Key Points
HLA-matched siblings are better than HLA-matched unrelated donors for patients with good performance scores
Survival rates are comparable after HLA-matched sibling and unrelated donor transplantations for patients with poor performance scores
Abstract
Older patients are increasingly undergoing allogeneic hematopoietic transplantation. A relevant question is whether outcomes can be improved with a younger allele-level 8/8 HLA-matched unrelated donor (MUD) rather than an older HLA-matched sibling (MSD). Accordingly, transplants in leukemia/lymphoma patients age ≥50 years were analyzed comparing outcomes for recipients of MSD ≥50 (n = 1415) versus MUD <50 years (n = 757). Risks of acute graft-versus-host disease (GVHD) grade 2 to 4 (hazard ratio [HR], 1.63; P < .001), 3 to 4 (HR, 1.85; P < .001), and chronic GVHD (HR, 1.48; P < .0001) were higher after MUD compared with MSD transplants. The effect of donor type on nonrelapse mortality (NRM), relapse, and overall mortality was associated with performance score. For patients with scores of 90 or 100, NRM (HR, 1.42; P = .001), relapse (HR, 1.45; P < .001), and overall mortality (HR, 1.28; P = .001) risks were higher after MUD transplants. For patients with scores below 90, NRM (HR, 0.96; P = .76), relapse (HR, 0.86; P = .25), and overall mortality (HR, 0.90; P = .29) were not significantly different after MUD and MSD transplants. These data favor an MSD over a MUD in patients age ≥50 years.
Introduction
HLA-matched unrelated donors (MUD) have become the most common donor source for allogeneic hematopoietic cell transplants (HCTs).1 Many recent reports have found nearly equivalent outcomes for MUD transplants compared with allotransplants from HLA-matched siblings (MSD), in large part because of the adoption of high resolution, HLA allele-level typing.2 These studies have demonstrated comparable rates for overall and relapse-free survival as well as treatment-related mortality, albeit with slightly higher rates for acute and chronic graft-versus-host disease (GVHD) in unrelated donor recipients.3-7
There has been a progressive increase in the age of recipients undergoing allogeneic HCTs, primarily because of the use of reduced-intensity and nonmyeloablative preparative regimens. Patients older than age 50 years now represent the fastest growing age group, including a marked rise in the subset of patients older than age 60 years.1 Most centers attempt to identify an MSD before considering an MUD. For older-age patients, use of a sibling donor, who usually is of an age similar to that of the patient, can be problematic because of the common presence of comorbidities as well as concerns regarding the regenerative potential of stem and immune cells from older donors. Several studies have also found donor age to be a risk factor for the development of acute and chronic GVHD, with higher rates seen with the use of older donors.8-10
Kollman and colleagues9 analyzed the impact of donor age on transplant outcomes in a retrospective review of roughly 7000 unrelated bone marrow transplants facilitated by the National Marrow Donor Program between 1987 and 1999. Receipt of a transplant from a donor between the ages of 18 and 30 years was independently associated with less acute and chronic GVHD and improved survival when compared with recipients of donors age >30 years.9 Similar results have been seen in single-center reviews with older-age donors (>30 years), resulting in higher rates for acute and chronic GVHD.8,10
An important question for older recipients is whether outcomes are improved with an MSD (who is generally about the same age) or a younger MUD. The goal of this study was to answer this question to guide donor selection for transplant recipients older than age 50 years.
Patients and methods
Patients
The Center for International Blood and Marrow Transplant Research (CIBMTR) is a voluntary working group of more than 450 transplantation centers that contribute detailed data on consecutive allogeneic and autologous hematopoietic cell transplantation to a Statistical Center at the Medical College of Wisconsin in Milwaukee, Wisconsin, and the National Marrow Donor Program Coordinating Center in Minneapolis, Minnesota. Participating centers are required to report all transplants consecutively, and compliance is monitored by on-site audits. Patients are followed longitudinally. All patients provided written informed consent in accordance with the Declaration of Helsinki for data submission and research participation. The Institutional Review Boards of the Medical College of Wisconsin and the National Marrow Donor Program approved this study.
