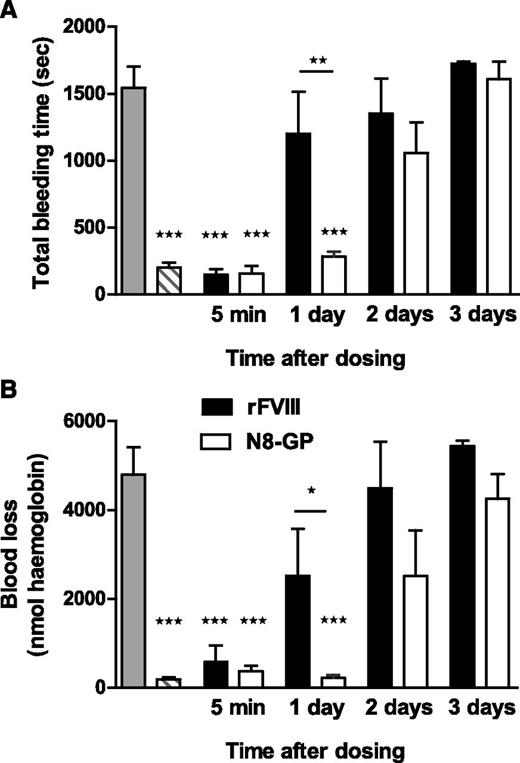
Prolonged effect of N8-GP in FVIII-deficient mice
In the tail bleeding model (Figure 5), a significant difference in bleeding time and blood loss was observed between N8-GP and FVIII 1 day after dosing (P < .01 and P < .05, respectively). N8-GP normalized bleeding time and blood loss after 1 day, at which point the hemostatic effect of FVIII had disappeared. No effect was seen 3 days after dosing.
Prolonged effect of N8-GP in a tail bleeding model in FVIII-deficient mice. The mice were dosed intravenously with 200 U/kg of N8-GP (open bars) or FVIII (Advate, solid bars), and total bleeding time (A) and blood loss (B) were measured in the tail bleeding model after 5 minutes and 1, 2, or 3 days after treatment. The gray bars correspond to FVIII-deficient mice, and the hatched bars to normal mice (C57BL/6). Data are mean ± SEM of n = 6 to 12 mice per group. ***P < .001 indicates significant difference from FVIII-deficient mice. *P < .05 and **P < .01 indicate significant differences between mice treated with FVIII and N8-GP.
Prolonged effect of N8-GP in a tail bleeding model in FVIII-deficient mice. The mice were dosed intravenously with 200 U/kg of N8-GP (open bars) or FVIII (Advate, solid bars), and total bleeding time (A) and blood loss (B) were measured in the tail bleeding model after 5 minutes and 1, 2, or 3 days after treatment. The gray bars correspond to FVIII-deficient mice, and the hatched bars to normal mice (C57BL/6). Data are mean ± SEM of n = 6 to 12 mice per group. ***P < .001 indicates significant difference from FVIII-deficient mice. *P < .05 and **P < .01 indicate significant differences between mice treated with FVIII and N8-GP.
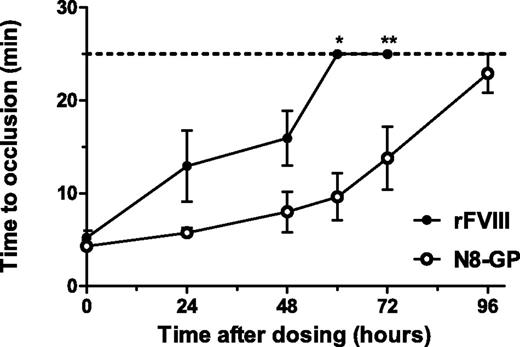
In the FeCl3-induced injury model (Figure 6), the damage of the vessel leads to initiation of coagulation, clot formation, occlusion of the vessel, and eventually the stopping of blood flow in normal mice. No occlusion occurs in untreated FVIII-deficient mice.26 In FVIII-deficient mice receiving N8-GP, the occlusion time was 5.8 ± 0.5 minutes at 24 hours and increased to 13.8 ± 3.4 minutes at 72 hours after dosing. The occlusion time in FVIII-treated mice was 13.0 ± 3.4 minutes and 15.9 ± 2.9 minutes after 24 and 48 hours, respectively, whereas no occlusions were observed 60 and 72 hours after administration. In all mice treated with N8-GP, occlusion was observed 24 hours after dosing, whereas only 67% of the mice treated with FVIII occluded. After 72 hours, occlusion was still seen in 63% of the mice treated with N8-GP, whereas no occlusion was observed 60 and 72 hours after administration of FVIII.
