Key Points
Confirms the prognostic effect of NOTCH1 mutations in pediatric T-cell lymphoblastic lymphoma in a large and independent cohort.
Provides the scientific basis for using NOTCH1 mutations and chromosome 6q alterations as stratification criterion in patients with T-cell lymphoblastic lymphoma.
Abstract
Probability of event-free survival (pEFS) in pediatric T-cell lymphoblastic lymphoma is about 80%, whereas survival in relapsed patients is very poor. No stratification criteria have been established so far. Recently, activating NOTCH1 mutations were reported to be associated with favorable prognosis, and loss of heterozygosity at chromosome 6q (LOH6q) was reported to be associated with increased relapse risk. The current project was intended to evaluate the prognostic effect of these markers. Mutations in hot spots of NOTCH1 and FBXW7 were analyzed in 116 patients. Concerning LOH6q status, 118 patients were investigated, using microsatellite marker analysis, in addition to an earlier reported cohort of 99 available patients. Ninety-two cases were evaluable for both analyses. All patients were treated with T-cell lymphoblastic lymphoma-Berlin-Frankfurt-Münster group (BFM)-type treatment. LOH6q was observed in 12% of patients (25/217) and associated with unfavorable prognosis (pEFS 27% ± 9% vs 86% ± 3%; P < .0001). In 60% (70/116) of the patients, NOTCH1 mutations were detected and associated with favorable prognosis (pEFS 84% ± 5% vs 66% ± 7%; P = .021). Interestingly, NOTCH1 mutations were rarely observed in patients with LOH in 6q16. Both prognostic markers will be used as stratification criteria in coming Non-Hodgkin Lymphoma-BFM trials.
Introduction
Lymphoblastic T-cell lymphoma (T-LBL) is the second most common subtype of non-Hodgkin lymphoma (NHL) in children and adolescents. With current treatment regimens, event-free survival (EFS) rates of 75% to 85% are achieved.1-7 Despite these acceptable EFS rates, concepts of risk-adjusted treatment could not be realized because of the lack of prognostic parameters. However, such parameters are highly needed, as survival rates in relapsed patients with T-LBL are only about 10%,8 and therefore identification of high-risk patients for targeted treatment intensification is desired to prevent relapses. In contrast, current treatment regimens are associated with significant acute and long-term toxicity, which might be avoidable if treatment intensity can be reduced for low-risk patients without affecting the outcome. Apart from stage of disease at diagnosis, which is an insufficient parameter for T-LBL, as more than 95% of patients are diagnosed with advanced stages of disease, no clinical parameter for treatment stratification could be validated to date.
Little is known about the pathogenesis and genetic changes in pediatric T-LBL, mostly because of the lack of adequate samples for biological research. In recent studies, activating mutations of NOTCH1 and/or FBXW7 (N/Fpos) have been observed in about 50% of pediatric patients with T-cell lymphoblastic leukemia (T-ALL; for a complete overview of publications dealing with this topic, see supplemental Table 1 on the Blood website).9-19 The evolutionary highly conserved NOTCH1 signaling pathway is involved in the regulation of many cellular processes; for example, early T-cell development.20,21 The transmembrane protein NOTCH1 is activated by the contact of NOTCH1-ligands (Delta-like 1-4, Jagged1, or Jagged2), which trigger the proteolytic cleavages releasing the protein’s active part, intracellular NOTCH1 (ICN1).20,21 ICN1 translocates to the nucleus, where it acts as part of a transcriptional complex regulating the expression of diverse target genes. The ICN1 level in the cell is decreased by degradation through ubiquitination. The responsible E3 ubiquitin ligase is the tumor suppressor FBXW7.20,21 The reported mutations occur in well-described hot spots in specific exons, leading to increased ICN1 in T-ALL cell lines9,22,23 and primary T-ALL cells.14 Until now, only 4 studies analyzed pediatric patients with T-LBL, including fewer than 90 patients in total (supplemental Table 1).11,17,24,25
Other genetic markers that might be of prognostic relevance are alterations of chromosome 6q. In previous studies, we could show in a limited test cohort of patients that loss of heterozygosity (LOH) at chromosome 6q14-24 (LOH6q) is associated with a significantly increased risk for relapse.26,27 Therefore, NOTCH1 and/or FBXW7 mutations, as well as alterations of chromosome 6q, are candidate genetic markers with potential prognostic relevance in pediatric T-LBL. To validate their effect in conjunction with NHL-Berlin-Frankfurt-Münster group (BFM) treatment strategies, we analyzed a large cohort of uniformly treated patients. The prognostic relevance of both alterations for pediatric patients suffering from T-LBL is presented, which will be the scientific basis for the implementation of both molecular markers as stratification criteria in upcoming clinical trials of the NHL-BFM study group.
Methods
Patients
Between April 1995 and July 2012, a total of 438 pediatric patients with T-LBL were registered in the NHL-BFM study center after written informed consent. Lymphoma and germline material sufficient for DNA isolation for LOH analysis was available from 217 of these patients. Lymphoma material sufficient for DNA isolation for analysis of NOTCH1 and/or FBXW7 was available from 116 of the patients. A total of 241 representative pediatric patients with T-LBL have been analyzed, with clinical characteristics similar to those of the 197 patients who did not have adequate material. The only exceptions were the younger age in the analyzed cohort and the poorer general conditions at diagnosis in all analyzed patients and the patients analyzed for NOTCH1 and FBXW7 (Table 1). For the trials NHL-BFM 95 and EURO-LB 02, in which patients were recruited, the accompanying molecular research on pediatric LBL has been approved by the ethical committees of the Hannover Medical School and Justus-Liebig University Giessen, Germany. These studies were conducted in accordance with the Declaration of Helsinki.
