Key Points
In this study, African American MM patients have a significantly lower frequency of IgH translocations than European American patients.
Abstract
Epidemiological data have suggested that African American (AA) persons are twice as likely to be diagnosed with multiple myeloma (MM) compared with European American (EA) persons. Here, we have analyzed a set of cytogenetic and genomic data derived from AA and EA MM patients. We have compared the frequency of IgH translocations in a series of data from 115 AA patients from 3 studies and 353 EA patients from the Eastern Cooperative Oncology Group (ECOG) studies E4A03 and E9487. We have also interrogated tumors from 45 AA and 196 EA MM patients for somatic copy number abnormalities associated with poor outcome. In addition, 35 AA and 178 EA patients were investigated for a transcriptional profile associated with high-risk disease. Overall, based on this cohort, genetic profiles were similar except for a significantly lower frequency of IgH translocations (40% vs 52%; P = .032) in AA patients. Frequency differences of somatic copy number aberrations were not significant after correction for multiple testing. There was also no significant difference in the frequency of high-risk disease based on gene expression profiling. Our study represents the first comprehensive comparisons of the frequency and distribution of molecular alterations in MM tumors between AA and EA patients. ECOG E4A03 is registered with ClinicalTrials.gov, number NCT00098475. ECOG E9487 is a companion validation set to the ECOG study E9486 and is registered with the National Institutes of Health, National Cancer Institute, Clinical Trials (PDQ), number EST-9486.
Introduction
Multiple myeloma (MM) is a hematologic malignancy resulting from the proliferation and accumulation of clonal plasma cells (PCs) in the bone marrow (BM). It is well established that MM is almost always preceded by monoclonal gammopathy of undetermined significance (MGUS).1,2
MM is the most common hematologic cancer affecting African American (AA) persons. Epidemiological data reveal that AA persons are diagnosed with MGUS and MM two to three times more frequently compared with European American (EA) patients.3 Reasons for this disparity are not well defined; however, evidence of racial dissimilarities in characteristics of MGUS and MM suggests a biological cause. In a study of 4 million veterans, the age-adjusted prevalence rate for MGUS was three times greater for AA than EA patients.3 Another investigation showing increased risk of MGUS in Ghanaian men when compared with the white population of Olmsted County, Minnesota, validated this finding.4 Furthermore, an independent study comparing the prevalence of MGUS in AA and EA women of equal socioeconomic status revealed a twofold increase in black women.5 Elevated mortality rates of twofold or greater have also been observed in AA versus EA patients (age-adjusted death rates per 100 000 patients from 1975 to 2006 in the United States).6
Clinical variations of MGUS and MM based on race have also been reported. AA patients with MGUS have lower levels of M-protein, an earlier age of onset, and a lower prevalence of IgM gammopathy when compared with EA patients.7 A recent investigation conducted by Weiss et al. reported a lower proportion of higher-risk MGUS in AA compared with EA patients when determined by the serum-free light-chain assay.8 Furthermore, AA patients have higher disease-specific and overall survival rates, but the steady improvements in survival over recent years experienced by EA patients have not been observed.9 Considered collectively, these differences support the concept of possible biological distinctions of MGUS and MM between these groups.
Cytogenetics and genomics have been used to define subtypes of MM. Two major karyotypic subtypes have been identified in approximately one-half of MM cases each: hyperdiploid MM (H-MM) and nonhyperdiploid MM (NH-MM). H-MM is characterized by trisomies of odd-numbered chromosomes (3, 5, 7, 9, 11, 15, 19, and 21). NH-MM is characterized by the increased frequency of IgH translocations with multiple chromosomal partners, including t(11;14)(q13;q32), t(4;14)(p16;q32), and t(14;16)(q32;q23), among others. Patients diagnosed with the NH-MM subtype have been shown to have a poorer prognosis.10-12 These two major subtypes of MM can be observed from the early stages of disease such as MGUS.13
Gene-expression profiling (GEP) has defined subgroups at high and low risk of adverse outcomes.14-16 The University of Arkansas Medical Science group identified a set of 70 probes associated with high-risk MM by correlating gene expression extremes with early disease-related deaths in 532 newly diagnosed MM patients.16 High risk was based on the expression patterns of 70 genes predominately located on chromosome 1 that were found to be upregulated on the q-arm and downregulated on the p-arm.16
To date, little is known about the biological similarities or differences among AA and EA patients with MM. Given the clinical differences of MM between AA and EA patients and the known genomic heterogeneity of MM tumors, we sought to identify for the first time molecular and genomic differences between these 2 groups. We have assessed differences in IgH translocations and the major genetic subtypes of the disease, H-MM and NH-MM. Furthermore, we have analyzed array-based copy-number data from an independent set of nearly 250 MM tumors for population frequency differences in somatic aberrations mapping to known regions of poor prognosis that have been reported in previous studies. In addition, matching GEP data from these 250 MM patients were used to detect the presence of the 70-gene high-risk expression signature. The frequency of each event was compared between groups using statistical tests. To our knowledge, this is the first systematic study to assess the degree of molecular and genomic differences in MM tumors from AA and EA patients.