Prolonged effect of N8-GP in a FeCl3-induced injury model in FVIII-deficient mice. Occlusion time after FeCl3-induced injury was measured 5 minutes (acute effect), 24 hours, 48 hours, 60 hours, 72 hours, and 96 hours after dosing of 280 U/kg N8-GP (open circles) or FVIII (Advate; closed circles). The dotted line indicates the observation period for the experiments; during this period, no occlusion was recorded in the vehicle-treated mice. Mean and SEM of n = 6 to 10 mice per group are shown. *P < .01; **P < .05 N8-GP compared with FVIII at individual time points.
Prolonged effect of N8-GP in a FeCl3-induced injury model in FVIII-deficient mice. Occlusion time after FeCl3-induced injury was measured 5 minutes (acute effect), 24 hours, 48 hours, 60 hours, 72 hours, and 96 hours after dosing of 280 U/kg N8-GP (open circles) or FVIII (Advate; closed circles). The dotted line indicates the observation period for the experiments; during this period, no occlusion was recorded in the vehicle-treated mice. Mean and SEM of n = 6 to 10 mice per group are shown. *P < .01; **P < .05 N8-GP compared with FVIII at individual time points.
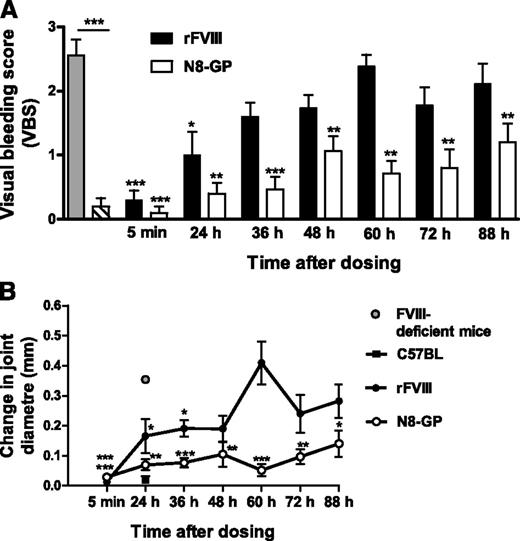
Both N8-GP and FVIII prevented bleeding (Figure 7A) and the concomitant joint swelling (Figure 7B) in the needle-induced joint bleeding model when the injury was induced 5 minutes after dosing. N8-GP prevented the increase in joint diameter at all time points analyzed (up to 88 hours), whereas the effect of FVIII lasted up to 36 hours (Figure 7B). The VBS in the N8-GP–treated groups was significantly lower compared with that of FVIII-treated mice when the animals were dosed 36 to 88 hours before injury (Figure 7A). Thus, the data demonstrate a prolonged duration of effect of N8-GP compared with FVIII.
Prolonged effect of N8-GP in a joint bleeding model in FVIII-deficient mice. N8-GP (A, open bars; B, open circles) or FVIII (Advate) (A, solid bars; B, solid circles) were administered intravenously to the mice at 280 U/kg, and needle-induced joint bleeding in the knee induced at the time points indicated after dosing. The gray bar (A) or gray circle (B) correspond to FVIII-deficient mice, and the hatched bar (A) and solid square (B) to normal mice (C57BL/6). The bleeding was evaluated 24 hours after injury, and VBS (A) and change in joint diameter (B) determined. A VBS of 3 indicates tense and distorted knees, whereas 0 designates a virtually nonaffected knee. Data are mean and SEM of 10 mice per group at time points 5 minutes, 24 hours, 72 hours, and 88 hours, and 15 mice per group at 36 to 60 hours after injury. *P < .05; **P < .01; and *** P < .001 indicate significant difference between treated and nontreated FVIII-deficient mice. Above the bar in (A), ***P < .001 indicates significant difference between FVIII-deficient and C57BL/6 control mice.