Inclusion criteria
Included are patients age 50 years or older who received transplants from 1995 to 2005 for a hematologic malignancy. Two cohorts were identified: (1) recipients of an HLA-matched transplantation with donors age 50 years or older (MSD ≥50 years) and (2) recipients of an HLA-matched unrelated graft from donors age younger than 50 years (MUD <50 years). All unrelated donor-recipient pairs were matched at HLA-A, -B, -C, and -DRB1 at the allele-level (8/8 HLA-match), and 91% were also matched at HLA-DQ. Allele-level HLA typing was performed retrospectively by the National Marrow Donor Program at a central laboratory on banked donor-recipient samples.11 Excluded are recipients of prior autologous and allogeneic transplants, mismatched unrelated donor transplants, and ex vivo T-cell depleted grafts.
End points
Incidences of grade 2 to 4 acute and chronic GVHD were based on reports from each transplantation center by using standard criteria.12,13 Nonrelapse mortality (NRM) was defined as death not related to disease recurrence or progression and relapse was defined as disease recurrence based on morphologic evaluation. Overall mortality (inverse of overall survival [OS]) was defined as death from any cause. Treatment failure (inverse of disease-free survival [DFS] was defined as relapse or death from any cause.
Statistical analysis
Patient, disease, and transplant-related characteristics for the two cohorts were compared by using χ2 statistics for categorical variables and the Kruskal-Wallis test for continuous variables (Table 1). The probability of OS was calculated by using the Kaplan-Meier estimator. The probabilities of acute and chronic GVHD, NRM, and relapse were calculated by using the cumulative incidence estimator to accommodate competing risks. For NRM, relapse was the competing risk, and for relapse, the competing risk was NRM. For hematopoietic recovery and chronic GVHD, death without the event was the competing risk. For acute GVHD, death without the event or disease recurrence prior to onset of acute GVHD was the competing risk. For analysis of OS, death from any cause was considered an event. For analysis of DFS, relapse or death from any cause was considered an event. In all analyses, data on patients without an event were censored at last follow-up.
Cox proportional hazard regression models were constructed for acute and chronic GVHD, NRM, relapse, treatment failure, and overall mortality. Results are expressed as hazard ratio (HR) with 95% confidence interval (CI). Multivariate models were built by using stepwise selection procedure. Proportional hazards assumption was tested for all variables considered in multivariate analysis. Final models were stratified by variables that failed to meet the proportional hazards assumption. The main effect term, donor source (MSD, HLA-matched sibling donor age ≥50 years vs MUD HLA-matched unrelated donor age <50 years) was held in all steps of model building, regardless of level of significance. First-order interactions between the main effect and the other variables were tested in multivariate models; when significant, an interaction term was built and retained in models. There was a significant interaction between performance score (90-100 vs ≤80) and donor source for the following outcomes: NRM, relapse, treatment failure, and OS. Therefore, for these outcomes, the main effect variable was built as MSD, performance score 90 to 100 vs MSD, performance score ≤80 vs MUD, performance score 90 to 100 vs MUD, performance score ≤80. Interactions between this main effect term and other covariates in the final model were tested and there were none. Interactions between other variables in the final model were also tested and there were none. The effect of patient age on OS was tested as a continuous variable, and the optimal cut point was determined statistically; mortality risks were higher in patients age 60 years or older compared with younger patients. We did not observe differences in survival between those age 60 to 69 years and those age 70 to 75 years. Other variables tested included patient age (50-59 vs 60-75 years), disease (acute myeloid leukemia [AML] vs acute lymphoblastic leukemia [ALL] vs myelodysplastic syndrome [MDS] vs chronic myeloid leukemia [CML] vs non-Hodgkin lymphoma [NHL] vs chronic lymphocytic leukemia [CLL]), disease status at transplantation (remission vs relapse), interval from diagnosis to transplantation (≤12 vs >12 months), conditioning regimen (ablative vs reduced intensity/nonablative), in vivo T-cell depletion (yes vs no), GVHD prophylaxis (cyclosporine-containing vs tacrolimus-containing), graft type (bone marrow vs peripheral blood progenitor cells), donor-recipient sex match (female donor–male recipient vs others), donor-recipient cytomegalovirus sero-status (donor negative/recipient negative vs donor positive/recipient negative vs donor negative/recipient positive vs donor positive/recipient positive), and year of transplant (1995-1999 vs 2000-2005). There was no significant effect of transplantation center on OS, tested by using the frailty model. All analyses were performed using SAS, version 9.1 (SAS Institute Cary, NC).