Clinical characteristics of patients according to different analysis subgroups
| . | Analyzed . | P value . | LOH analyzed . | P value . | LOH6q . | P value . | NOTCH1 analyzed . | P value . | NOTCH1 . | P value . | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No . | Yes . | No . | Yes . | Negative . | Positive . | No . | Yes . | Negative . | Positive . | ||||||||||||||||
| N . | % . | N . | % . | N . | % . | N . | % . | N . | % . | N . | % . | N . | % . | N . | % . | N . | % . | N . | % . | ||||||
| Total | 197 | 100 | 241 | 100 | 221 | 100 | 217 | 100 | 192 | 100 | 25 | 100 | 322 | 100 | 116 | 100 | 46 | 100 | 70 | 100 | |||||
| Sex | .90405 | .25512 | .74315 | .61698 | .47935 | ||||||||||||||||||||
| Male | 140 | 71.1 | 170 | 70.5 | 151 | 68.3 | 159 | 73.3 | 140 | 72.9 | 19 | 76.0 | 230 | 71.4 | 80 | 69.0 | 30 | 62.5 | 50 | 71.4 | |||||
| Female | 57 | 28.9 | 71 | 29.5 | 70 | 31.7 | 58 | 26.7 | 52 | 27.1 | 6 | 24.0 | 92 | 28.6 | 36 | 31.0 | 16 | 34.8 | 20 | 28.6 | |||||
| Age | .03746 | .09623 | .26578 | .18768 | .00601 | ||||||||||||||||||||
| <10 Years | 95 | 48.2 | 137 | 56.8 | 110 | 49.8 | 122 | 56.2 | 110 | 57.3 | 12 | 48.0 | 168 | 52.2 | 64 | 55.2 | 17 | 37.0 | 47 | 67.1 | |||||
| 10-14 Years | 69 | 35.0 | 82 | 34.0 | 76 | 34.4 | 75 | 34.6 | 63 | 32.8 | 12 | 48.0 | 108 | 33.5 | 43 | 37.1 | 24 | 52.2 | 19 | 27.1 | |||||
| ≥15 Years | 33 | 16.8 | 22 | 9.1 | 35 | 15.8 | 20 | 9.2 | 19 | 9.9 | 1 | 4.0 | 46 | 14.3 | 9 | 7.8 | 5 | 10.9 | 4 | 5.7 | |||||
| Stage | .70742 | .75102 | .68258 | .41989 | .60010 | ||||||||||||||||||||
| Stage I | 1 | 0.6 | — | — | 1 | 0.5 | — | — | — | — | — | — | 1 | 0.3 | — | — | — | — | — | — | |||||
| Stage II | 5 | 2.8 | 5 | 2.3 | 5 | 2.5 | 5 | 2.5 | 4 | 2.3 | 1 | 4.5 | 9 | 3.1 | 1 | 1.0 | — | — | 1 | 1.6 | |||||
| Stage III | 130 | 73.9 | 165 | 75.0 | 149 | 75.3 | 146 | 73.7 | 129 | 73.3 | 17 | 77.3 | 219 | 75.3 | 76 | 72.4 | 32 | 76.2 | 44 | 69.8 | |||||
| Stage IV | 40 | 22.7 | 50 | 22.7 | 43 | 21.7 | 47 | 23.7 | 43 | 24.4 | 4 | 18.2 | 62 | 21.3 | 28 | 26.7 | 10 | 23.8 | 18 | 28.6 | |||||
| Bone marrow involvement | .98354 | .73479 | .73872 | .92278 | .74030 | ||||||||||||||||||||
| No | 162 | 82.2 | 198 | 82.2 | 183 | 82.8 | 177 | 81.6 | 156 | 81.3 | 21 | 84.0 | 265 | 82.3 | 95 | 81.9 | 37 | 80.4 | 58 | 82.9 | |||||
| Yes | 35 | 17.8 | 43 | 17.8 | 38 | 17.2 | 40 | 18.4 | 36 | 18.8 | 4 | 16.0 | 57 | 17.7 | 21 | 18.1 | 9 | 19.6 | 12 | 17.1 | |||||
| Mediastinal tumor | .96553 | .59650 | .55776 | .58700 | .39877 | ||||||||||||||||||||
| No | 21 | 10.7 | 26 | 10.8 | 22 | 10.0 | 25 | 11.5 | 23 | 12.0 | 2 | 8.0 | 33 | 10.2 | 14 | 12.1 | 7 | 15.2 | 7 | 10.0 | |||||
| Yes | 176 | 89.3 | 215 | 89.2 | 199 | 90.0 | 192 | 88.5 | 169 | 88.0 | 23 | 92.0 | 289 | 89.8 | 102 | 87.9 | 39 | 84.8 | 63 | 90.0 | |||||
| CNS | .78466 | .48870 | .28498 | .08988 | .63681 | ||||||||||||||||||||
| CNS negative | 163 | 96.4 | 187 | 95.9 | 183 | 96.8 | 167 | 95.4 | 146 | 94.8 | 21 | 100 | 269 | 97.1 | 81 | 91.3 | 35 | 94.6 | 46 | 92.0 | |||||
| CNS positive | 6 | 3.6 | 8 | 4.1 | 6 | 3.2 | 8 | 4.6 | 8 | 5.2 | — | — | 8 | 2.9 | 6 | 6.9 | 2 | 5.4 | 4 | 8.0 | |||||
| General condition at diagnosis | .01461 | .10402 | .07316 | .01602 | .19669 | ||||||||||||||||||||
| General condition <5 | 177 | 90.8 | 190 | 82.6 | 194 | 89.0 | 173 | 83.6 | 156 | 85.2 | 17 | 70.8 | 282 | 88.7 | 85 | 79.4 | 36 | 85.7 | 49 | 75.4 | |||||
| General condition = 5 | 18 | 9.2 | 40 | 17.4 | 24 | 11.0 | 34 | 16.4 | 27 | 14.8 | 7 | 29.2 | 36 | 11.3 | 22 | 20.6 | 6 | 14.3 | 16 | 24.6 | |||||
| Status | |||||||||||||||||||||||||
| Death resulting from initial complications | 1 | 0.5 | 1 | 0.4 | 1 | 0.5 | 1 | 0.5 | 1 | 0.5 | — | — | 2 | 0.6 | — | — | — | — | — | — | |||||
| Nonresponse/progression/relapse | 21 | 10.6 | 40 | 16.6 | 21 | 9.6 | 40 | 18.5 | 23 | 11.9 | 17 | 68.0 | 40 | 12.4 | 21 | 18.1 | 12 | 26.1 | 9 | 12.9 | |||||
| Toxic death | 6 | 3.0 | 3 | 1.2 | 8 | 3.6 | 1 | 0.5 | 1 | 0.5 | — | — | 7 | 2.2 | 2 | 1.7 | 1 | 2.2 | 1 | 1.4 | |||||