Prolonged effect of N8-GP in a joint bleeding model in FVIII-deficient mice. N8-GP (A, open bars; B, open circles) or FVIII (Advate) (A, solid bars; B, solid circles) were administered intravenously to the mice at 280 U/kg, and needle-induced joint bleeding in the knee induced at the time points indicated after dosing. The gray bar (A) or gray circle (B) correspond to FVIII-deficient mice, and the hatched bar (A) and solid square (B) to normal mice (C57BL/6). The bleeding was evaluated 24 hours after injury, and VBS (A) and change in joint diameter (B) determined. A VBS of 3 indicates tense and distorted knees, whereas 0 designates a virtually nonaffected knee. Data are mean and SEM of 10 mice per group at time points 5 minutes, 24 hours, 72 hours, and 88 hours, and 15 mice per group at 36 to 60 hours after injury. *P < .05; **P < .01; and *** P < .001 indicate significant difference between treated and nontreated FVIII-deficient mice. Above the bar in (A), ***P < .001 indicates significant difference between FVIII-deficient and C57BL/6 control mice.
Discussion
The current standard prophylactic treatment regimen in hemophilia A typically requires injection of FVIII 3 times per week or every other day to maintain a sufficient circulating level to prevent frequent bleeds and preserve joint function. Previous attempts to produce a longer-acting FVIII based on the native FVIII have been hampered by loss of potency and product heterogeneity. FVIII circulates as a pro-cofactor that is activated by thrombin at the site of vessel wall injury in a process where the B-domain is released in the formation of FVIIIa. The objective of the present studies was to investigate whether PEGylation, a technology known to extend the half-life of therapeutic proteins, could be directed to the B-domain to allow generation of fully active native FVIIIa following activation by thrombin. To avoid modification of the peptide, the target for PEGylation was a unique O-glycan at position 750 located in the B-domain of FVIII (turoctocog alfa14 ). A gentle enzymatic 3-step process was developed. In the first step, all sialic acid residues are removed with a sialidase. In the second step, an O-glycan–specific sialyltransferase, ST3GalI, selectively transfers a single PEGylated sialic acid (10-, 20-, 40-, or 80-kDa PEG) onto the B-domain O-glycan at position 750. In the final step, the sialyltransferase ST3GalIII caps all free galactose residues with a terminal sialic acid.
All glycoPEGylated FVIII conjugates maintained high-affinity binding to VWF and FVIII activity (FVIII:C) indistinguishable from that of native FVIII when measured in the standard chromogenic assay. However, the specific activity in a 1-stage clot assay varied with different aPTT reagents; that is, for FVIII conjugated with a 40-kDa PEG (N8-GP), specific activities were similar to what was obtained using chromogenic assay to threefold lower depending on the aPTT reagent used (ie, from 9303 ± 428 U/mg to 3001 ± 430 U/mg). Similar observations have been described for PEGylated FVIII-K1804C8 and may be an assay artifact as prolonged aPTT clotting times have been reported after PEGylation of proteins not involved in coagulation.32 The exact mechanism for the PEG aPTT reagent-specific interference is not clear. Apparently, ellagic acid–containing reagents tend to give less interference than silica; however, these observations need to be confirmed in more elaborated studies.
When the pharmacokinetics was evaluated in FVIII- and VWF-deficient mice, there was a clear beneficial effect of increasing PEG sizes from 10 to 20 kDa and even further to the branched 40-kDa PEG; however, conjugating an 80-kDa PEG provided no additional improvement. FVIII conjugated with a 40-kDa PEG (N8-GP) was further characterized and demonstrated an approximately twofold longer half-life in mice, rabbits, and monkeys, whereas the effect was less pronounced in rats. N8-GP had similar FVIII cofactor activity, rate of activation by thrombin in the absence of VWF, rate of APC-mediated inactivation, and affinity for VWF as native FVIII. The rate of N8-GP activation in the presence of VWF appeared reduced in the in vitro assay used. However, in the tail bleeding model in FVIII-deficient mice, the efficacy and potency of N8-GP were not different from FVIII (Figure 4). Shear stress induces unfolding of VWF,33 and it could be hypothesized that flow reduces the impact of VWF on the activation of FVIII under physiological conditions.
The duration of hemostatic effect was evaluated using 3 different models in FVIII-deficient mice at various time points after administration of equimolar doses of N8-GP and FVIII. The effect of N8-GP was maintained at time points where the effect of FVIII was no longer present, corresponding to the longer half-life of N8-GP.
