Key Points
Serine protease inhibitor serpinB1 protects neutrophils by inhibition of their own azurophil granule protease cathepsin G.
Granule permeabilization in neutrophils leads to cathepsin G–mediated death upstream and independent of apoptotic caspases.
Bone marrow (BM) holds a large reserve of polymorphonuclear neutrophils (PMNs) that are rapidly mobilized to the circulation and tissues in response to danger signals. SerpinB1 is a potent inhibitor of neutrophil serine proteases neutrophil elastase (NE) and cathepsin G (CG). SerpinB1 deficiency (sB1−/−) results in a severe reduction of the BM PMN reserve and failure to clear bacterial infection. Using BM chimera, we found that serpinB1 deficiency in BM cells was necessary and sufficient to reproduce the BM neutropenia of sB1−/− mice. Moreover, we showed that genetic deletion of CG, but not NE, fully rescued the BM neutropenia in sB1−/− mice. In mixed BM chimera and in vitro survival studies, we showed that CG modulates sB1−/− PMN survival through a cell-intrinsic pathway. In addition, membrane permeabilization by lysosomotropic agent l-leucyl-l-leucine methyl ester that allows cytosolic release of granule contents was sufficient to induce rapid PMN death through a CG-dependent pathway. CG-mediated PMN cytotoxicity was only partly blocked by caspase inhibition, suggesting that CG cleaves a distinct set of targets during apoptosis. In conclusion, we have unveiled a new cytotoxic function for the serine protease CG and showed that serpinB1 is critical for maintaining PMN survival by antagonizing intracellular CG activity.
Introduction
Polymorphonuclear neutrophil (PMN) granulocytes are essential components of the innate immune response to infection. PMNs are relatively short-lived leukocytes that originate from hematopoietic stem cells in the bone marrow (BM) in a process called granulopoiesis. Granulopoiesis proceeds through a proliferative phase followed by a maturation phase. After maturation, the BM retains a large reserve of mature PMNs, which includes over 90% of the mature PMNs in the body while only a small proportion (1%-5%) is in the blood.1,2 Even in noninflammatory conditions, granulopoiesis is remarkable as >1011 PMNs are produced daily in an adult human, only to be disposed of, largely unused, a few hours later.3 There is evidence that the majority of PMNs produced never reach circulation and die within the BM.4 Congenital or acquired forms of neutropenia are associated with the highest risks of bacterial and fungal infection,5 indicating a strong evolutionary pressure to maintain granulopoiesis at high levels and sustain a large mobilizable pool of PMNs in the BM.
In steady state, PMNs die by apoptosis, a form of programmed cell death that allows for the safe disposal of aging PMNs and their potentially toxic cargo. Like in other cells, caspases participate in the initiation, amplification, and execution steps of apoptosis in PMNs.6,7 Interestingly, noncaspase cysteine proteases calpain and cathepsin D were reported to induce PMN apoptosis through activation of caspases.8,,-11 In addition, PMNs carry a unique set of serine proteases (neutrophil serine proteases [NSPs]) including elastase (NE), cathepsin G (CG), and proteinase-3 (PR3) stored active in primary granules. There is strong evidence for a role of NSPs in killing pathogens and inducing tissue injury when released extracellularly.12,-14 In contrast, the function of NSPs in PMN homeostasis and cell death remains elusive. In particular, no defects in granulopoiesis or PMN homeostasis have been reported in mice deficient in cathepsin G (CG−/−),15 neutrophil elastase (NE−/−),16,17 or dipeptidylpeptidase I (DPPI−/−), which lack active NSPs.18 We have recently shown that mice lacking the serine protease inhibitor serpinB1 (sB1−/−) have reduced PMN survival in the lungs following Pseudomonas infection and that these mice have a profound reduction in mature PMN numbers in the BM.19,20 SerpinB1, also known as monocyte NE inhibitor, is expressed at high levels in the cytoplasm of PMNs and is one of the most potent inhibitors of NE, CG, and PR3.21,22 In this study, we tested the hypothesis that serpinB1 promotes PMN survival by inhibiting 1 or several NSPs, and we discovered a novel regulatory pathway in PMN homeostasis in vivo.
Methods
Mice
Generation of sB1−/− (Serpinb1atm1.1Cben) mice in 129S6/SvEvTac (129S6) and backcrossing in the C57BL/6J background were described previously.19,20 CG−/− (Ctsgtm1Ley)15 and DPPI−/− (Ctsctm1Ley)23 mice were provided by Christine Pham (Washington University, St. Louis, MO). NE−/− (B6J-Elanetm1Sds),24 green fluorescent protein (GFP+) transgenic (B6-Tg(CAG-EGFP)1Osb), and CD45.1 (B6.SJL-PtprcaPepcb/BoyJ) mice were obtained from The Jackson Laboratory. All animal studies were approved by the Cantonal Veterinary Office of Bern and conducted in accordance with the Swiss federal legislation on animal welfare.
Flow cytometry and cell sorting
Leukocyte counts and hematopoietic lineage differential analysis of blood and BM were performed in 8-week-old mice. Equal numbers of males and females were included for each line. Blood (70 µL) was collected from the retroorbital sinus of sedated mice using heparinized microhematocrit tubes. Total blood leukocyte, erythrocyte, and platelet counts were performed at the central clinical laboratories of the veterinary faculty of the University of Bern. BM cells were harvested from femurs by flushing with cation-free Hank’s balanced salt solution supplemented with 1% fetal calf serum and counted in a Neubauer chamber. Isolated BM cells and whole blood were stained with fluorescently labeled antibodies (Biolegend and BD Biosciences) and analyzed on a FACSCalibur flow cytometer (BD Biosciences) as described previously.25 Briefly, leukocyte subset percentages were determined within CD45+ cells as PMNs (CD11b+Ly-6Ghi), myelocytes (CD11b+Ly-6GnegSSChiCD115neg), monocytes (CD115+), and B cells (CD19+ or CD45RB220+). Data were analyzed and presented using FlowJo (TreeStar). Flow sorting of PMNs, B cells, and myelocytes was performed on single-cell suspensions of BM leukocytes using a FACSAria II sorter (BD Biosciences) at the flow cytometry core facility of the Department of Clinical Research (University of Bern).