| Death (other) | — | — | 1 | 0.4 | 1 | 0.5 | — | — | — | — | — | — | — | — | 1 | 0.9 | — | — | 1 | 1.4 | |||||
| Second malignancy | 7 | 3.6 | 6 | 2.5 | 7 | 3.2 | 6 | 2.8 | 5 | 2.6 | 1 | 4.0 | 10 | 3.1 | 3 | 2.6 | 2 | 4.3 | 1 | 1.4 | |||||
| . | Analyzed . | P value . | LOH analyzed . | P value . | LOH6q . | P value . | NOTCH1 analyzed . | P value . | NOTCH1 . | P value . | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No . | Yes . | No . | Yes . | Negative . | Positive . | No . | Yes . | Negative . | Positive . | ||||||||||||||||
| N . | % . | N . | % . | N . | % . | N . | % . | N . | % . | N . | % . | N . | % . | N . | % . | N . | % . | N . | % . | ||||||
| Total | 197 | 100 | 241 | 100 | 221 | 100 | 217 | 100 | 192 | 100 | 25 | 100 | 322 | 100 | 116 | 100 | 46 | 100 | 70 | 100 | |||||
| Sex | .90405 | .25512 | .74315 | .61698 | .47935 | ||||||||||||||||||||
| Male | 140 | 71.1 | 170 | 70.5 | 151 | 68.3 | 159 | 73.3 | 140 | 72.9 | 19 | 76.0 | 230 | 71.4 | 80 | 69.0 | 30 | 62.5 | 50 | 71.4 | |||||
| Female | 57 | 28.9 | 71 | 29.5 | 70 | 31.7 | 58 | 26.7 | 52 | 27.1 | 6 | 24.0 | 92 | 28.6 | 36 | 31.0 | 16 | 34.8 | 20 | 28.6 | |||||
| Age | .03746 | .09623 | .26578 | .18768 | .00601 | ||||||||||||||||||||
| <10 Years | 95 | 48.2 | 137 | 56.8 | 110 | 49.8 | 122 | 56.2 | 110 | 57.3 | 12 | 48.0 | 168 | 52.2 | 64 | 55.2 | 17 | 37.0 | 47 | 67.1 | |||||
| 10-14 Years | 69 | 35.0 | 82 | 34.0 | 76 | 34.4 | 75 | 34.6 | 63 | 32.8 | 12 | 48.0 | 108 | 33.5 | 43 | 37.1 | 24 | 52.2 | 19 | 27.1 | |||||
| ≥15 Years | 33 | 16.8 | 22 | 9.1 | 35 | 15.8 | 20 | 9.2 | 19 | 9.9 | 1 | 4.0 | 46 | 14.3 | 9 | 7.8 | 5 | 10.9 | 4 | 5.7 | |||||
| Stage | .70742 | .75102 | .68258 | .41989 | .60010 | ||||||||||||||||||||
| Stage I | 1 | 0.6 | — | — | 1 | 0.5 | — | — | — | — | — | — | 1 | 0.3 | — | — | — | — | — | — | |||||
| Stage II | 5 | 2.8 | 5 | 2.3 | 5 | 2.5 | 5 | 2.5 | 4 | 2.3 | 1 | 4.5 | 9 | 3.1 | 1 | 1.0 | — | — | 1 | 1.6 | |||||
| Stage III | 130 | 73.9 | 165 | 75.0 | 149 | 75.3 | 146 | 73.7 | 129 | 73.3 | 17 | 77.3 | 219 | 75.3 | 76 | 72.4 | 32 | 76.2 | 44 | 69.8 | |||||
| Stage IV | 40 | 22.7 | 50 | 22.7 | 43 | 21.7 | 47 | 23.7 | 43 | 24.4 | 4 | 18.2 | 62 | 21.3 | 28 | 26.7 | 10 | 23.8 | 18 | 28.6 | |||||
| Bone marrow involvement | .98354 | .73479 | .73872 | .92278 | .74030 | ||||||||||||||||||||
| No | 162 | 82.2 | 198 | 82.2 | 183 | 82.8 | 177 | 81.6 | 156 | 81.3 | 21 | 84.0 | 265 | 82.3 | 95 | 81.9 | 37 | 80.4 | 58 | 82.9 | |||||
| Yes | 35 | 17.8 | 43 | 17.8 | 38 | 17.2 | 40 | 18.4 | 36 | 18.8 | 4 | 16.0 | 57 | 17.7 | 21 | 18.1 | 9 | 19.6 | 12 | 17.1 | |||||
| Mediastinal tumor | .96553 | .59650 | .55776 | .58700 | .39877 | ||||||||||||||||||||
| No | 21 | 10.7 | 26 | 10.8 | 22 | 10.0 | 25 | 11.5 | 23 | 12.0 | 2 | 8.0 | 33 | 10.2 | 14 | 12.1 | 7 | 15.2 | 7 | 10.0 | |||||
| Yes | 176 | 89.3 | 215 | 89.2 | 199 | 90.0 | 192 | 88.5 | 169 | 88.0 | 23 | 92.0 | 289 | 89.8 | 102 | 87.9 | 39 | 84.8 | 63 | 90.0 | |||||
| CNS | .78466 | .48870 | .28498 | .08988 | .63681 | ||||||||||||||||||||
| CNS negative | 163 | 96.4 | 187 | 95.9 | 183 | 96.8 | 167 | 95.4 | 146 | 94.8 | 21 | 100 | 269 | 97.1 | 81 | 91.3 | 35 | 94.6 | 46 | 92.0 | |||||
| CNS positive | 6 | 3.6 | 8 | 4.1 | 6 | 3.2 | 8 | 4.6 | 8 | 5.2 | — | — | 8 | 2.9 | 6 | 6.9 | 2 | 5.4 | 4 | 8.0 | |||||
| General condition at diagnosis | .01461 | .10402 | .07316 | .01602 | .19669 | ||||||||||||||||||||
| General condition <5 | 177 | 90.8 | 190 | 82.6 | 194 | 89.0 | 173 | 83.6 | 156 | 85.2 | 17 | 70.8 | 282 | 88.7 | 85 | 79.4 | 36 | 85.7 | 49 | 75.4 | |||||
| General condition = 5 | 18 | 9.2 | 40 | 17.4 | 24 | 11.0 | 34 | 16.4 | 27 | 14.8 | 7 | 29.2 | 36 | 11.3 | 22 | 20.6 | 6 | 14.3 | 16 | 24.6 | |||||
| Status | |||||||||||||||||||||||||
| Death resulting from initial complications | 1 | 0.5 | 1 | 0.4 | 1 | 0.5 | 1 | 0.5 | 1 | 0.5 | — | — | 2 | 0.6 | — | — | — | — | — | — | |||||
