Key Points
Stromal OPN anchors leukemia cells in prodormancy BM niches.
Inhibiting this interaction leads dormant cells to proliferate, sensitizing them to chemotherapy.
Abstract
Malignant cells may evade death from cytotoxic agents if they are in a dormant state. The host microenvironment plays important roles in cancer progression, but how niches might control cancer cell dormancy is little understood. Here we show that osteopontin (OPN), an extracellular matrix molecule secreted by osteoblasts, can function to anchor leukemic blasts in anatomic locations supporting tumor dormancy. We demonstrate that acute lymphoblastic leukemia (ALL) cells specifically adhere to OPN in vitro and secrete OPN when localized to the endosteal niche in vivo. Using intravital microscopy to perform imaging studies of the calvarial bone marrow (BM) of xenografted mice, we show that OPN is highly expressed adjacent to dormant tumor cells within the marrow. Inhibition of the OPN-signaling axis significantly increases the leukemic cell Ki-67 proliferative index and leads to a twofold increase in tumor burden in treated mice. Moreover, using cell-cycle–dependent Ara-C chemotherapy to produce minimal residual disease (MRD) in leukemic mice, we show that OPN neutralization synergizes with Ara-C to reduce detectable BM MRD. Taken together, these data suggest that ALL interacts with extracellular OPN within the malignant BM, and that this interaction induces cell cycle exit in leukemic blasts, protecting them from cytotoxic chemotherapy.
Introduction
Acute lymphoblastic leukemia (ALL) in adults initially responds well to induction chemotherapy, with greater than 80% of patients attaining a complete remission (CR). Unfortunately, most initial CRs are short lived, and overall survival rate is only 30% to 40% for adults who are diagnosed before age 60 years.1 Although outcomes in the pediatric population are better, a significant number of patients still experience relapsed or refractory disease.2 Relapses in both populations are believed to be the outgrowth of minimal residual disease (MRD) that is not completely eliminated by chemotherapy. Indeed, it has been demonstrated that patients with the lowest levels of detectable MRD at CR have the best prognosis and least likelihood of relapse.2 Strategies to overcome resistance and reduce MRD may therefore have the potential to increase overall survival duration.
Antiapoptotic signals from the host tissue microenvironment are increasingly recognized as important mechanisms of malignant cell survival against chemotherapy. Our previous work using the Nalm-6 model of ALL has shown that the bone marrow (BM) microenvironment plays a critical role in disease spread and in the dysregulation of normal hematopoiesis that occurs during leukemic growth.3,4 To metastasize and outcompete native BM cells, leukemic cells co-opt normal signaling mechanisms within hematopoietic stem cell (HSC) niches. At least 2 distinct HSC niches, one perivascular and one endosteal (or bony), exist in the BM.5 In the basal state, interactions between HSCs and specific cells and molecules within these niches modulate HSC transit and self-renewal.6,7 Although the anatomic and molecular distinctions between these niches are controversial, evidence suggests that the endosteal niche maintains a population of quiescent HSCs with high reconstitution potential, whereas the perivascular niche harbors more activated HSCs.8,9
Stromal cell-derived factors, including cytokines and extracellular matrix proteins (ECMs), play a significant role in niche regulation of the benign HSC population. One important protein expressed by endosteal osteoblasts and implicated in HSC migration and self-renewal is osteopontin (OPN).5,10-14 Extracellular OPN is a multidomain-secreted glycoprotein that can function as a soluble cytokine or chemokine as well as an adhesive component of the ECM. It is bound by multiple cell surface receptors including CD44, αVβ3, (α4, α9, α5)β1, and α4β7 integrins.15-18 OPN expression is necessary for proper trans-marrow migration and lodgment of HSCs at the endosteal surface, a process mediated by α4β1 and α9β1 integrins.19,20 Furthermore, HSC engagement with OPN constrains HSC pool size, and there is an increased number of cycling progenitors in the marrow and peripheral blood of OPN−/− mice.20,21
The role of OPN has been extensively investigated in solid malignancies, with multiple studies showing that OPN positively regulates tumor cell proliferation and metastasis.16,22-25 Despite the well-defined functions for OPN in solid tumor and benign HSC biology, scarce data exist regarding OPN and leukemia. A limited number of studies of patients with acute myeloid leukemia (AML) demonstrate that increased BM transcript and serum OPN levels correlate with decreased overall survival duration, suggesting a function for OPN in progression of leukemia.26,27 Using murine xenograft models in combination with highly sensitive in vivo microscopic examinations of the calvarial BM, we set out to investigate the role of OPN in ALL, and in particular, the persistence of MRD after chemotherapy.