BM chimera
For the generation of chimeric mice, recipient mice were lethally irradiated from a cesium source with 10 Gy using a Gammacell 40 Exactor (Theratronics). BM cells (10-20 × 106) from 8- to 13-week-old donor mice were injected into the retroorbital sinus of recipient mice 18 hours after irradiation. Antibiotics (1% Cotrim; Spirig Pharma AG) were added to the drinking water for 4 weeks, and BM and blood populations were analyzed at least 8 weeks after BM transfer. Reconstitution efficiency was always >95% based on flow cytometry analysis of recipient cells (CD45.1, CD45.2, or GFP). In mixed BM chimera, the relative percentage of wild-type (WT; CD45.1) and sB1−/− (CD45.2) cell subsets was determined as described in supplemental Figure 3 (available on the Blood website). Because B-cell numbers were not different in WT and sB1−/− mice and engrafted with a similar efficiency, B cells provided an internal control population to estimate the actual percentage of hematopoietic stem cell input of each genotype (percentage of input) transplanted in each GFP mouse.
Western blot
Sorted cells were washed and lysed (107/mL) in radioimmunoprecipitation assay buffer with protease inhibitor cocktail (Roche). Lysates were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis under reducing conditions and immunoblotted using rabbit antiserum to serpinB1.20 Blots were stripped and restained with anti–β-actin antibody (Cell Signaling Technology).
In vitro survival assays
BM cells were isolated by flushing femurs and tibias of 8- to 22-week-old mice with Hank’s balanced salt solution. PMNs were enriched using a magnetic negative selection kit (EasySep; StemCell Technologies). Purity and viability of the purified neutrophils were assessed by flow cytometry and were typically 70% to 95% and >90%, respectively. Purified neutrophils were cultured in Dulbecco’s modified Eagle medium (4mM L-Glut, 25mM D-Glucose, 1mM sodium pyruvate) (Gibco; Life Technologies) containing 1% fetal calf serum at 0.5-1 × 106 cells per mL in the presence or absence of the pan-caspase inhibitor Q-VD-OPh (SM Biochemicals LLC), CG inhibitor I (JNJ-10311795; RWJ-355871) (Merck Chemicals), or l-leucyl-l-leucine methyl ester (LLME) (G-2550; Bachem). Cytotoxic effects of human CG (Athens Research & Technology) were evaluated in serum-free medium. Effects of LLME were investigated using whole BM after erythrocyte lysis by NH4Cl. At the indicated time points, cells were harvested and viability was assessed using Annexin V–fluorescein isothiocyanate (FITC) and 7-aminoactinomycin D (7AAD). After gating on Ly-6G+ events, viable cells were identified as FSChi7AADnegAnnexinVneg, early apoptotic as FSChi7AADnegAnnexinV+, and late apoptotic/necrotic as 7AAD+, respectively (supplemental Figure 1).
In vivo administration of G-CSF