| Nonresponse/progression/relapse | 21 | 10.6 | 40 | 16.6 | 21 | 9.6 | 40 | 18.5 | 23 | 11.9 | 17 | 68.0 | 40 | 12.4 | 21 | 18.1 | 12 | 26.1 | 9 | 12.9 | |||||
| Toxic death | 6 | 3.0 | 3 | 1.2 | 8 | 3.6 | 1 | 0.5 | 1 | 0.5 | — | — | 7 | 2.2 | 2 | 1.7 | 1 | 2.2 | 1 | 1.4 | |||||
| Death (other) | — | — | 1 | 0.4 | 1 | 0.5 | — | — | — | — | — | — | — | — | 1 | 0.9 | — | — | 1 | 1.4 | |||||
| Second malignancy | 7 | 3.6 | 6 | 2.5 | 7 | 3.2 | 6 | 2.8 | 5 | 2.6 | 1 | 4.0 | 10 | 3.1 | 3 | 2.6 | 2 | 4.3 | 1 | 1.4 | |||||
Presented are absolute numbers and rates for patients fulfilling certain criteria as well as p(χ)-values evaluating the differences. Data given refer to patients with successful investigation of the respective criterion. General condition at diagnosis was evaluated in 5 steps according to Karnofsky score, poor performance status (general condition = 5) was defined as Karnofsky score 0-20%. CNS, central nervous system.
Diagnosis and therapy
Diagnosis of T-LBL cases was carried out according to World Health Organization Classification of Hematological Malignancies and recommendations from the European childhood lymphoma pathology panel.28,29 Patients were treated according to an ALL-BFM-type treatment strategy for LBL, as described previously.2
Mutational analysis of NOTCH1 and/or FBXW7
Lymphoma DNA at the time of initial diagnosis was isolated from frozen cells (tumors or pleural/pericardial effusions) or paraffin-embedded tumor biopsies. Mutational hot spots were amplified using standard polymerase chain reaction (PCR) conditions and OneTaq Hot Start 2× Master Mix (New England Biolabs, Frankfurt/Main, Germany). Primers (sequences given in supplemental Table 2) were synthesized by Eurofins MWG Operon (Ebersberg, Germany). PCR products were sequenced by LGC Genomics (Berlin, Germany) or on an ABI 3130xl Genetic Analyzer (Life Technologies, Darmstadt, Germany), either directly or after subcloning into Topo TA cloning vectors (Life Technologies, Darmstadt, Germany). Base-pair substitutions were verified twice, and frameshift mutations once.
LOH analysis
LOH analysis was carried out essentially as described previously.27 Cases with fewer than 3 informative results were excluded because of insufficient DNA quantity and/or quality. LOH positivity was defined as at least 2 adjacent informative microsatellite markers with LOH. LOH data on 108 patients have been reported previously26,27 ; because of the lack of adequate material for validation, data of 9 patients have been excluded, and the remaining 99 patients represent the test cohort.
Statistical analysis
Probability of EFS (pEFS) was calculated according to Kaplan and Meier30 with differences compared by log-rank test.31 pEFS was calculated from the date of diagnosis to first event (death from any cause, relapse, resistant disease, or second malignancy) or to the date of last follow-up. Patients lost to follow-up were censored at date of last follow-up-examination. Cumulative incidence functions for relapse were constructed by the method of Kalbfleisch and Prentice32 and compared with Gray’s test.33 Differences in the distribution of individual parameters among patient subsets were analyzed using the χ2 test or Fisher’s exact test. Multivariate analyses were calculated using standard methods.34
Results
Five-year pEFS was 80% ± 2% for the total cohort of 438 patients. For the 241 evaluable patients with T-LBL, pEFS was 80% ± 3%, for the 116 patients with investigated NOTCH1 and/or FBXW7 status it was 77% ± 4%, and for the 217 patients with investigated LOH6q status it was 79% ± 3%. The median follow-up was 5.5 years for the entire group. In line with the treatment regimen, 6 of 241 analyzed patients (6/217 in LOH6q group and 3/116 in NOTCH1/FBXW7 group) received intensified high-risk courses analog to the ALL-BFM protocol because of insufficient tumor response at day 33 of induction. Clinical characteristics for all analyzed patients are given in Table 1.