Materials and methods
Cell lines and culture
The green fluorescent protein (GFP)–expressing clone of Nalm-6 was generated and cultured as described previously.28
In vitro adhesion assay
Recombinant human OPN was thrombin-cleaved and plates coated at 20 μg/mL. Nalm-6-GFP or primary ALL cells were resuspended in adhesion buffer (1 μM of PMA and 2 mM of MnCl2) and were allowed to adhere for 2 hours.
In vivo OPN neutralization
Severe combined immunodeficiency (SCID) mice were injected via t.v. with 1 mg/kg of anti-mouse and 2 mg/kg of anti-human OPN or isotype control (day 0). At 24 hours after the injection, mice were engrafted with Nalm-6 GFP. Mice were re-treated on day 7 and day 14 and were euthanized on day 21.
Intravital microscopic imaging
Imaging was performed as described previously.28
In vivo Ara-C MRD studies
Mice were engrafted with DiR-labeled Nalm-6 GFP on day 0. On day 10, mice were injected via t.v. with 1 mg/kg of anti-mouse and 2 mg/kg of anti-human OPN or isotype control. Mice were treated with Ara-C (500 mg/m2 IP) on days 10 to 14 and days 24 to 28; imaging was performed on day 35.
All animal experiments were performed following guidelines approved by the Institutional Animal Care and Use Committee at the University of Chicago–protocol #71675.
Detailed methods are provided in supplemental Materials.
Results
Human ALL blasts highly express OPN when localized to the endosteal niche
To the best of our knowledge, OPN expression in ALL BM has not been described previously. Therefore, we performed OPN immunohistochemistry (IHC) studies on diagnostic BM biopsies from 6 patients with precursor-B ALL. BM biopsies from patients with lymphoma but with no pathological evidence of BM infiltration served as controls. OPN expression was detected in leukemia cells from all 6 patients with ALL, suggesting that OPN upregulation is a common event in ALL. Furthermore, as shown in Figure 1A, a gradient of OPN expression was detected in several leukemic marrows, with OPN-expressing blasts localized near trabecular bone surfaces in areas of high endosteal matrix OPN deposition. This result suggests that OPN levels are elevated in the endosteal niche in ALL, and that this niche may specifically recruit OPN-expressing blasts or expresses factors that induce OPN expression in adjacent lymphoblasts.
Human ALL blasts express high levels of OPN when localized to the endosteal niche, express OPN receptors, and adhere to tcOPN in vitro. (A) Representative micrographs of OPN IHC analysis of BM biopsies from control patients and patients with pre-B ALL. Leukemic lymphoblasts were found to express OPN in 6 of 6 ALL cases examined, and representative images are shown of 3 ALL cases and 1 control case. A gradient of OPN expression was detected in the leukemic marrows, with high OPN-expressing blasts localized near the trabecular bone surfaces. (B) Flow cytometry analysis of 6 primary ALL samples demonstrating variable expression of known OPN receptors. (C) In vitro adhesion assay to tcOPN of the 6 primary ALL samples from (B) demonstrating significant adhesion to OPN of blasts isolated from patients 1 to 3. (D) Intravital confocal imaging of the calvarial marrow of mice engrafted with DiR-labeled primary ALL cells (ALL #3 from Figure 1B-C). Mice were engrafted 42 days before imaging. Dormant DiR-retaining cells are represented in green; extracellular OPN is represented in red. The far right panel is a magnification of panel 3. A significant portion of dye-retaining blasts was found co-localized with or adjacent to areas of high OPN expression (n = 3).