Results
Patient, disease, and transplant characteristics
Patient, disease, and transplant characteristics are shown in Table 1. All patients were older than 50 years at transplantation. A total of 1415 patients received MSD transplants from their siblings also age 50 years or older and 757 patients received MUD transplants from 8/8 HLA-matched unrelated adult donors age younger than 50 years. Ninety-one percent of 8/8 HLA-matched MUD transplants (692 of 757) were matched at HLA-A, -B, -C, -DRB1, and -DQ (10/10 HLA-matched). The median ages of patients in the two cohorts were comparable: 57 years (range, 50-75 years) for MSD transplants and 56 years (range, 50-74 years) for MUD transplants. Roughly a quarter of patients in both groups were older than age 60 years. The median ages of sibling and unrelated donors were 58 years (range, 50-85 years) and 34 years (range, 19-49 years), respectively. Disease characteristics, including disease type and disease status at transplantation, of the two cohorts differed. Compared with MSD recipients, MUD recipients were more likely to have AML (40% vs 44%) and less likely to have CML (17% vs 14%) or NHL (17% vs 14%) (P = .010). MSD recipients were more likely to be in remission at transplantation compared with MUD recipients (52% vs 46%) (P = .010). As expected, time from diagnosis to transplantation was less in the MSD cohort with 32% of patients receiving transplant within 6 months versus 25% in the MUD group (P = .005). The median time to transplantation in the MSD group was 10 months (range, <1-239 months) and in the MUD group, it was 11 months (range, 1-196 months). The use of antithymocyte globulin ATG (29% vs 20%; P < .001), bone marrow grafts (39% vs 18%; P < .001), and tacrolimus-based GVHD prophylaxis (57% vs 24%; P < .001) were all more common in the MUD group. Female donors for male recipients were more common in MSDs (26% vs 16%; P < .001) as was the percentage of patients who had a transplant from 1995 to 1999 (13% vs 27%; P < .001), while there were more dual cytomegalovirus donor-negative/recipient-negative pairs in the MUD group (31% vs 19%; P < .001). The median number of transplants per center for both donor types was 4; for the MSD group, the range was 1 to 76 transplants, and for the MUD group, the range was 1 to 95 transplants. The median follow-up for survivors was 56 months (range, 3-156 months) for MSD age >50 years and 49 months (range, 12-145 months) for the MUD age <50 years groups.
Acute and chronic GVHD
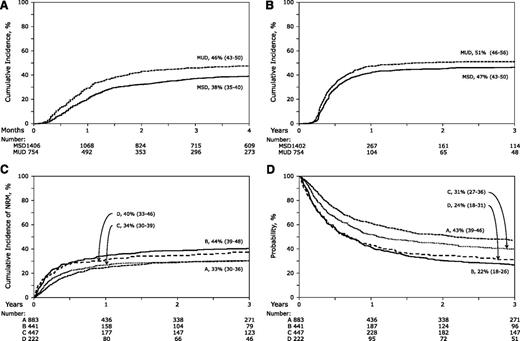
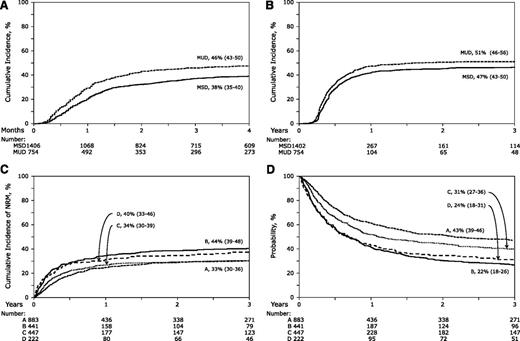
The day 100 probability of acute grade 2 to 4 GVHD was higher at 46% (95% CI, 43%-50%) after MUD transplantation compared with 38% (95% CI, 35%-40%) after MSD transplantation (P < .001) (Figure 1A). The corresponding probabilities of acute grade 3 to 4 GVHD were 29% (95% CI, 26%-32%) after MUD transplantation compared with 21% (95% CI, 19%-23%) after MSD transplantation (P < .001). The results of multivariate analysis, stratified by year of transplantation (1995-1999 vs 2000-2005) are shown in Table 2. Risks of grade 2 to 4 and 3 to 4 acute GVHD were higher after MUD compared with MSD transplantations. The effect of donor source was independent of other variables associated with acute GVHD. Grade 2 to 4 acute GVHD risks were higher in patients who did not receive in vivo T-cell depletion (HR, 1.45; 95% CI, 1.20-1.72; P < .001) but who did receive cyclosporine-containing