NOTCH1 and FBXW7 analyses
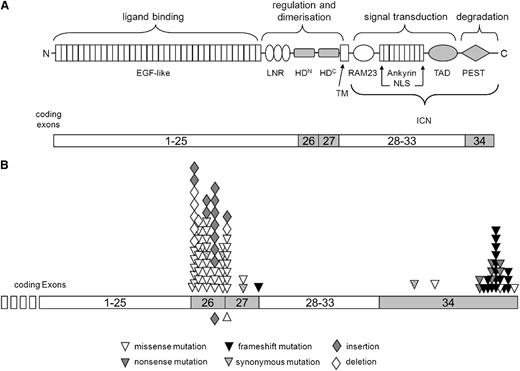
Six exons, which have been identified as mutational hot spots in pediatric T-ALL,9-19 were sequenced: for NOTCH1, exons 26 (encoding the N-terminal part of the heterodimerization [HD] domain), 27 (encoding the C-terminal part of the HD domain), and 34 (encoding the transactivation domain (TAD) and PEST domain (sequence rich in proline [P], glutamic acid [E], serine [S] and threonine [T]); amplification in fragments 34a, 34b, and 34c); and for FBXW7, exons 9, 10, and 12 (all 3 encoding fragments of the 8 WD40 domains). Sequencing was successful in 116 pediatric patients with T-LBL, with evaluable sequences of all PCRs for 112 cases and of all PCRs except NOTCH1-exon 34a because of insufficient amplification of minor-quality DNA for 4 cases. In 70 of 116 cases (60%), mutations in NOTCH1 could be identified: 46 cases showed mutations in exon 26 (66%), 13 in exon 27 (19%), 2 in the TAD domain (3%), and 26 in the PEST domain (37%) (Figure 1).
Overview about the mutations found in NOTCH1. The domain structure of NOTCH1, the functions of these domains, and the respective coding exons are schematically depicted (A). Analyzed exons and domains are highlighted. Mutations detected in this study are illustrated with respect to their position in the exons of the gene (B). Symbols below the exon line indicate homozygous mutation. Modified after Weng et al9 , Zuurbier et al14 , and Wagener and Müller.35 EGF, epidermal growth factor; HD, heterodimerization domain; LNR, LIN12/NOTCH repeats; NLS, nuclear localization sequence; TAD, transactivation domain. TM, transmembrane domain.
Overview about the mutations found in NOTCH1. The domain structure of NOTCH1, the functions of these domains, and the respective coding exons are schematically depicted (A). Analyzed exons and domains are highlighted. Mutations detected in this study are illustrated with respect to their position in the exons of the gene (B). Symbols below the exon line indicate homozygous mutation. Modified after Weng et al9 , Zuurbier et al14 , and Wagener and Müller.35 EGF, epidermal growth factor; HD, heterodimerization domain; LNR, LIN12/NOTCH repeats; NLS, nuclear localization sequence; TAD, transactivation domain. TM, transmembrane domain.
35 In 2 cases, mutations in exons 26 and 27 were detectable concurrently; in 13 cases, mutations in exons 26 and 34; and in 2 cases, mutations in exons 27 and 34.
Twenty-one (18%) of the 116 analyzed patients showed mutations in FBXW7: 14 in exon 9 (67%), 6 in exon 10 (29%) and 4 in exon 12 (19%) (supplemental Figure 1). Two cases had mutations both in exons 9 and 10, and 1 patient had mutations in exons 9 and 12. Seventeen (15%) of 116 patients showed mutations in both genes; in a single case, mutations occurred in parallel in the PEST domain of NOTCH1 and in FBXW7. There, the mutation in FBXW7-exon 12 was a frameshift instead of the normally occurring missense mutations. Included in the total of 91 detected aberrations are 3 synonymous mutations: 1 each in exon 26, exon 27, and the TAD (Figure 1). A detailed overview of the identified aberrations is given in supplemental Figure 2.
The clinical features of the 74 N/Fpos patients were similar to those of the 42 patients without NOTCH1 and/or FBXW7 mutations (N/Fneg); the only exception was the overrepresentation of younger patients in the N/Fpos group (Table 1). The significant correlation of both, the 5-year pEFS (84% ± 5% in NOTCH1 mutated [Npos] patients vs 66% ± 7% in NOTCH1 nonmutated [Nneg] patients; P = .021) and the 5-year cumulative incidence (CI) of nonresponse/progression/relapse (15% ± 5% in Npos compared with 27% ± 7% in Nneg; P = .05), showed the prognostic effect of NOTCH1 mutations within our cohort (Figure 2).
Five-year probability of EFS (A) and 5-year cumulative incidence of nonresponse/progression/relapse (B), according to NOTCH1 mutational status.
Five-year probability of EFS (A) and 5-year cumulative incidence of nonresponse/progression/relapse (B), according to NOTCH1 mutational status.
Similar results were observed taking into account NOTCH1 and/or FBXW7 mutations (supplemental Figure 3) but not regarding FBXW7 mutations alone (supplemental Figure 4). pEFS was also calculated according to the underlying mutations: In our cohort of 116 analyzed patients, no prognostic relevance of the position of mutation could be confirmed (Table 2). Data from subgroup analyses according to treatment protocols are given in supplemental Table 3.
Overview about pEFS at 5 years, according to position of mutations
| Position of mutation . | Number of patients with the respective mutation . | pEFS at 5 years . |
|---|---|---|
| Only in HD domain | 27 | 92% ± 5% |
| Only in PEST domain | 11 | 76% ± 15% |
| Only in HD + PEST domain | 14 | 85% ± 10% |
| Only in FBXW7 | 4 | 75% ± 22% |
| Only in HD + FBXW7 | 15 | 76% ± 12% |
| Position of mutation . | Number of patients with the respective mutation . | pEFS at 5 years . |
|---|---|---|
| Only in HD domain | 27 | 92% ± 5% |
| Only in PEST domain | 11 | 76% ± 15% |
| Only in HD + PEST domain | 14 | 85% ± 10% |
| Only in FBXW7 | 4 | 75% ± 22% |
| Only in HD + FBXW7 | 15 | 76% ± 12% |
P(Log-Rank) = .89; not considered for calculation are 1 case with a single mutation in TAD, 1 case with mutations in TAD and FBXW7, and 1 case with mutations in HD, PEST domain, and FBXW7.