Human ALL blasts express high levels of OPN when localized to the endosteal niche, express OPN receptors, and adhere to tcOPN in vitro. (A) Representative micrographs of OPN IHC analysis of BM biopsies from control patients and patients with pre-B ALL. Leukemic lymphoblasts were found to express OPN in 6 of 6 ALL cases examined, and representative images are shown of 3 ALL cases and 1 control case. A gradient of OPN expression was detected in the leukemic marrows, with high OPN-expressing blasts localized near the trabecular bone surfaces. (B) Flow cytometry analysis of 6 primary ALL samples demonstrating variable expression of known OPN receptors. (C) In vitro adhesion assay to tcOPN of the 6 primary ALL samples from (B) demonstrating significant adhesion to OPN of blasts isolated from patients 1 to 3. (D) Intravital confocal imaging of the calvarial marrow of mice engrafted with DiR-labeled primary ALL cells (ALL #3 from Figure 1B-C). Mice were engrafted 42 days before imaging. Dormant DiR-retaining cells are represented in green; extracellular OPN is represented in red. The far right panel is a magnification of panel 3. A significant portion of dye-retaining blasts was found co-localized with or adjacent to areas of high OPN expression (n = 3).
Primary ALL cells express OPN receptors and adhere to thrombin-cleaved OPN in vitro
To our knowledge, whether ALL cells can interact with extracellular OPN has not been tested previously. Using flow cytometry, we analyzed a set of primary human BM B-ALL cells for surface expression of known OPN receptors.18 As shown in Figure 1B, hALL cells express multiple OPN cell surface receptors, including the α4β1, α5β1, and α9β1 integrins and CD44. α4 and α5 integrins appeared to have the highest cell surface expression, whereas α9β1 integrin and CD44 expression were relatively weak.
To test whether ALL cells can functionally interact with OPN, we performed in vitro adhesion assays. Three of six primary patient samples demonstrated significant adhesion of blasts to thrombin-cleaved OPN, the predominant form of the protein in the BM microenvironment (Figure 1C). Interestingly, two thirds of the OPN nonadherent samples were from patients presenting with high peripheral white blood cell counts.
Dormant primary leukemia cells localize to areas of high OPN expression in vivo
It has been reported that BM OPN induces HSC quiescence by serving as a chemotactic factor that recruits and anchors HSCs to the prodormancy endosteal microenvironment.20 Given our observation that OPN expression was elevated in endosteal regions of human ALL BM, we hypothesized that OPN might similarly regulate leukemic blast dormancy in these niches. To test this hypothesis, we first investigated whether quiescent leukemic cells preferentially co-localize with areas of high OPN expression in the tumor microenvironment. BM blasts from a patient with pre-B ALL were purified by FACS and labeled with a fluorescent membrane dye that is retained in dormant, noncycling cells but is diluted to undetectable levels in proliferating cells.29 Cells were then engrafted via tail vein into NSG mice.
To determine the 3-dimensional relationship between leukemic cells and the specific anatomic locations in the marrow where extracellular OPN is expressed, we used intravital confocal microscopy studies to perform imaging of the calvarial BM of mice. This approach allows us to detect soluble OPN, which is typically lost during the processing of tissue sections. Leukemic mice were injected with murine/human cross-reactive fluorescence-labeled anti-OPN antibodies approximately 20 hours before imaging on post-engraftment day 42. As shown in Figure 1D, the extracellular OPN signal is detected in the NSG mice but not in the OPN null mice or NSG mice injected with AF647-labeled isotype control antibody (supplemental Figure 1), confirming the specificity of the antibody. Imaging of the entire calvarial BM revealed that the majority of dye-retaining cells were present in regions of high OPN expression, and that a large number of these cells directly co-localized with OPN (Figure 1D). Dye-retaining cells were also noted to be intensely bright, suggesting that they had undergone very few divisions in vivo (supplemental Figure 2). These data suggested the possibility of a functional connection between dormant leukemic cells and extracellular OPN.