Patients and methods
Patients
Research samples came from four sources: the Mayo Clinic, the Multiple Myeloma Research Consortium (MMRC) Tissue Bank as a component of the Multiple Myeloma Genomics Initiative,17 and 2 ECOG studies, E4A03 and E9487.18-22 All samples used through the Mayo Clinic were obtained under appropriate consent in accordance with the Declaration of Helsinki and approved by the Mayo Clinic Scottsdale Institutional Review Board. Microarray data from the MMRC was obtained from the Multiple Myeloma Genomics Portal, a publicly available database (http://www.broadinstitute.org/mmgp). Additional microarray data used in this study was deposited in the Gene Expression Omnibus at the National Center for Biotechnology Information and is accessible through GEO Series accession number GSE44271 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE44271).
A clinical description of ECOG patients has been published previously.18,23 Patients were grouped according to self-identified racial demographics. The cases used for each analysis are summarized in Tables 1 and 2, and are listed in more detail in supplemental Tables 1 and 2 available on the Blood website.
IgH translocations
Assessment of IgH translocations from MM tumors was conducted using a cIg–fluorescence in situ hybridization (FISH) approach as published previously.20 In brief, MM cells were identified by cytoplasmic anti-κ or anti-λ staining concomitantly with the FISH technique (cIg-FISH). Cytospin slides were used as targets with probes capable of detecting IgH translocations. All cases with available material for FISH were also tested to determine frequencies of the 3 most common chromosome partners: t(11;14)(q13;q32), t(4;14)(p16;q32), and t(14;16)(q32;q23).
Array-based comparative genomic hybridization
Array-based comparative genomic hybridization (aCGH) was conducted using Agilent Human Genome CGH 244A Oligo microarrays (accession number: GSE44271) (Agilent Technologies, Santa Clara, CA), which have an average probe spacing of 8.9 KB. Briefly, 800 nanograms (ng) of DNA obtained from CD138+ selected plasma cells and DNA from a normal female reference (Promega, Madison, WI) were independently digested with Bovine DNAse I (Ambion, Austin, TX) and directly labeled with Cy5 and Cy3, respectively, using the BioPrime Array CGH Genomic Labeling Module (Invitrogen, Carlsbad, CA). Labeled DNA was purified using Vivaspin 500 columns (Satorius Stedim Biotech, Goettingen, Germany). Equal amounts of labeled, purified tumor and reference DNA were hybridized to the microarray in a rotary oven at 65°C for 40 hours at a rotation speed of 20 rpm. The slides were washed according to the manufacturer’s protocol and scanned with the Agilent G2505B scanner using the default settings. Scanner images were extracted using Feature Extraction software version 10.1 (Agilent Technologies). Log2 data were imported into Agilent DNA Analytics 4.0.81 software for visualization and quality assessment. Samples with a DLRSpread value of 0.3 or greater (QC Metrics; DNA Analytics software) were excluded from the analysis. QC metric values are listed for samples analyzed by aCGH in supplemental Table 3. Chromosome aberrations were identified using the ADM-2 algorithm (threshold = 10.5) and 2 probe 0.3 log2 filters.
The H-MM phenotype was defined as the presence of additional copies of at least two of the following odd-numbered chromosomes: 3, 5, 7, 9, 11, 15, 19, and 21. Upon visualization, samples with scatter plot amplitudes of 0.3 log2 or greater distributed across 3 or more of the odd-numbered chromosomes listed before were considered H-MM. The remaining samples were labeled “NH-MM” (supplemental Table 4). In addition, the frequencies of genomic aberrations previously reported to be associated with poor prognosis were analyzed for each patient. The frequency of the following events was calculated: 1q gain24,25 ; deletions of 1p,24,25 p18,25,26 8p,27 13q,21,24,28 14q,29 16q,29,30 17p,27 22q29 ; p18 homozygous deletion25 ; and chromosome 13 monosomy21,24 (supplemental Tables 5 and 6). The dataset was also stratified by H-MM and NH-MM subtypes and the analysis was repeated.
Gene-expression profiling
GEP was performed on RNA extracted from CD138+ selected plasma cells using the Affymetrix U133 Plus 2.0 Array (Affymetrix, Santa Clara, CA) as previously described.17 Gene expression raw intensity values were generated using the MAS 5.0 algorithm; values were log2-transformed and analyzed using GeneSpring GX 10.0 software (Agilent Technologies). GEP data were used to determine the 70-gene index,16 and results were expressed as the number of times the high-risk signature was detected and the percentage of the occurrence in each ethnic group.