LOH analyses
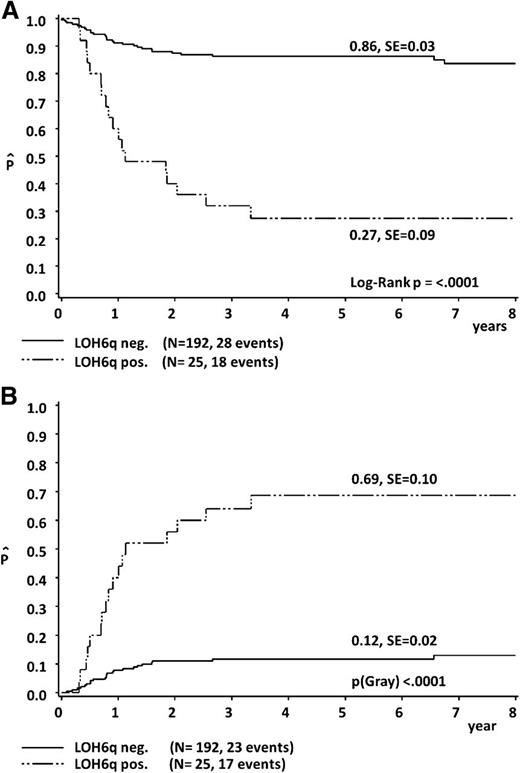
Fragment-length analysis of germline and corresponding tumor DNA from a total of 217 patients with T-LBL (99 patients of the test cohort26,27 and 118 new patients of the validation cohort) was successful for a total of 4058 marker analyses, with LOH in 206 markers, retention of heterozygous patterns in 2680 markers, homozygous patterns in 1090 markers, and microsatellite instability in 82 markers. LOH of at least 2 neighboring markers (separation by any result but retention of heterozygosity permitted) was detected in 25 (12%) of the analyzed 217 cases. In 4 cases, LOH affected all informative markers, whereas interstitial regions of LOH were detected in the remaining 21 patients. In 2 cases, 2 regions of LOH were identified. According to previous findings, in 22 of the 25 cases the putative LOH region included at least one of the adjacent markers D6S1284, D6S1716, and D6S1717 on chromosomal band 6q16, representing the common deleted region (supplemental Figure 5). Clinical characteristics were similar in the 25 LOH6q-positive (LOH6qpos) patients compared with the 192 LOH6q-negative patients (LOH6qneg; Table 1).
In the outcome analysis, LOH6qpos was associated with a significantly inferior 5-year pEFS of 27% ± 9% compared with 86% ± 3% for LOH6qneg cases (P < .0001). This was mainly because of an increased CI of nonresponse/progression/relapse being 69% ± 10% in LOH6qpos vs 12% ± 2% in LOH6qneg (P < .0001) (Figure 3). There was no difference in the pEFS (80% ± 4% vs 79% ± 4%; P = .92) and CI of nonresponse/progression/relapse (18% ± 4% vs 18% ± 4%; P = .96) between the 99 patients of the test cohort and the 118 patients of the validation cohort. Data concerning pEFS of subgroups according to treatment protocol are given in supplemental Table 3.
Five-year probability of EFS (A) and 5-year cumulative incidence of nonresponse/progression/relapse (B), according to LOH6q status.
Five-year probability of EFS (A) and 5-year cumulative incidence of nonresponse/progression/relapse (B), according to LOH6q status.
NOTCH1/FBXW7 and LOH6q
In 92 patients, adequate material was available to perform both genetic marker studies. Fifty-three of 92 cases were Npos, whereas LOH6qpos was detected in 11 of 92 patients. Except in 3 cases, both genetic alterations did not occur in parallel (P[Fisher’s Exact] = .033). In 2 of 3 cases, LOH at chromosome 6q did not span the chromosomal region 6q16 potentially relevant for unfavorable outcome. As a consequence, only 1 of 92 patients showed in parallel a favorable activating NOTCH1 mutation together with a poor prognostic LOH6q16. NOTCH1 mutations were not generally associated with a reduced incidence of deletions or LOH. The incidence of LOH of chromosome 9p at the locus of CDKN2A and CDKN2B, which has been investigated previously,36 was observed in 16 of 26 Npos cases compared with 7 of 16 Nneg cases (P[Fisher’s Exact] = .21). Of these 92 patients with results of both experimental analyses, 81 cases were LOH6qneg and 11 cases were LOH6qpos. Data concerning outcome analysis of this subgroup are given in supplemental Table 3.
Within the cohort, 8 (21%) of 39 cases were Nneg and LOH6qpos, whereas only 3 (6%) of 53 cases were Npos and LOH6qpos concurrently. To exclude a dependency of both markers and to evaluate the strength of their predicting relevance, LOH6q status, NOTCH1 status, and FBXW7 status were subjected to a multivariate analysis, together with the clinical parameter “general condition at diagnosis.” Poor performance status, defined as a Karnofsky score between 0% and 20%, was associated with unfavorable prognosis in pediatric patients with T-LBL of the NHL-BFM group in a univariate analysis (B.B., M.Z., and A.R., unpublished results). Within this multivariate analysis, both LOH6q status and NOTCH1 mutation status remained independent prognostic factors (Table 3).
Multivariate analysis of pEFS at 5 years for LOH6q status, NOTCH1 status and FBXW7 status and general condition at diagnosis
| Parameters . | HR . | LL . | UL . | p(χ) . |
|---|---|---|---|---|
| General condition at diagnosis | 0.93 | 0.27 | 3.23 | 0.907 |
| LOH6q | 2.79 | 1.08 | 7.21 | 0.035 |
| NOTCH1 mutation | 0.36 | 0.14 | 0.91 | 0.031 |
| FBXW7 mutation | 1.13 | 0.31 | 4.09 | 0.854 |
| Parameters . | HR . | LL . | UL . | p(χ) . |
|---|---|---|---|---|
| General condition at diagnosis | 0.93 | 0.27 | 3.23 | 0.907 |
| LOH6q | 2.79 | 1.08 | 7.21 | 0.035 |
| NOTCH1 mutation | 0.36 | 0.14 | 0.91 | 0.031 |
| FBXW7 mutation | 1.13 | 0.31 | 4.09 | 0.854 |
HR, hazard ratio; LL, lower limit; UL, upper limit.