Mice received daily intraperitoneal injections of recombinant murine granulocyte colony-stimulating factor (G-CSF; 500 ng per mouse) (Invitrogen) or control saline for 5 days. Blood and BM were harvested 24 hours after the last injection and analyzed by flow cytometry as described above in “Flow cytometry and cell sorting”.
Statistical analysis
All analyses were performed using GraphPad Prism Mac 4.0c. A value of P < .05 was considered statistically significant.
Results
SerpinB1 neutropenia is rescued by BM transfer
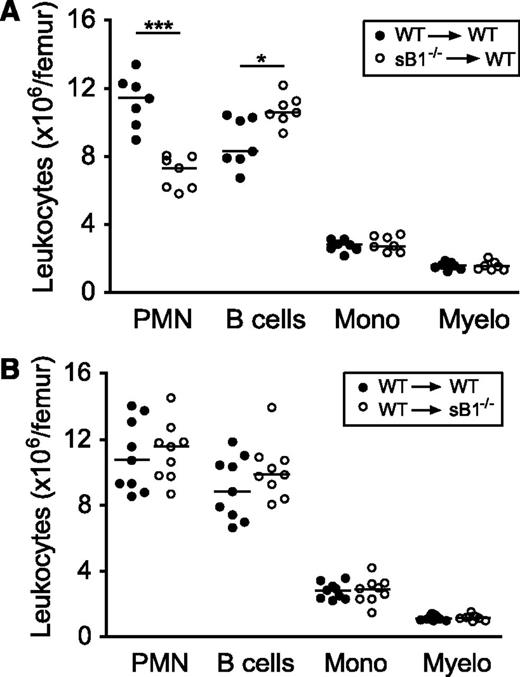
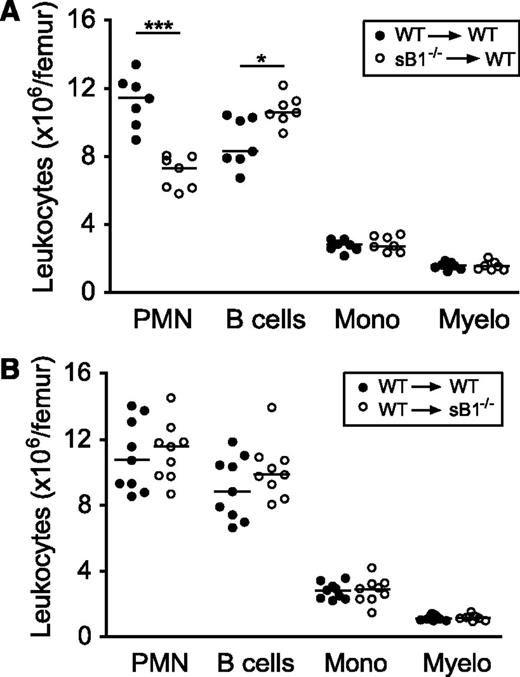
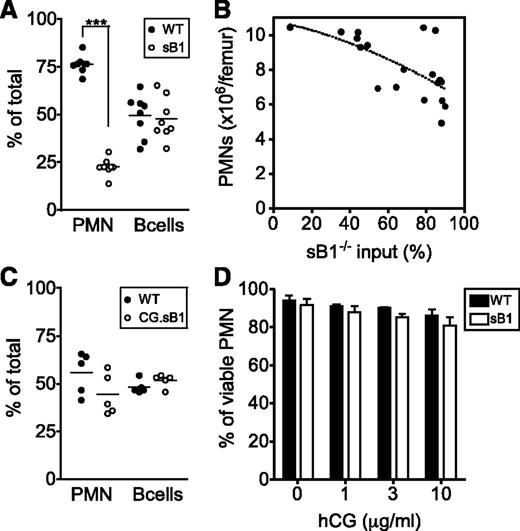
We have previously shown that serpinB1 is expressed at highest levels in the granulocyte lineage and that sB1−/− mice have a profound reduction in BM mature PMN numbers despite normal granulopoiesis.19 Because serpinB1 is also expressed in many tissues and cell types,21,26 we generated BM chimera to investigate the contribution of nonhematopoietic cells in sustaining PMN survival. WT mice were lethally irradiated and were transferred with WT or sB1−/− BM cells. Eight to 10 weeks after BM transfer, mice reconstituted with sB1−/− cells recapitulated the defective PMN reserve of sB1−/− mice, whereas the mice reconstituted with WT cells had normal numbers of BM PMNs, indicating that serpinB1 deficiency in BM cells is sufficient to induce BM neutropenia in vivo (Figure 1A). In the reverse experiment, transfer of WT BM cells in irradiated sB1−/− mice restored a normal PMN reserve in the BM (Figure 1B). Overall, these results demonstrate that serpinB1 deficiency in the BM compartment is necessary and sufficient to reproduce the neutropenic defect of sB1−/− mice.
Defective PMN reserve in BM chimera depends on serpinB1 deficiency in the hematopoietic compartment. Flow cytometry analysis of major BM leukocyte subsets of lethally irradiated mice was performed 8 to 10 weeks after BM transfer. (A) Irradiated WT (CD45.1) mice were transferred with WT (●) or sB1−/− (○) BM cells. (B) Irradiated WT (●) and sB1−/− (○) mice both CD45.2 were transferred with WT (CD45.1) BM cells. Each circle represents leukocyte numbers for 1 mouse and horizontal line indicates the median. Median subsets numbers were compared by the Mann-Whitney test (*P < .05; ***P < .001).
Defective PMN reserve in BM chimera depends on serpinB1 deficiency in the hematopoietic compartment. Flow cytometry analysis of major BM leukocyte subsets of lethally irradiated mice was performed 8 to 10 weeks after BM transfer. (A) Irradiated WT (CD45.1) mice were transferred with WT (●) or sB1−/− (○) BM cells. (B) Irradiated WT (●) and sB1−/− (○) mice both CD45.2 were transferred with WT (CD45.1) BM cells. Each circle represents leukocyte numbers for 1 mouse and horizontal line indicates the median. Median subsets numbers were compared by the Mann-Whitney test (*P < .05; ***P < .001).