Nalm-6 pre-B ALL cells express multiple OPN receptors and adhere to thrombin-cleaved OPN in vitro via α4β1 integrins
To explore the functional role of OPN in leukemogenesis, we used the well-described Nalm-6 xenograft SCID mouse model. This model generates disease that mirrors human ALL, with metastasis occurring in the BM, spleen, and central nervous system.3,30 Importantly for proliferation studies, engraftment rates are virtually 100%, and clinical disease develops within a highly reproducible time frame. The disease is also predictably responsive to Ara-C chemotherapy in vivo. This model presents the additional advantage to microenvironment investigations in that the SCID strain does not exhibit the multiple myeloid and cytokine abnormalities present in NOD-SCID or NSG mouse BM.31 Moreover, reliable engraftment of Nalm-6 occurs without preirradiation or other BM conditioning that induces marked changes in cell adhesion molecule and cytokine profiles. Finally, proliferative cells that have diluted their membrane label can be visualized in vivo through constitutive expression of virally transduced fluorescent protein constructs.
To validate Nalm-6 cells as a model for our investigations, we first analyzed their expression of OPN cell surface receptors by flow cytometry. Similar to primary ALL, Nalm-6 express the α4β1, α5β1, and α9β1 integrins, although they do not express CD44 (Figure 2A). Neither Nalm-6 nor primary ALL cells express the αv or β7 integrins. To test whether Nalm-6 cells can functionally interact with OPN, we performed in vitro adhesion assays to OPN using fibronectin, an α4β1 ligand, as a positive control. Nalm-6 strongly adhered to thrombin-cleaved OPN but showed weak adhesion to full-length OPN (Figure 2B). Pretreatment with an OPN-neutralizing antibody specifically inhibited Nalm-6 adhesion to thrombin-cleaved OPN but not to fibronectin (Figure 2C).
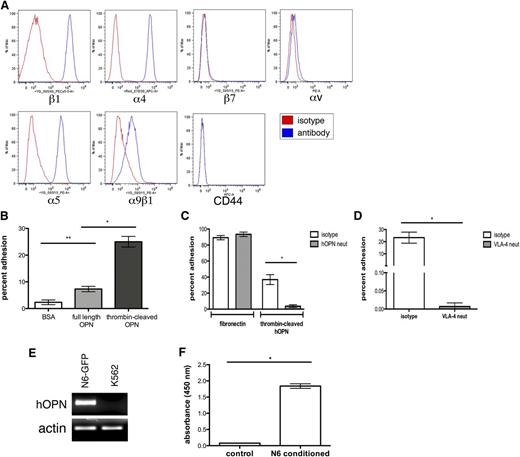
Nalm-6 GFP secrete OPN and express functional OPN receptors. (A) Flow cytometry analysis of Nalm-6 GFP reveals cell surface expression of the OPN receptors β1, α4, α5, and α9β1, but no expression of the β7, αV or CD44 receptors. (B) Nalm-6 GFP adhere strongly to thrombin-cleaved OPN (tcOPN) in vitro, but weakly to full-length hOPN, *P = .015, **P = .035. (C) Nalm-6 GFP adhesion to the α4β1 ligand fibronectin in vitro is unaffected by OPN neutralization, whereas Nalm-6 GFP adhesion to tcOPN is specifically inhibited by OPN-neutralizing antibodies, *P < .001. (D) Anti-VLA-4 receptor antibodies inhibit almost all Nalm-6 GFP adhesion to tcOPN in vitro, demonstrating that VLA-4 is the major integrin receptor responsible for Nalm-6 tcOPN adhesion, *P < .0001. (E) RT-PCR analysis shows human OPN (hOPN) mRNA transcripts in Nalm-6 GFP. K562, an AML cell line, was used as a negative control. (F) hOPN ELISA analysis of conditioned media from Nalm-6 GFP demonstrates their secretion of OPN, *P < .001.