A Cox proportional hazards model was used to obtain the estimates and the 95% confidence interval of the relative risk for prognostic factors. In multivariate analysis considering general condition at diagnosis, which is itself a statistically significant poor-prognosis parameter in pediatric T-LBL (B.B., M.Z., and A.R., unpublished data), the parameters LOH6q and mutation in NOTCH1 remain significant.
Discussion
Gaining an insight into the genetic field of pediatric T-LBL is hampered by the scarcity of patient material for molecular biological research. Until now, no biological marker could be validated as a prognostic parameter for treatment stratification. Thus, the primary objective of this study was to search for valid stratification criteria for patients with T-LBL. Here, we analyzed the mutational status in hot spots of NOTCH1 and FBXW7 and copy number alterations on chromosome 6q14-24 in a total of 241 pediatric patients with T-LBL. This cohort of patients, which is to our knowledge the largest series of pediatric T-LBL available for molecular genetic studies so far, was registered in the NHL-BFM study center and uniformly diagnosed and treated according to NHL-BFM treatment strategies. The current study validated LOH6qpos as a poor-prognosis marker (5-year pEFS 27% ± 9% vs 86% ± 3% in LOH6qneg patients) within 217 investigated patients. This confirms that the unfavorable prognosis that was reported previously is indeed associated with the patients with T-LBL of the NHL-BFM studies and not a result of any bias in sample selection. The strength of this marker is also its very high significance (P < .0001). Until now, we have not been aware of any molecular or clinical marker distinguishing 2 prognostic groups in pediatric T-LBL with such strength. These results are so convincing that the LOH6q status will serve as a stratification criterion to identify very high risk patients in future clinical trials with involvement of the NHL-BFM group. With the help of this marker, a subgroup of less than 15% of patients can be defined in which more than 40% of relapses occur. Reducing the incidence of relapse in this small subgroup will have significant effect on the pEFS of the whole cohort.
Concerning NOTCH1 and/or FBXW7 mutation status, 4 papers were published including 54, 14, 11, and 9 pediatric patients with T-LBL.11,17,24,25 This study, which included 116 evaluable patients, strengthened the input to that field. The detected mutations in the current study were mainly either mismatch mutations or small in-frame deletions/insertions in the HD domain and nonsense or frameshift mutations in the PEST domain of NOTCH1, as well as mismatch mutations affecting 3 essential positions within the canonical binding pocket of FBXW7.23,37 This was in agreement with the published data in pediatric T-LBL.
More is known about the frequency, type, and prognostic relevance of NOTCH1 and/or FBXW7 mutations in pediatric patients with T-ALL.9-19 T-LBL and T-ALL are often considered to be closely related or 2 manifestations of 1 disease; however, recent publications provide evidence for molecular differences between them.24,27,38,38-42 Among others, differences in the common deleted region and, most likely because of that, in the prognostic effect of chromosome 6q alterations between T-ALL and T-LBL are described.27 This might be a distinguishing feature between both T-ALL and T-LBL and may be relevant with regard to the different clinical manifestations.
Comparison of the incidence, type, and position of NOTCH1 and/or FBXW7 mutations obtained in the current study with the published data on T-ALL revealed no difference between the 2 diseases. This fits with the common understanding that the NOTCH1 pathway is responsible for early T-cell development, which is disturbed both in T-ALL and T-LBL. Several studies indicate a favorable prognosis for patients with T-ALL with the NOTCH1 mutation compared with nonmutated patients9-19 (supplemental Table 1). However, available data on T-ALL are not absolutely consistent, as the effect of the mutation might depend on the applied treatment. The analyses of the 116 T-LBL reported here also showed a favorable prognosis for Npos cases.
The data presented are very similar to those reported for pediatric patients with T-ALL who were treated according to the ALL-BFM treatment regimen,10,15 which is comparable to the treatment administered to patients with T-LBL from the NHL-BFM group. In their hands, the favorable prognostic relevance of NOTCH1 mutations can be separated from the relevance of FBXW7 mutations too, as observed in our cohort. This supports the hypothesis of the prognostic effect of activating NOTCH1 mutations being influenced by the applied treatment. Furthermore, it confirms that in the context of BFM-type treatment, NOTCH1 mutations can be used as a prognostic marker to subgroup patients into risk groups.
The diverging distances between the 5-year pEFS (84% ± 5% vs 66% ± 7%) and the 5-year CI of nonresponse/progression/relapse (15% ± 5% vs 27% ± 7%) could be suggestive of a higher nonrelapse mortality rate in Npos patients. However, as it is depicted in Table 1, rates for treatment-related mortality, death, or secondary malignancies are similar between Npos and Nneg patients.
A recent report on patients with T-ALL showing an inverse association between the strength of the NOTCH1 and/or FBXW7 mutation and the strength of the favorable prognostic relevance14 could not be confirmed in the current cohort of pediatric patients with T-LBL. This might be explained by significant differences in the treatment administered to the patients with T-LBL in the current study and the patients with T-ALL in the cited manuscript Dutch Childhood Oncology Group, which modify the prognostic effect to a certain extent.
The way NOTCH1 and/or FBXW7 mutations alter the chemosensitivity of the cells is not yet understood. To our knowledge, only a single study reported upregulation of several chemotherapy-relevant genes modulating the response to methotrexate, doxorubicin, vincristine, 6-mercaptopurine, and L-asparaginase in N/Fpos cases of pediatric T-ALL.12 Thus, the effect of modulated chemosensitivity of N/Fpos patients might depend on the polychemotherapy regimen concerning selection, dose, and schedule of drug administration. Further analyses and a search for mutations in genes involved in regulation of chemoresistance are inevitable to clarify the underlying mechanisms.