Statistical analysis
The Fisher’s exact test31 was used to compare the frequency of each event between AA and EA MM patients. P values were calculated for comparisons of translocation and GEP data. For FISH and GEP analyses, P values ≤ .05 were regarded as statistically significant. For aCGH analyses, P values were adjusted for multiple testing (n = 11) using the Benjamini-Hochberg method.32
Results
Comparison of IgH translocation status between AA and EA MM tumors
The prevalence of IgH translocations in tumors from AA and EA patients was compared using the cIg-FISH break-apart strategy. The numbers of patients used for each analysis are provided in Tables 1 and 2. Based on the cases studied, 40% (46/115) of AA patients demonstrated evidence of an IgH translocation compared with 52% (183/353) of EA patients (P = .032) (Table 3). The distributions of 3 common translocation partners—t(4;14), t(11;14), and t(14;16)—were also assessed in independent analyses and were not significantly different between the 2 groups (P = .304, .553, and .256, respectively). IgH translocations in which no identifiable chromosome partners could be found were designated 14qNOS, and comparisons were also not statistically significant (P = 1.000) (Table 3).
Comparisons of somatic copy number changes between AA and EA MM tumors
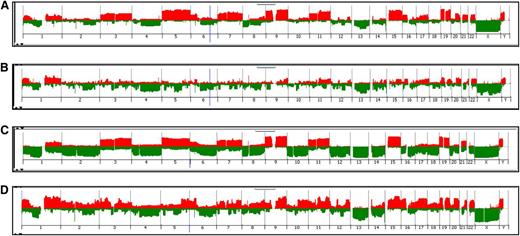
Next we proceeded to analyze cases for genomic gain or loss using aCGH. To classify MM tumors as H-MM or NH-MM, we used data from 45 AA and 196 EA MM patients (Figure 1; supplemental Table 4). H-MM and NH-MM occurred at similar frequencies among AA (H-MM 53%; NH-MM 47%) and EA (H-MM; 53%; NH-MM 48%) patients. These results are similar to the previously reported frequencies of H-MM and NH-MM.33-35 It is notable that the classification is capable of discerning H-MM by virtue of whole-chromosome gains but cannot readily identify differences in the incidence of IgH translocations.
Classification of AA and EA patients by MM subtype using aCGH. Penetrance plots are shown depicting chromosome gains (red) and losses (green) from the analysis of tumor DNA from MM patients. Data were visualized using DNA Analytics software (Agilent). Patients were grouped by race and stratified into the 2 major subtypes, H-MM and NH-MM. Subtype frequencies were compared between the 2 groups: (A) AA H-MM (53%), (B) AA NH-MM (47%), (C) EA H-MM (53%), and (D) EA NH-MM (47%).
Classification of AA and EA patients by MM subtype using aCGH. Penetrance plots are shown depicting chromosome gains (red) and losses (green) from the analysis of tumor DNA from MM patients. Data were visualized using DNA Analytics software (Agilent). Patients were grouped by race and stratified into the 2 major subtypes, H-MM and NH-MM. Subtype frequencies were compared between the 2 groups: (A) AA H-MM (53%), (B) AA NH-MM (47%), (C) EA H-MM (53%), and (D) EA NH-MM (47%).
We further examined whether there are differences in the frequency of copy number alterations that have been previously reported to be associated with poor prognosis between AA and EA patients. Among this cohort, differences in the frequencies of the aberrations studied were not significant when all patients were analyzed and P values were corrected for multiple testing (Table 4). The dataset was then stratified according to the H-MM and NH-MM subtypes and the analysis was repeated. We observed lower frequencies of chromosome 1q gain in H-MM tumors from AA patients (29% vs 60%; P = .007) and 14q deletion in NH-MM AA patients (24% vs 56%; P = .008) compared with EA patients (Tables 5 and 6). However, after correction for multiple testing, the differences were not statistically significant. These analyses are provided in more detail in supplemental Tables 5 and 6.
Assessment of GEP-based high-risk signature among AA and EA MM tumors
We conducted GEP analysis to assess the frequency of high-risk tumors from AA and EA MM patients based on the University of Arkansas for Medical Sciences 70-gene high-risk signature16 (Table 7; supplemental Table 1). Our analysis revealed that 31% (11/35) of AA patients and 34% (61/178) of EA patients had tumors with high-risk signatures (P = .846). When samples were stratified into H-MM and NH-MM subtypes, no significant differences were found.