GVHD prophylaxis regimens (HR, 1.24; 95% CI, 1.06-1.44; P = .007) compared with tacrolimus-containing regimens and in patients transplanted in relapse (HR, 1.19; 95% CI, 1.04-1.36; P = .011) compared with those transplanted in remission. Risks of grade 3 to 4 acute GVHD were also higher after cyclosporine-containing compared with tacrolimus-containing GVHD prophylaxis regimens (HR, 1.54; 95% CI, 1.26-1.88; P < .001). Risks of grade 2 to 4 acute GVHD (HR, 1.21; P = .04) was marginally higher after transplantation of peripheral blood but not risk of grade 3 to 4 acute GVHD (HR, 1.15; P = .24).
The probabilities of acute and chronic GVHD, non-relapse mortality, and overall survival. (A)The unadjusted cumulative incidence of grade 2 to 4 acute GVHD for recipients of MSD transplants with donors age 50 years or older and MUD transplants with donors age younger than 50 years. (B) The unadjusted cumulative incidence of chronic GVHD for recipients of MSD transplants with donors age 50 years or older and MUD transplants with donors age younger than 50 years. (C) The unadjusted cumulative incidences of NRM for recipients of MSD transplants with donors age 50 years or older and performance scores 90 or 100 (group A), MSD transplants with donors age 50 years or older and performance scores 80 or lower (group B), MUD transplants with donors age younger than 50 years and performance scores 90 or 100 (group C) and MUD transplants with donors age younger than 50 years and performance scores 80 or lower (group D). (D) Probabilities of OS adjusted for conditioning regimen, patient age, disease, and disease status for recipients of MSD transplants with donors age 50 years or older and performance scores 90 or 100 (group A), MSD transplants with donors age 50 years or older and performance scores 80 or lower (group B), MUD transplants with donors age younger than 50 years and performance scores 90 or 100 (group C) and MUD transplants with donors age younger than 50 years and performance scores of 80 or lower (group D).
The probabilities of acute and chronic GVHD, non-relapse mortality, and overall survival. (A)The unadjusted cumulative incidence of grade 2 to 4 acute GVHD for recipients of MSD transplants with donors age 50 years or older and MUD transplants with donors age younger than 50 years. (B) The unadjusted cumulative incidence of chronic GVHD for recipients of MSD transplants with donors age 50 years or older and MUD transplants with donors age younger than 50 years. (C) The unadjusted cumulative incidences of NRM for recipients of MSD transplants with donors age 50 years or older and performance scores 90 or 100 (group A), MSD transplants with donors age 50 years or older and performance scores 80 or lower (group B), MUD transplants with donors age younger than 50 years and performance scores 90 or 100 (group C) and MUD transplants with donors age younger than 50 years and performance scores 80 or lower (group D). (D) Probabilities of OS adjusted for conditioning regimen, patient age, disease, and disease status for recipients of MSD transplants with donors age 50 years or older and performance scores 90 or 100 (group A), MSD transplants with donors age 50 years or older and performance scores 80 or lower (group B), MUD transplants with donors age younger than 50 years and performance scores 90 or 100 (group C) and MUD transplants with donors age younger than 50 years and performance scores of 80 or lower (group D).