Nalm-6 GFP secrete OPN and express functional OPN receptors. (A) Flow cytometry analysis of Nalm-6 GFP reveals cell surface expression of the OPN receptors β1, α4, α5, and α9β1, but no expression of the β7, αV or CD44 receptors. (B) Nalm-6 GFP adhere strongly to thrombin-cleaved OPN (tcOPN) in vitro, but weakly to full-length hOPN, *P = .015, **P = .035. (C) Nalm-6 GFP adhesion to the α4β1 ligand fibronectin in vitro is unaffected by OPN neutralization, whereas Nalm-6 GFP adhesion to tcOPN is specifically inhibited by OPN-neutralizing antibodies, *P < .001. (D) Anti-VLA-4 receptor antibodies inhibit almost all Nalm-6 GFP adhesion to tcOPN in vitro, demonstrating that VLA-4 is the major integrin receptor responsible for Nalm-6 tcOPN adhesion, *P < .0001. (E) RT-PCR analysis shows human OPN (hOPN) mRNA transcripts in Nalm-6 GFP. K562, an AML cell line, was used as a negative control. (F) hOPN ELISA analysis of conditioned media from Nalm-6 GFP demonstrates their secretion of OPN, *P < .001.
It has recently been reported that thrombin cleavage of OPN exposes a cryptic integrin-binding epitope on the N-terminal cleavage fragment that is recognized by α4β1 and α9β1.19,20 To further define the receptors involved in OPN adhesion, Nalm-6 cells were pretreated with α4β1-neutralizing antibodies. Blockade of very late activation antigen-4 (VLA-4; α4β1) almost entirely abrogated adhesion to thrombin-cleaved OPN, suggesting that Nalm-6 binding to OPN is mediated principally by VLA-4 (Figure 2D).
Our data from IHC staining of human ALL BM biopsies demonstrated that lymphoblasts, like BM stromal cells, can express OPN. Investigations of solid tumor and myeloma models have shown that these malignant cells both produce and secrete OPN16,17,27 Therefore, we tested whether Nalm-6 cells synthesize and secrete OPN in vitro. As seen in Figure 2, Nalm-6 cells express OPN messenger RNA (mRNA) transcripts (Figure 2E) and secrete OPN protein into culture media (Figure 2F). Multiple additional ALL cell lines and primary ALL cells were also demonstrated to express OPN mRNA transcripts (supplemental Figure 3). These results suggested that leukemia itself could be a source of ECM OPN in the BM microenvironment in vivo.
The leukemia BM microenvironment contains increased levels of both leukemia- and host-derived OPN
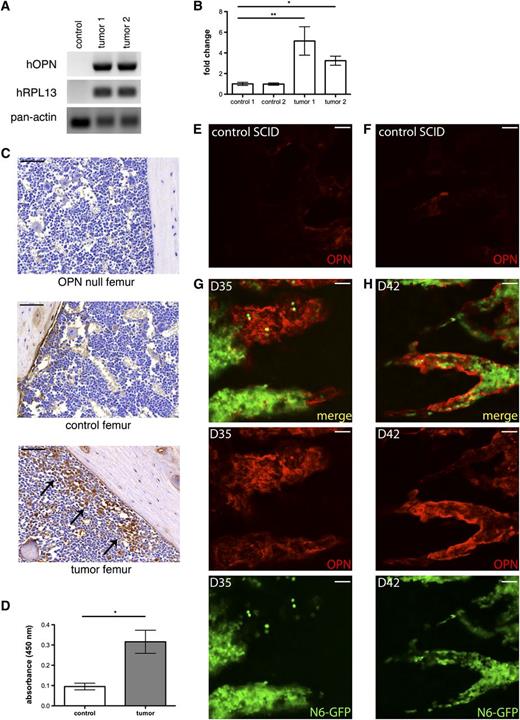
Having shown that Nalm-6 cells express OPN in vitro, we next tested whether Nalm-6 express and secrete OPN in vivo and whether leukemic engraftment affects host OPN production. Using primers specific to human OPN mRNA, we performed real-time polymerase chain reaction (PCR) analysis on control nonengrafted mouse and Nalm-6 leukemic mouse BM. As shown in Figure 3A, human OPN transcripts are readily detected in the leukemic BM, demonstrating that Nalm-6 continue to express OPN while resident within the marrow. In addition, IHC analysis demonstrates blast-specific OPN expression in the tumor microenvironment (Figure 3C). Interestingly, similar to our findings in primary ALL BM, this expression is highest in leukemic cells that are near the OPN-rich endosteum, suggesting that Nalm-6 OPN production may itself be regulated by the niche.