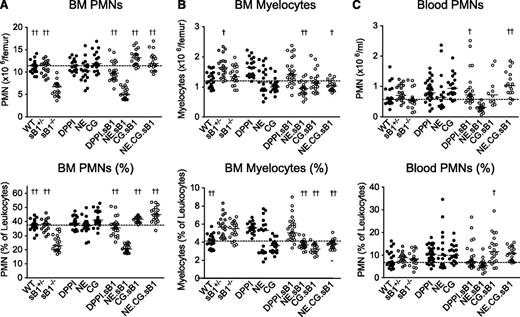
CG regulates neutrophil numbers in the BM
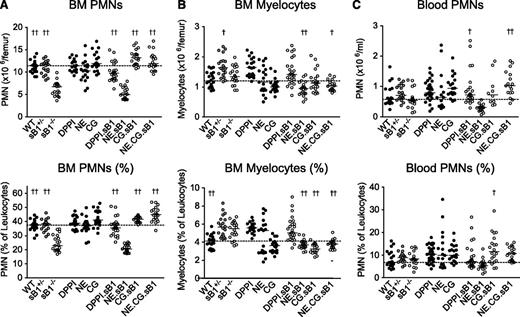
Because serpinB1 is an efficient inhibitor of NE, CG, and PR3, we then examined PMN numbers in mice deficient in 1 or several NSPs in combination with serpinB1 deletion. As expected, sB1−/− mice had significantly reduced numbers and percentage of mature PMNs in the BM compared with WT and heterozygous sB1+/− mice. In addition, PMN numbers were normal in mice deficient in either DPPI, NE, or CG (Figure 2A). DPPI is not inhibited by serpinB1 but is required for the activation of all NSPs, and no NSP activity is detectable in DPPI−/− mice.18,23 PMN counts in DPPI−/−.sB1−/− BM were significantly higher than in sB1−/− BM, suggesting that 1 or several NSPs contribute to the PMN survival defect. To examine the role of NSPs in this process, we crossed several NSP-deficient strains with sB1−/− mice. We found that NE.CG.sB1−/− mice had normal PMN numbers indicating that these NSPs play a key role in the defective phenotype of sB1−/− PMNs (Figure 2A). Furthermore, CG.sB1−/− mice showed normal PMN numbers whereas NE.sB1−/− mice retained the BM neutropenia phenotype indicating that CG, but not NE, plays a significant role in the death of sB1−/− PMNs (Figure 2A). In addition, the double-deficient NE.sB1−/− mice had significantly lower BM myelocyte numbers than sB1−/− mice while the myelocyte numbers in singly deficient NE−/− and sB1−/− BM were normal (Figure 2B). These results suggest that NE may promote myeloid cell proliferation, an activity that is revealed only when serpinB1 is absent. This complex interaction between sB1 and NE requires further investigation. On the other hand, B-cell and monocyte numbers and relative percentage in the BM were largely similar in all genotypes (supplemental Figure 2). Total numbers of blood leukocytes, erythrocytes, and platelets were normal in mice deficient in NSPs and/or serpinB1 (supplemental Figure 3). PMN numbers in blood were normal in sB1−/− mice in steady state and combined deficiency of NSPs did not significantly alter these numbers (Figure 2C). Taken together, our results indicate that serpinB1 likely sustains the survival of postmitotic PMNs through its interaction with CG.
PMN and myelocyte numbers in BM and blood of mice deficient in NSPs and serpinB1. Cell counts (top panels) and percentages (bottom panels) were determined by flow cytometry analysis for (A) BM PMNs, (B) myelocytes, and (C) blood PMNs in 8-week-old mice. Each circle represents the value for 1 mouse; horizontal bars indicate median value for each genotype (BM: N = 19-25; blood: N = 18-35). The dotted line across the graph shows the median for WT mice. For each subset, median values of double and triple knockout mice lacking sB1 and 1 or several NSPs (○) were analyzed by 1-way ANOVA followed by Dunnett’s comparison relative to reference column for sB1−/− mice (†P < .05; ††P < .01). ANOVA, analysis of variance.
PMN and myelocyte numbers in BM and blood of mice deficient in NSPs and serpinB1. Cell counts (top panels) and percentages (bottom panels) were determined by flow cytometry analysis for (A) BM PMNs, (B) myelocytes, and (C) blood PMNs in 8-week-old mice. Each circle represents the value for 1 mouse; horizontal bars indicate median value for each genotype (BM: N = 19-25; blood: N = 18-35). The dotted line across the graph shows the median for WT mice. For each subset, median values of double and triple knockout mice lacking sB1 and 1 or several NSPs (○) were analyzed by 1-way ANOVA followed by Dunnett’s comparison relative to reference column for sB1−/− mice (†P < .05; ††P < .01). ANOVA, analysis of variance.
CG-mediated PMN death is cell intrinsic
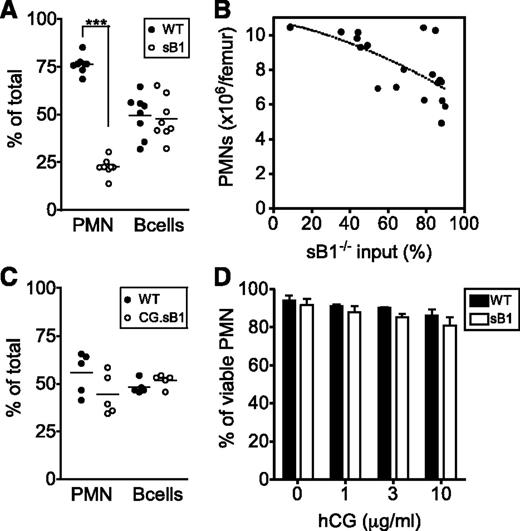
We next generated mixed BM chimeras to measure PMN competitive survival as well as to evaluate the potential bystander effects of CG or serpinB1 released in the BM environment on the overall PMN survival in vivo. First, lethally irradiated GFP+ mice were reconstituted with a 1:1 mixture of WT (CD45.1) and sB1−/− (CD45.2) BM cells and analyzed 8 to 10 weeks after reconstitution. Recipient GFP+ cells were always <3% and excluded from the analysis (supplemental Figure 4). B cells of WT and sB1−/− origin were found in similar proportions in the BM, reflecting similar input of hematopoietic stem cells from the 2 donor genotypes. In contrast, the proportion of WT PMNs was significantly greater than the proportion of sB1−/− PMNs, indicating a robust survival advantage of the WT PMNs and negligible bystander effect of WT cells on sB1−/− PMN survival (Figure 3A). Second, we transferred irradiated GFP+ mice with skewed ratios of WT and sB1−/− BM. We found that total PMN numbers in the BM negatively correlated with the percentage of sB1−/− input (reflected by the percentage of B cells of sB1−/− origin): when sB1−/− input was low, total PMN counts were similar to those of WT mice; conversely, when sB1−/− input was high, PMN numbers corresponded to those of sB1−/− mice (Figure 3B). Third, we found no survival defect of CG.sB1−/− PMNs compared with WT PMNs in vivo when reconstituted at equal ratios into GFP+ mice (Figure 3C). Finally, isolated BM PMNs were incubated in vitro with various concentrations of human CG. Extracellular CG failed to induce a significant increase in sB1−/− PMN death in vitro compared with WT PMNs (Figure 3D). Taken together, these results suggest that CG-mediated death in sB1−/− PMNs is cell intrinsic.