The 3-year probabilities of chronic GVHD were 51% (95% CI, 46%-56%) after MUD compared with 47% (95% CI, 43%-50%) after MSD transplantation (P = .12; Figure 1B). Multivariate analysis shows that chronic GVHD was higher after MUD compared with MSD transplantation after adjusting for other significant factors associated with chronic GVHD (Table 2). The effect of donor source on chronic GVHD is independent of other variables associated with chronic GVHD. Regardless of donor source, chronic GVHD risks were higher in patients who did not receive in vivo T-cell depletion (HR, 1.69; 95% CI, 1.43-2.00; P < .001) and with transplantation of peripheral blood progenitor cells (HR, 1.23; 95% CI, 1.06-1.44; P = .008).
NRM
During model building for NRM, relapse, and overall mortality, we observed a significant interaction between donor source and patient performance score. Consequently, multivariate models were built considering donor source and patient performance score for these outcomes. Compared with recipients of MSD transplantations with good performance score (90-100), NRM was significantly higher after MUD transplantations (HR, 1.42; 95% CI, 1.15-1.74; P = .001; Table 3; Figure 1C). Among patients with performance score less than 90, NRM risks were not significantly different after MSD and MUD transplantations (HR, 0.96; 95% CI, 0.74-1.24; P = .76). NRM risks were higher for those transplanted in relapse compared with those transplanted in remission (HR, 1.37; 95% CI, 1.18-1.58; P < .001), in those receiving myeloablative regimens compared with those receiving reduced-intensity regimens (HR, 1.46; 95% CI, 1.26-1.69; P < .001), in male recipients who received grafts from female donors (HR, 1.47; 95% CI, 1.25-1.72; P < .001), and in those who received cyclosporine-containing GVHD prophylaxis compared with those who received tacrolimus-containing regimens (HR, 1.30; 95% CI, 1.10-1.53; P = .002).
Relapse
Compared with recipients of MSD transplantations with good performance score, relapse risks were significantly higher after MUD transplantations (HR, 1.45; 95% CI, 1.19-1.76; P < .001; Table 3). Among patients with poor performance score, relapse risks were not significantly different after MSD and MUD transplantations (HR, 0.86; 95% CI, 0.66-1.12; P = .25). Relapse risks were higher for those transplanted in relapse compared with those transplanted in remission (HR, 1.93; 95% CI, 1.63-2.27; P < .001), in those receiving reduced-intensity regimens compared with those receiving myeloablative regimens (HR, 1.39; 95% CI, 1.18-1.65; P < .001), and in transplantations performed from 2000 to 2005 compared with the earlier period (HR, 1.37; 95% CI, 1.09-1.70; P = .006). Transplantation period was highly correlated with conditioning regimen; only 38% of transplantations in 2000-2005 used myeloablative regimens compared with 85% in the earlier period (P < .001). Relapse risks were lowest for CLL compared with AML, ALL, MDS, CML, and NHL.
OS and DFS
Among patients with good performance score, overall mortality (HR, 1.29; 95% CI, 1.11-1.49; P < .001) and treatment failure (HR, 1.34; 95% CI, 1.16-1.54; P < .001) were significantly higher after MUD compared with MSD transplantations (Table 3; Figure 1D). Among patients with poor performance score, mortality risks (HR, 0.90; 95% CI, 0.75-1.09; P = .29) and treatment failure (HR, 0.88; 95% CI, 0.73-1.06; P = .18) were not significantly different after MSD and MUD transplantations. The overall mortality model was stratified by conditioning regimen. Mortality risks were higher in patients age 60 years or older compared with those age 50-59 years (HR, 1.22; 95% CI, 1.07-1.38; P = .002) and for those transplanted in relapse compared with those transplanted in remission (HR, 1.60; 95% CI, 1.42-1.81; P < .001). Treatment failure was higher for patients transplanted in relapse (HR, 1.65; 95% CI, 1.47-1.86; P < .001) and for those with in vivo T-cell depletion (HR, 1.18; 95% CI, 1.04-1.33; P = .008). Mortality and treatment failure risks were lower for CLL compared with AML, ALL, MDS, CML, and NHL.