Discussion
To what extent potential somatic molecular differences in MM between AA and EA patients contribute to dissimilarities in incidence and outcome has remained unanswered and understudied. Here, we used a comprehensive analysis of genomic data from a cohort of AA and EA patients including IgH translocation status, aCGH, and GEP to provide a look at the biological similarities and differences in MM tumors from these patients.
In our study, we found a significantly lower incidence of chromosome translocations in AA compared with EA patients using FISH. In addition, using aCGH data, we found similar frequencies of H-MM and NH-MM tumors. This investigation also revealed no statistically significant differences in the frequency of several genomic events associated with poor prognosis when assessing patients independent and dependent of global ploidy status (NH-MM vs. H-MM). Finally, GEP revealed no significant differences in the prevalence of high-risk signatures.
Fewer translocation events would indicate that AA patients have a biological tendency toward less aggressive versions of the disease. However, using the presented data, we cannot formally assign all remaining patients without an IgH translocation to the H-MM category, given the criteria, ie, having at least 3 trisomies. Therefore, we cannot exclude other possibilities. Some of these would include other variant forms of MM being more common among AA patients such as immunoglobulin light-chain locus translocations or genetic categories yet to be defined. Indeed our results are indirectly supported by the finding of improved overall survival of AA patients with MM, something likely in subsets of patients with a lower prevalence of IgH translocations.36,37
Aside from translocation events, other factors may be considered that account for the differences in MM between AA and EA patients. Distinctions in the frequency of somatic activating mutations such as those previously reported in KRAS,38 NRAS,38 TP53,39 and NFκB signaling pathway members,40 and the more novel mutations recently found by Chapman et al,17 in FAM46C, DIS3, and the kinase, BRAF by whole genome sequencing, may reveal clues to the increased incidence seen in AA patients. In addition, mechanisms involved in DNA methylation such as the overexpression of the histone methyltransferase, MMSET, and the activation of the histone demethylase, UTX, as well as epigenetic silencing of VHL, TP53, CDKN2A, and TGFBR2 have been linked to MM41 and may differentially occur in AA and EA patients. Moreover, a recent study of the myeloma kinome by Tiedemann et al42 found that the G-protein coupled-receptor kinase, GRK6, is lethal to 6 of 7 myeloma cell lines when inhibited by small interfering RNA. Examination of the kinome of tumors from AA MM patients may identify novel prognostic targets. Furthermore, dissimilarities in the activation of signaling pathways by cytokines, growth factors, and chemokines of the bone marrow microenvironment could possibly explain the differences observed between these 2 ethnic groups.
One limitation of this study is the lack of clinical data regarding diagnosis and treatment of all patients, which may affect the detection of certain chromosomal abnormalities. Another limitation is the low number of AA patients within our cohort when compared with EA patients, despite of the disparity in incidence for AA patients. Recruitment of greater numbers of AA MM patients for clinical research that involves comprehensive genomic analyses is crucial to understanding the effects of the biological differences of MM within these ethnic groups.
In summary, with this cohort we have identified a significantly lower frequency of chromosome translocations in AA patients. To our knowledge, this is the first report to comprehensively compare the genomic composition of MM derived from a cohort of both AA and EA patients to help determine whether molecular differences exist in tumors from these 2 populations. Although our results will need to be validated and confirmed in larger independent cohorts, these data provide the first glimpse into potential biological factors that might help explain some of the differences in incidence and outcomes previously observed between AA and EA MM patients.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Multiple Myeloma Research Consortium (grants SPORE CA90297052, P01 CA62242, R01 CA83724, and ECOG CA 21115T); Predolin Foundation; Mayo Clinic Cancer Center; and the Mayo Foundation. This research was also funded in part by Damon Runyon Cancer Research Fund (to R.F.).
Authorship
Contribution: A.B., E.B., G.A., S.A.V.W., S. Jacobus, D.L., T.T., A.D., L.P., S.K., K.H., D.V., S.V.R., and D.F.J. performed the research; A.B., E.B., S. Jacobus, S. Jung, J.C., and R.F. analyzed results; A.B. and E.B. created the figures; A.B., E.B., D.L., T.T., A.D., J.L., L.P., S.K., K.H., D.V., S.V.R., D.F.J., J.C., and R.F. designed the research; and A.B., E.B., J.C., and R.F. wrote the manuscript.
Conflict-of-interest disclosure: Relevant to this work, Dr Fonseca has received a patent for the prognostication of MM based on genetic categorization of the disease. He has received consulting fees from Medtronic, Otsuka, Celgene, Genzyme, BMS, and AMGEN. He also has sponsored research from Cylene and Onyx. The remaining authors declare no competing financial interests.
Correspondence: Rafael Fonseca, Mayo Clinic Scottsdale, 13400 East Shea Blvd., Collaborative Research Building, 3-006, Scottsdale, AZ 85259; e-mail: fonseca.rafael@mayo.edu.