CG-mediated sB1−/− PMN death is cell intrinsic. (A) The relative percentage of cells from each donor is shown for PMNs and B cells from irradiated GFP+ mice 8 to 10 weeks after transfer of 1:1 mixture of WT (CD45.1) and sB1−/− (CD45.2) BM. Data points are indicated for each mouse and means were compared by paired the Student t test (***P < .001). (B) Total PMN numbers in BM of irradiated GFP+ mice are shown 8 to 10 weeks after transplant with varying amounts (1:1 or 1:4) of BM cells of WT and sB1−/− donors relative to the percentage of sB1−/− input. The dotted line indicates negative correlation between sB1−/− input and total PMN numbers. (C) The relative percentage of cells from each donor is shown for PMNs and B cells from irradiated GFP+ mice 8 to 10 weeks after transfer of 1:1 mixture of WT (CD45.1) and CG.sB1−/− (CD45.2) BM. Data points are indicated for each mouse and means were compared by the paired Student t test. (D) Survival of WT and sB1−/− PMNs in vitro in the presence of human CG for 3 hours in serum-free medium. Percentage of live cells (mean ± SD) of 2 to 5 independent experiments were compared by 2-way ANOVA with the Bonferroni posttest.
CG-mediated sB1−/− PMN death is cell intrinsic. (A) The relative percentage of cells from each donor is shown for PMNs and B cells from irradiated GFP+ mice 8 to 10 weeks after transfer of 1:1 mixture of WT (CD45.1) and sB1−/− (CD45.2) BM. Data points are indicated for each mouse and means were compared by paired the Student t test (***P < .001). (B) Total PMN numbers in BM of irradiated GFP+ mice are shown 8 to 10 weeks after transplant with varying amounts (1:1 or 1:4) of BM cells of WT and sB1−/− donors relative to the percentage of sB1−/− input. The dotted line indicates negative correlation between sB1−/− input and total PMN numbers. (C) The relative percentage of cells from each donor is shown for PMNs and B cells from irradiated GFP+ mice 8 to 10 weeks after transfer of 1:1 mixture of WT (CD45.1) and CG.sB1−/− (CD45.2) BM. Data points are indicated for each mouse and means were compared by the paired Student t test. (D) Survival of WT and sB1−/− PMNs in vitro in the presence of human CG for 3 hours in serum-free medium. Percentage of live cells (mean ± SD) of 2 to 5 independent experiments were compared by 2-way ANOVA with the Bonferroni posttest.
CG-mediated PMN death proceeds independent of caspase activity
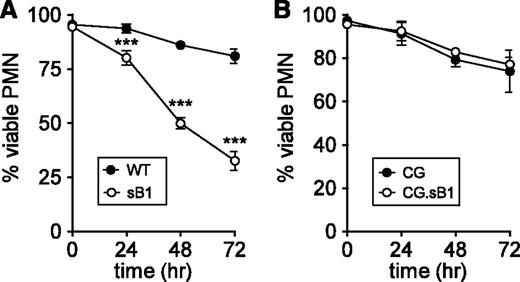
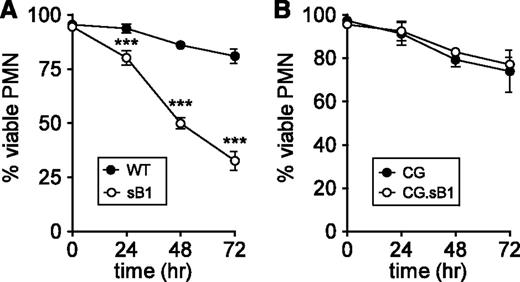
Neutrophil apoptosis whether triggered by intrinsic or extrinsic pathways culminates in the activation of effector caspases.6,7 We previously reported that sB1−/− PMNs isolated from the BM show increased spontaneous apoptosis and necrosis compared with WT PMNs after 16 hours ex vivo.19,20 To investigate whether increased sB1−/− PMN death was dependent on caspase activity, purified BM neutrophils were cultured in the presence of the pan-caspase inhibitor Q-VD-OPh.27 In the absence of Q-VD-OPh, we found no significant difference in the survival of WT and sB1−/− PMNs (survival of 31% at 24 hours; 5% at 48 hours; <1% at 72 hours in both genotypes). Caspase inhibition significantly extended the survival of both WT and sB1−/− PMNs compared with untreated PMNs. However, caspase inhibition was significantly less effective in increasing sB1−/− PMN survival compared with WT PMNs, with 32% vs 81% survival at 72 hours, respectively (Figure 4A). We then attempted to block CG activity using the cell-permeable CG inhibitor I.28 Although CG inhibitor I at 10 and 30µM led to a small but significant increase in survival of sB1−/− PMNs, we observed simultaneous toxicity on WT PMN survival (supplemental Figure 5), thus precluding further increase in inhibitor concentration. Because granule levels of CG are high in PMNs, sufficient concentrations of CG inhibitor may not have been reached to fully block CG activity. Therefore, we investigated whether genetic deletion of CG would affect sB1−/− PMN survival. We found that in the doubly deficient CG.sB1−/− mice the absence of CG completely rescued the sB1−/− PMN survival defect, even in the presence of Q-VD-OPh (Figure 4B). Taken together, our results unveil a CG-dependent but caspase-independent death pathway in PMNs that is controlled by serpinB1.