In the current study, almost 100 cases were available for both LOH6q and NOTCH1 and/or FBXW7 analyses. Interestingly, only 3 cases were observed in which both alterations were detectable; in addition, in 2 of the 3 cases, LOH6q did not span the chromosomal band 6q16, which has been described as the critical region of LOH associated with poor outcome in T-LBL (supplemental Figure 5).26,27 As a consequence, only a single patient could be identified in whom both genetic alterations with opposite prognostic association could be observed. This patient received standard treatment and died 28 months after initial diagnosis because of multiple relapses. Except in this single case, good prognostic NOTCH1 mutations and poor prognostic LOH6q16 seem to occur mutually exclusive. In the multivariant analysis including the potential prognostic parameters LOH6q status, NOTCH1 status, and FBXW7 status and the general condition at diagnosis, both LOH6q status and NOTCH1 status turned out to be independent prognostic parameters, whereas general condition at diagnosis lost significance (Table 3). This observation strengthens the biological relevance of these 2 markers.
There are 2 hypotheses to explain the phenomenon of mutually exclusive genetic events in cancer cells: first, that these events are genetically redundant,43,44 which can also mean that they supply the same selective pressure for clonal expansion,45 and second, that both events confer drawbacks to these cells.43 Transferred into pediatric T-LBL, the exclusive occurrence of NOTCH1 mutations and LOH6q16 might be explained by the loss of a gene or regulatory region in 6q16 acting in the NOTCH1 pathway. Candidates might be POU3F2, which is described as an activator of the NOTCH1 pathway in melanoma cells46 ; FBXL4, which, similar to FBXW7, is a member of the F-box protein family; and UFL1, which is involved in regulation of the NFκB pathway.47 NFκB itself is involved in T-cell development and regulated by the NOTCH1 pathway.20,47,49 Concerning the second hypothesis, the transfer of survival drawbacks or the provision of proapoptotic signals to the cell, putative candidates in 6q16 are suggested to be either involved in tumorigenesis or implicated in a wide range of highly relevant cellular processes. Examples are the tumor suppressor protein TSG1; the G protein–coupled receptors GPR63 and MCHR2; 2 proteins that are involved in the mitochondrial respiratory chain (namely, NDUFAF4 and COQ3); CCNC, which encodes cyclin C regulating the quiescence of human hematopoietic stem/progenitor cells50 ; and the DNA repair protein MMS22L.
It would be interesting to analyze the association of the described genetic alterations with early treatment response, especially as insufficient response after induction is the only indication for treatment intensification in the current NHL-BFM protocol. However, the 6 patients fulfilling this criterion (6/217 patients in the LOH6q group and 3/116 patients in the NOTCH1/FBXW7 group) are far too few to allow the performance of valid and meaningful statistical tests. Unfortunately, in the current NHL-BFM protocols, neither positron emission tomography–computed tomography nor flow minimal residual disease/minimal disseminated disease analyses are included, so these questions need to be solved prospectively.
Very recently, a study was published by E. Macintyre’s group dealing, among other things, with the analysis of mutations in NOTCH1 and/or FBXW7 and the deletion of CASP8AP2 (also known as FLASH) at chromosome 6q15.25 Fifty-five percent of 54 pediatric patients with T-LBL, having also received a uniformly BFM-type therapy, were N/Fpos, which was significantly associated with a more favorable outcome. Monoallelic deletions of CASP8AP2 could be observed in 18% of cases but did not display any prognostic significance. The current report confirms relevant findings concerning the prognostic effect of NOTCH1 mutations in an independent cohort. Importantly, the present study analyzed more than twice as many patients, and thus provided a much broader, more detailed, and more meaningful insight into this matter.
In conclusion, the present study reports mutations in NOTCH1 and/or FBXW7 as well as the status of LOH6q in the largest series of pediatric patients with T-LBL published to date. The 2 genetic variations seem to occur mutually exclusive and are both associated with the outcome of the patient, albeit with the opposite effect. Although the prognostic relevance of LOH6q seems to be stronger than the one of NOTCH1 mutational status, the difference in pEFS according to NOTCH1 status is statistically significant, and the number of analyzed patients is large enough. Thus, the available data from our own group presented in this manuscript, together with the published data in the literature, are so convincing that the NHL-BFM group aims at implementing these genetic markers as stratification criteria into the next treatment protocol. The prognostic power of both markers will be reflected by a gradual modification of the treatment plan: Nneg cases will receive moderate treatment intensification, whereas LOH6qpos cases will receive relevant treatment intensification.
Thus, our current manuscript presents the scientific basis for 2 different molecular prognostic markers that will be used for stratification in the next clinical trials of the NHL-BFM group. This will help improve the prognosis of pediatric patients with T-LBL, as it is the first time that stratification other than according to clinical parameters for T-LBL will be used. In addition to the NHL-BFM, other groups and other treatment centers might evaluate, validate, and use the parameters described in the current report.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Claudia Keller, Ute Jacobsen, and Franziska Greene for excellent technical assistance, as well as the physicians and nurses in the participating centers of the NHL-BFM group. The authors thank Gabriele Buck (cytomorphology), Ulrike Meyer, and Bettina Paul (data management), as well as Sareetha Kailayangiri (manuscript editing).
The work was supported by Forschungshilfe Peiper, Giessen, Germany.
Authorship
Contribution: B.R.B. conceived the study, designed and performed the research, analyzed and interpreted the data, and wrote the manuscript. M.R. designed and performed the research, analyzed and interpreted the data, and wrote the manuscript. M.Z. analyzed and interpreted the data and performed the statistical analysis. D.K. performed the research and analyzed and interpreted the data. I.O., F.N., G.W., A.A., G.E., and W.K. provided patient material and reviewed the cases. A.R. conceived the study, provided patient material, reviewed the cases, and analyzed and interpreted the data. B.B. conceived the study, provided patient material, reviewed the cases, designed and performed the research, analyzed and interpreted the data, and wrote the manuscript. All authors read and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Birgit Burkhardt, NHL-BFM Study Center, University Children’s Hospital Münster, Pediatric Hematology and Oncology, Domagkstrasse 24, Münster D-48149, Germany; e-mail: birgit.burkhardt@ukmuenster.de.