Ninety-one percent of MUD transplants (692 of 757) were 10/10 HLA-matched. We compared outcomes after 10/10 MUD transplants to those after MSD transplants, and the results were consistent with the main analysis (data not shown). Because only 65 transplants were matched at HLA-A, -B, -C, and -DRB1 and mismatched at HLA-DQ (9/10 HLA-matched), we were not able to compare outcomes after these transplants to 10/10 HLA-matched transplants.
Donor and recipient age and outcomes
Because patient age is correlated with donor age in the group of patients who received MSD transplants, we explored for a donor age cutoff in this population; after adjusting for performance score, conditioning regimen, disease, and disease status, overall mortality risks were higher in patients who received grafts from their siblings age 67 years or older compared with those who received grafts from siblings age 50 to 66 years (HR, 1.47; 95% CI, 1.19-1.82; P < .001). Relapse risks but not GVHD or NRM were also higher in patients who received grafts from their siblings age 67 years or older compared with those who received grafts from younger siblings (HR, 1.55; 95% CI, 1.18-2.05; P = .002). Among unrelated donors, we did not identify an age cut point associated with survival (19-29 vs 30-39 vs 40-49 years; P = .55). To further explore the effect of patient age and donor source/donor age, five mutually exclusive groups were created (Table 4). Patients were grouped as age 50 to 59 and 60 years or older on the basis of our observation of higher mortality risks in patients age 60 years or older. Donors were grouped as follows: MSD younger than age 67 years, MSD age 67 years or older (on the basis of our observation of higher mortality risks with donors age 67 years or older), and MUD. After adjusting for other significant factors, risks of acute and chronic GVHD were lower in patients age 50 to 59 years after MSD transplantations compared with patients age 50 to 59 years after MUD transplantations on multivariate analysis, but without a survival advantage. In the older age group, compared with recipients of MUD transplantation, those who received grafts from MSDs age younger than 67 years had lower risks of acute and chronic GVHD and overall mortality on multivariate analysis. Similarly, GVHD risks were lower for those who received grafts from MSDs age 67 years or older compared with MUDs, but without a survival advantage.
Discussion
This past decade has seen a marked rise in the average age of patients receiving HCT and increasing numbers of MUD transplants. Recent analyses of MUD and MSD transplants have found comparable survival outcomes (albeit with higher rates for acute and chronic GVHD) for patients with various forms of leukemia. The fact that the age of the donor has an impact on GVHD and survival raises questions regarding the use of older MSDs vs younger MUDs. It is important to determine which donor source is preferable when given a choice. The results of this report indicate that for patients older than age 50 years, the risk of acute and chronic GVHD is higher after MUD compared with MSD transplants. Donor type and patient performance score influenced NRM, relapse, DFS, and OS. For patients with performance scores of 90 or 100, NRM and relapse risks were higher after MUD compared with MSD transplants. Consequently, DFS and OS were significantly lower after MUD transplants. Conversely, for patients with lower performance scores, NRM, relapse, DFS, and OS were not different after MUD and MSD transplants.