sB1−/− PMN death mediated by CG does not require caspase activity. Survival of isolated BM PMNs in the presence of 50µM pan-caspase inhibitor Q-VD-OPh was measured by flow cytometry (supplemental Figure 1) at indicated time points for (A) WT and sB1−/− PMNs (N = 3) and (B) CG−/− and CG−/−.sB1−/− PMNs (N = 2). Data are shown as mean ± SD and was analyzed by 2-way ANOVA followed by the Bonferroni posttest (***P < .001).
sB1−/− PMN death mediated by CG does not require caspase activity. Survival of isolated BM PMNs in the presence of 50µM pan-caspase inhibitor Q-VD-OPh was measured by flow cytometry (supplemental Figure 1) at indicated time points for (A) WT and sB1−/− PMNs (N = 3) and (B) CG−/− and CG−/−.sB1−/− PMNs (N = 2). Data are shown as mean ± SD and was analyzed by 2-way ANOVA followed by the Bonferroni posttest (***P < .001).
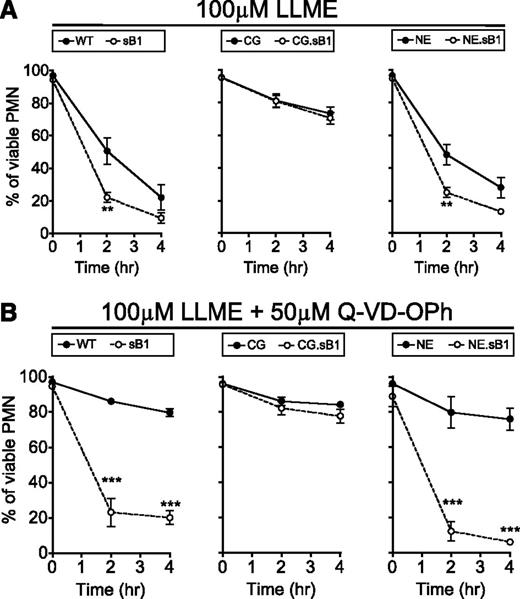
Granule membrane permeabilization induces CG-mediated death in PMNs
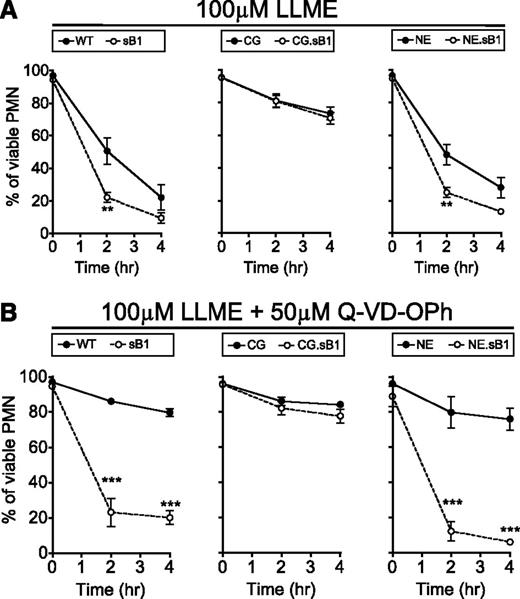
To test whether granule disruption contributes to the serpinB1-regulated CG-dependent cell death, BM cells were treated with the lysosomotropic agent LLME. LLME accumulates in lysosomes where the acyl transferase activity of DPPI generates hydrophobic (Leu-Leu)n-OMe polymers that induce lysosomal membrane permeabilization (LMP) and cytotoxicity in granule-bearing cells such as cytotoxic T lymphocytes, NK cells, and myeloid cells.29,30 Although the cytotoxic effect of LLME and other methyl ester derivatives on PMNs has long been described, the contribution of PMN granule serine proteases to this process following LMP is unknown. First, we confirmed that DPPI deficiency was completely protective against LLME-induced cell death in PMNs (data not shown). Then, we found that LLME significantly decreased the survival of WT PMNs; however, this effect was even more pronounced in sB1−/− PMNs compared with WT PMNs (Figure 5A left panel). In contrast, survival of CG−/− and CG.sB1−/− PMNs was identical to each other but significantly greater than that of WT PMNs (P < .001 after 2 and 4 hours) (Figure 5A middle panel). Finally, NE.sB1−/− PMNs were more sensitive to LLME compared with NE−/− PMNs but deficiency in NE alone had no further effect on PMN survival in the presence of LLME when compared with WT PMN survival (Figure 5A right panel) and the reduced survival of NE.sB1−/− PMNs is similar to that of sB1−/− PMNs, reflecting mainly the absence of serpinB1 (Figure 5A right panel). In the presence of caspase inhibitor Q-VD-OPh, a similar pattern was observed between the genotypes (Figure 5B). Furthermore, Q-VD-OPh failed to improve the survival of sB1−/− and NE.sB1−/− PMNs, whereas the survival of WT and NE−/− PMNs was significantly increased. In mice lacking CG, caspase inhibition had a modest effect on PMN survival (Figure 5B). These results suggest that the cytoplasmic release of CG upon LMP likely initiates cell death upstream and independent of caspases and this effect is especially evident when CG activity is unopposed in the absence of serpinB1.
LMP induces CG-mediated death in PMNs. BM cells of indicated genotypes were incubated with (A) 100μM LLME only or (B) 100μM LLME and 50μM Q-VD-OPh. Percentage of surviving PMNs was determined by flow cytometry. Data are shown as mean ± SEM (N = 3-5) and was analyzed by 2-way ANOVA followed by the Bonferroni posttest (*P < .05, **P < .01, ***P < .001).