An important question in relation to the PEGylation is the mechanism by which PEG reduces clearance of a much larger molecule (FVIII) than the PEG moiety itself. The observed PEG-size dependency of pharmacokinetics in VWF-deficient mice, combined with the significantly reduced LRP and cell binding of N8-GP, indicates that the mechanism for reduced clearance is largely mediated by shielding against interactions with clearance receptors. The reduced binding of N8-GP to LRP was primarily driven by a slower on-rate. This indicates that PEGylation resulted in shielding of the interaction between N8-GP and LRP; however, once bound, the complex did not dissociate faster, suggesting that the PEG moiety is not located directly in the LRP interface. Whether this is the actual mechanism behind the reduced clearance of N8-GP relative to FVIII remains to be fully elucidated. Distribution studies in rats34 demonstrated delayed uptake of N8-GP but no alteration of distribution between organs, suggesting that N8-GP may be cleared through the same pathways as FVIII, however, at a reduced rate.
Formation of inhibitors is a key concern in relation to FVIII therapy. Dendritic cells are APCs mediating uptake of foreign proteins for degradation and presentation to the adaptive immune system.16 Human monocyte-derived dendritic cells showed sevenfold reduced maximal binding capacity for N8-GP as compared with FVIII. This may be a result of shielding of the PEG moiety toward binding to surface receptors on the surface of the dendritic cells similar to the effect of PEG on binding to LRP and LRP-expressing cells. However, antagonists to LRP and macrophage mannose receptor, antibodies to DC-SIGN (CD209), and silencing RNA-mediated decrease in expression of these receptors all failed to prevent endocytosis of FVIII in dendritic cells31 ; thus, the responsible receptor(s) on the APCs remain(s) to be identified. It can be hypothesized that the reduced endocytosis of N8-GP in dendritic cells will subsequently reduce the presentation to FVIII-specific T cells in a manner similar to what has previously been described for FVIII with VWF35 or FVIII coformulated with an anti-C1–domain antibody KM33,31 as well as FVIII with mutations in the C1-domain.36 However, although KM33 and the C1-domain mutations result in delayed antibody formation of FVIII in mouse models, the correlation between these models and the human clinic remains to be established. Whether a reduction in immune response toward exogenous FVIII can be achieved by N8-GP therefore needs to be documented in a clinical setting.
The present data demonstrate that PEGylation of the O-linked glycan in the B-domain of FVIII (turoctocog alfa) is site specific and does not result in any other modification of the FVIII molecule. The biochemical functions of N8-GP are maintained, whereas the cellular uptake and in vivo clearance are reduced. In mice models, the potency and efficacy of N8-GP are indistinguishable from unmodified FVIII, whereas the duration of effect of N8-GP is prolonged. In conclusion, N8-GP has the potential to offer efficacious treatment and prevention of bleeds in hemophilia A at reduced dosing frequency. Clinical investigation of N8-GP is currently ongoing.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Fritz Poulsen and Dorthe Riis, Novo Nordisk A/S, for antigen analysis of in vivo samples, and Mette Loftager for supplementary analyses of FVIII activity in samples from mice. Furthermore, the authors acknowledge a large number of technicians for their excellent contributions.
Authorship
Contribution: H.R.S. and S.E.B. conceived the study, designed research, and wrote the manuscript; M.K. designed and performed in vitro research, analyzed data, and wrote the manuscript; D.M.K. designed, performed, and analyzed pharmacokinetic studies, except for pharmacokinetics of FVIII in cynomolgus monkeys, where W.K. designed the study and analyzed data; F.R. designed and performed the pharmacokinetic study addressing VWF levels and the FVIII-VWF complex; H.P. analyzed plasma samples; K.W.B., C.N.G., E.P., I.H., H.R.-N., A.K.K., L.T., and E.H.N.O. designed, performed, and analyzed in vitro research; C.J., A.B., and B.P. established the glycoPEGylation process; P.B.J., T.E., K.Ø., F.M., and H.L.H. designed, performed, and analyzed animal studies; M.T. and M.E. designed animal studies; and A.A.P. contributed with cell line construction.
Conflict-of-interest disclosure: All authors are employees of Novo Nordisk A/S.
Correspondence: Henning Stennicke, Biopharmaceuticals Research Unit, Novo Nordisk, Novo Nordisk Park, DK-2760 Måløv, Denmark; e-mail: hrse@novonordisk.com.
References
Author notes
H.R.S. and M.K. contributed equally to this study.