It is important to note that the population being studied strongly influences results. The earlier reports indicated a higher rate of acute and chronic GVHD but similar survival outcomes with transplants from unrelated donors compared with those from matched siblings.3-7 Recipient age influences survival outcomes following the development of GVHD, with older populations having poorer survival.14 The studies that showed relative equivalence of MSD and MUD transplantation primarily involved younger-age recipients than in this study, which, by design, focuses on patients age 50 years or older. The higher rates for acute and chronic GVHD in recipients of MUDs in this analysis may be related to greater genetic disparity for minor histocompatibility antigens. The donor source (MUD vs MSD) had a greater effect on development of GVHD and NRM than donor age.15-17
There are some differences in this study compared with previous analyses. In contrast to earlier studies, in this study, we could not identify an age cutoff that resulted in higher rates of acute or chronic GVHD after MUD transplants. Once again, differences in study populations likely explain the discrepancy in findings. The Kollman et al9 analysis, the largest of these reports, was conducted at a time when matching at six HLA loci, primarily through low-resolution typing, was the standard for donor selection and included donors up to the age of 60 years. Accordingly, the incidence of acute GVHD was higher in that report, and weak risk factors for its development were easier to identify in a very large study (7000 patients). In this study, only patients who received HCTs from an 8/8 high-resolution MUD were included. The goal of our study was to answer a clinically important question for older-age recipients: whether use of a younger MUD (age <50 years) would result in improved outcomes compared with use of an older MSD (age ≥50 years). It may be that removing the MUDs with the oldest age in our analysis resulted in our inability to identify the significance of donor age within the MUD cohort. Recently, the European Group for Blood and Marrow Transplantation (EBMT) published a report suggesting that transplants from younger MUDs (age <30 years) offer a survival advantage for patients with MDS.18 However, they observed no benefit with respect to relapse-free survival, NRM, or acute GVHD to explain the reported advantage seen in survival. Further, their analysis did not consider the effect of multiple comparisons when testing the effect of donor type and donor age, which questions the true significance of the borderline association they identified. We are unable to confirm these observations because only 10% of our patients had MDS (MSD, 166 patients; MUD, 98 patients).
Our data would call into question earlier findings associating GVHD with increasing donor age. In support of our findings, a murine study comparing the ability of T cells from mice of different ages to cause GVHD found an age-dependent decline in GVHD lethality with 100% GVHD lethality in mice that were recipients of young T cells, 75% lethality in those that were recipients of adult T cells, and no deaths in recipients of old T cells.19 In this study, T cells from older mice expressed lower levels of type 1 cytokines and had less cytolytic function. Further support lies in the fact that the number of peripheral blood CD4+CD25+ regulatory T cells has been shown to increase with age in healthy human volunteers.20 It is possible that grafts from older donors may contain a higher percentage of regulatory T cells, which may act to limit the development of clinically apparent GVHD. Clinical evidence has consistently shown recipient age to be a strong risk factor for development of GVHD, and murine models have attributed this to enhanced allo-stimulatory activity of host antigen-presenting cells in older (when compared with younger) mice.21,22 Murine data have demonstrated the importance of host antigen-presenting cells and donor effector T cells in the pathophysiology of GVHD.23-25 Because our study addressed the role of donor age in older-age recipients, one would expect that the combination of an older-age recipient and a younger-age donor would indeed result in high rates of GVHD.
In conclusion, when selecting a donor for patients who are age 50 years or older with performance scores of 90 or 100, priority should be given to a sibling donor age younger than 67 years rather than to a younger-age MUD. Similarly, for patients with lower performance scores and/or when the donor is age 67 years or older, lower acute and chronic GVHD rates after MSD compared with MUD transplantation favor an MSD when such a donor is available. An added advantage with an MSD is easy access to the donor, which is critical for patients with high-risk disease. It is noteworthy that older MSDs are more likely to have comorbidities that may preclude donation. The data also support transplantation of either bone marrow or peripheral blood progenitor cells, because graft type was not significantly associated with DFS and OS.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a Public Health Service grant (U24-CA76518) from the National Cancer Institute, the National Heart, Lung and Blood Institute, and the National Institute of Allergy and Infectious Diseases; a grant (HHSH234200637015C) from the Health Resources and Services Administration; and a grant (N00014-10-01-0204) from the Office of Naval Research, Department of Navy to the National Marrow Donor Program. Opinions, findings, and conclusions or recommendations expressed herein are those of the authors and do not reflect the views of the Office of Naval Research or the National Marrow Donor Program.
Authorship
Contribution: A.M.A., R.M.S., R.E.C., J.L.-R., and M.E. created the study design, interpreted data, and wrote the paper. F.K. prepared the dataset. J.L.-R. did the statistical analysis. F.R.A., A.A., J.B., S.M.D., F.K., M.J.L., H.M.L., J.L., M.-A.P., R.T.M., M.S., and E.K.W. interpreted data and critically reviewed the manuscript. All authors approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mary Eapen, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, 8701 Watertown Plank Rd, Milwaukee, WI 53226; e-mail: meapen@mcw.edu.