LMP induces CG-mediated death in PMNs. BM cells of indicated genotypes were incubated with (A) 100μM LLME only or (B) 100μM LLME and 50μM Q-VD-OPh. Percentage of surviving PMNs was determined by flow cytometry. Data are shown as mean ± SEM (N = 3-5) and was analyzed by 2-way ANOVA followed by the Bonferroni posttest (*P < .05, **P < .01, ***P < .001).
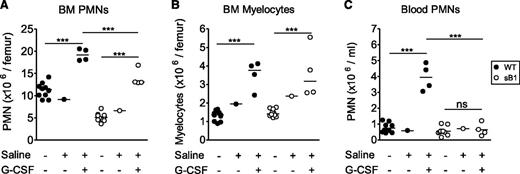
G-CSF therapy increases sB1−/− PMN numbers via enhanced granulopoiesis
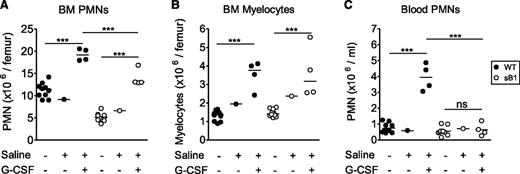
G-CSF therapy is an effective long-term treatment in many cases of severe congenital neutropenia and it is also used to prevent chemotherapy-induced febrile neutropenia by enhancing PMN production. In addition, G-CSF delays neutrophil apoptosis by differentially regulating proapoptotic and antiapoptotic factors.10 To test whether G-CSF could rescue sB1−/− PMN survival defect, WT and sB1−/− mice were treated with therapeutic doses of G-CSF or saline for 5 days and BM and blood PMNs were analyzed 24 hours after the last injection. Total counts of myelocytes and PMNs were significantly increased in the BM of treated mice compared with their respective untreated genotype controls (Figure 6A-B). The increase in myelocyte numbers was identical in G-CSF–treated WT and sB1−/− mice, indicating that G-CSF–induced granulopoiesis proceeds normally in sB1−/− myeloid progenitors (Figure 6B). However, PMN counts of treated sB1−/− mice remained significantly lower than that of WT mice (Figure 6A). More strikingly, blood PMN numbers were increased only in G-CSF–treated WT but not sB1−/−-treated mice. In G-CSF–treated sB1−/− mice, blood PMN numbers were identical to the baseline numbers for sB1−/− mice (Figure 6C; supplemental Figure 6). These results indicate that sB1−/− PMN precursor proliferation is normal in response to G-CSF therapy leading to expansion of the BM reserve to some extent. However, the lower number of PMNs in the BM and, particularly, the lack of a sustained blood neutrophilia suggest that G-CSF does not fully rescue the survival defect of mature sB1−/− PMNs.
In vivo G-CSF therapy increases PMN numbers in BM of sB1−/− mice. (A) BM PMN, (B) myelocyte, and (C) blood PMN numbers were determined 24 hours after recombinant murine G-CSF therapy for 5 consecutive days. Cell numbers of individual WT (●) and sB1−/− female mice (○), respectively. Untreated females (Figure 2 subset) were similar to saline control females and were compared with the G-CSF–treated mice. Statistical analysis was performed using 2-way ANOVA with Bonferroni posttest. (**P < .01; ***P < .001).
In vivo G-CSF therapy increases PMN numbers in BM of sB1−/− mice. (A) BM PMN, (B) myelocyte, and (C) blood PMN numbers were determined 24 hours after recombinant murine G-CSF therapy for 5 consecutive days. Cell numbers of individual WT (●) and sB1−/− female mice (○), respectively. Untreated females (Figure 2 subset) were similar to saline control females and were compared with the G-CSF–treated mice. Statistical analysis was performed using 2-way ANOVA with Bonferroni posttest. (**P < .01; ***P < .001).
Discussion
SerpinB1 is a member of the clade B serpins, a subfamily composed of leaderless proteins with nucleocytoplasmic localization. Clade B serpins are often expressed in cells that also carry target proteases, which led to the hypothesis that intracellular serpins protect against misdirected granule proteases and/or protect bystander cells from released proteases.31 We previously reported that deficiency in serpinB1 is associated with reduced PMN survival in the BM and at inflammatory sites.19,20 The evidence presented here demonstrates that the cytoprotective function of serpinB1 in PMNs is based on the inhibition of granule protease CG. Deficiency in CG was sufficient to rescue the defect of sB1−/− mice as illustrated by normal PMN counts in the BM of double knockout CG.sB1−/− mice. We also showed that the protease-serpin interaction occurred within PMNs. Indeed, WT PMNs had a greater survival over sB1−/− PMNs in mixed BM chimera, whereas the survival of CG.sB1−/− PMNs was similar to WT PMNs after BM transfer. SerpinB1 is an ancestral clade B serpin with a conserved specificity determining reactive center loop in all vertebrates.32 Furthermore, human and mouse serpinB1 have the same specificity for chymotrypsin-like and elastase-like serine proteases.21,22 Likewise, human and mouse CG have identical substrate specificities and the phenotype of CG−/− murine PMN can be rescued by human CG.33 Therefore, it is highly likely that the antagonistic functions of CG and serpinB1 in cellular homeostasis observed in mice can be extended to other species.
Extracellular CG was previously reported to promote detachment-induced apoptosis (anoikis) in human and mouse cardiomyocytes.34 This activity is mediated through the shedding and transactivation of epidermal growth factor receptor and downregulation of focal adhesion signaling.35,36 In our study, exogenous human CG also induced PMN death in vitro but these effects were not enhanced in sB1−/− PMNs and the neutropenia associated with serpinB1 deficiency was principally cell intrinsic. How intracellular CG induces PMN death remains to be fully investigated. However, our studies provide some indications on the potential pathways. Like other NSPs, the expression of CG is transcriptionally restricted to the promyelocyte stage during PMN development and NSPs are then stored in active form in primary azurophil granules.37 Because serpinB1 is equally efficient at inhibiting NE, CG, and PR3, it was surprising that deletion of CG alone was sufficient to achieve a complete reversal of the PMN survival defect in CG.sB1−/− mice. A possible explanation would be that CG gains access to targets more readily than other granule proteases. There is evidence that binding to serglycin proteoglycans differs between NE and CG resulting in altered sorting of NE but not CG into granules of serglycin-deficient PMNs.38 Different interactions with granule matrix may thus contribute to differential release of CG from the granules compared with other NSPs. However, because sB1−/− PMNs have similar levels of CG and NE as WT PMNs20 and because LLME-induced granule permeabilization likely releases all granule contents equally, we favor an alternative interpretation where CG specifically targets essential cellular components that are not cleaved by the other serpinB1-inhibitable granule proteases. Upon granule permeabilization, we found that CG can induce cell death upstream of caspases as well as independent of caspases. CG was previously shown to activate caspase-7 in vitro and it functions at neutral pH, which is consistent with a physiological role in the nucleocytoplasmic environment.39 Cell death induced by lysosomal/granule membrane permeabilization has previously been linked to cysteine cathepsins in other cell types. However, these proteases appear to depend on caspase activation to trigger apoptosis and they function poorly at neutral pH, questioning their potential role as regulators of cell death.40 In contrast, CG-mediated cell death is not completely blocked by caspase inhibition, which is a property reminiscent of granzymes in cytotoxic T cells.41 In fact, CG is phylogenetically most closely related to serine proteases granzyme B and H.42 Granzymes have numerous nuclear, mitochondrial, and cytoplasmic target proteins leading to cell death41 and we anticipate that this may also be the case for CG.
Whether intracellular release of CG induces death in other cell types remains to be investigated. CG and CG-inhibitory serpins, such as serpinB1 and serpinB6, are also expressed in monocytes, dendritic cells, and mast cells and the protease-serpin balance in these cells may be altered in specific stress conditions leading to cell death. Interestingly, human gzmH has a chymotrypsin-like activity similar to CG and was recently shown to be inhibited by serpinB1 in NK cells.43 However, mice have many homologs of gzmB and gzmH that have diverged from their human counterparts.44 Therefore, it is yet unclear whether mouse serpinB1 would also inhibit 1 or several mouse granzymes. In cancer cells, tumor necrosis factor-α–mediated apoptosis has been associated with induction of CG expression and apoptosis was partly prevented by transfection with human squamous cell carcinoma antigen 2 (SCCA2) (serpinB4), a serum marker for advanced squamous cell carcinoma tumors. SCCA2 inhibits CG, although with lower second order rate constants than serpinB1 or serpinB6.22,45 These findings, while only associative, are reminiscent of the upregulation of serpinB9 in tumor cells as a protective strategy against CTL death mediated by gzmB and perforin.46,-48 Whether serpin’s inhibition of CG activity in tumors contributes to antiapoptotic resistance of cancer cells should be interesting to investigate.
G-CSF therapy is successfully used to treat most congenital and acquired neutropenia through increased granulopoiesis, mobilization from the BM, and increased survival of PMNs. Prosurvival effects of G-CSF include the upregulation of antiapoptotic Bcl-2 family members, which act upstream of the mitochondria and the activation of effector caspases. In sB1−/− mice, G-CSF levels in serum are fourfold higher than in WT mice in steady state and this is accompanied by an upregulation of the antiapoptotic Bcl-2 family member Mcl-1 in sB1−/− PMNs.19 Here, G-CSF therapy significantly increased granulopoiesis in both WT and sB1−/− mice. However, the PMN numbers in treated sB1−/− BM and blood were significantly lower than those of treated WT mice, indicating only a partial rescue of the survival defect. This is consistent with our findings that CG-mediated death can proceed independent of caspases and can thus bypass antiapoptotic effects mediated by G-CSF.
CG has largely been studied in association with antimicrobial and inflammatory functions due to its presence in PMNs.12,-14,49 In this context, we have previously shown that serpinB1 contributes to prevent increased mortality and morbidity associated with production of inflammatory cytokines upon infection with Pseudomonas aeruginosa and influenza A virus.20,50 In this study, we demonstrate that serpinB1 inhibition of the primary granule protease CG in PMNs is essential for PMN survival and this ultimately regulates PMN numbers in vivo. Our findings also extend the roles of CG from antimicrobial and immunoregulatory functions to a novel role in inducing cell death.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr. Eileen Remold-O’Donnell for insightful discussions and for anti-serpinB1 antibody. The authors acknowledge Isabelle Wymann, Katrin Bissegger, and Svetlozar Tsonev for animal husbandry. The authors also thank Elisabeth Frei and Stephan Hirschi for excellent technical assistance.
This work was supported by grants from the Swiss National Fonds (310030-127464; C.B.), the EU/FP7 Marie Curie International Reintegration grant (C.B.), the Novartis Foundation (C.B.), the Berne University Research Foundation (C.B.), and partially by National Institutes of Health grant AI049261 (C.T.N.P.).
Authorship
Contribution: M.B. performed research, analyzed and interpreted data, performed statistical analysis, and drafted the manuscript; C.T.N.P. contributed vital reagents and contributed to the writing of the manuscript; and C.B. designed research, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Charaf Benarafa, Theodor Kocher Institute, Freiestrasse 1, 3006 Bern, Switzerland; e-mail: charaf.benarafa@tki.unibe.ch.