The tumor microenvironment contains increased amounts of both tumor and host OPN. (A) RT-PCR shows hOPN mRNA transcripts in tumor-engrafted mice. Human RPL13A and pan-species β actin housekeeping genes serve as loading controls. (B) Quantitative PCR analysis for murine OPN transcripts shows increased levels in Nalm-6 GFP mice, *P = .001, **P = .007. (C) IHC analysis of OPN expression in femurs from an OPN knockout mouse, a control nonengrafted SCID mouse and a SCID mouse with diffuse Nalm-6 GFP leukemic BM infiltration. OPN is detected at the endosteal surface in control mice. In tumor mice, OPN+ leukemic cells are localized near the endosteal niche (arrows). (D) ELISA analysis demonstrates hOPN in BM serum from leukemic mice; control n = 2, tumor n = 6, *P < .0001. (E-F) Intravital confocal microscopic examination of extracellular OPN (red) expression in control SCID mouse calvarial BM. (G-H) Representative images of intravital confocal microscopic study of extracellular OPN expression in the calvarial BM of Nalm-6 GFP (green)–engrafted SCID mice at D35 (G) and D42 (H). Control images E and F correspond to the regions imaged in panel G and H, respectively. These results indicate that extracellular OPN levels are most intense where tumor burden abuts bony margins. Scale bars equal 50 µm.
The tumor microenvironment contains increased amounts of both tumor and host OPN. (A) RT-PCR shows hOPN mRNA transcripts in tumor-engrafted mice. Human RPL13A and pan-species β actin housekeeping genes serve as loading controls. (B) Quantitative PCR analysis for murine OPN transcripts shows increased levels in Nalm-6 GFP mice, *P = .001, **P = .007. (C) IHC analysis of OPN expression in femurs from an OPN knockout mouse, a control nonengrafted SCID mouse and a SCID mouse with diffuse Nalm-6 GFP leukemic BM infiltration. OPN is detected at the endosteal surface in control mice. In tumor mice, OPN+ leukemic cells are localized near the endosteal niche (arrows). (D) ELISA analysis demonstrates hOPN in BM serum from leukemic mice; control n = 2, tumor n = 6, *P < .0001. (E-F) Intravital confocal microscopic examination of extracellular OPN (red) expression in control SCID mouse calvarial BM. (G-H) Representative images of intravital confocal microscopic study of extracellular OPN expression in the calvarial BM of Nalm-6 GFP (green)–engrafted SCID mice at D35 (G) and D42 (H). Control images E and F correspond to the regions imaged in panel G and H, respectively. These results indicate that extracellular OPN levels are most intense where tumor burden abuts bony margins. Scale bars equal 50 µm.
To determine whether OPN-producing leukemic cells secrete OPN into the tumor microenvironment, we performed human-specific OPN enzyme-linked immunosorbent assay (ELISA) analysis on BM serum from leukemic vs control mice. Human OPN was detected in the BM sera of tumor-engrafted mice (Figure 3D and supplemental Figure 4), confirming that leukemic blasts can secrete OPN into the BM microenvironment in vivo. To establish whether leukemic engraftment affects host stromal production of OPN, we next performed quantitative PCR analysis of murine OPN mRNA levels in leukemic and control mouse femurs. At 40 days post Nalm-6 GFP engraftment, murine OPN transcripts were significantly increased in leukemic BM (Figure 3B). Taken together, our data suggest that increased OPN production in the tumor microenvironment is derived from both invading tumor and reactive host stroma. In addition, the enriched expression of OPN by blasts localized to the endosteum suggests that tumor-stroma cross-talk within this niche provides a positive feedback loop for OPN expression.
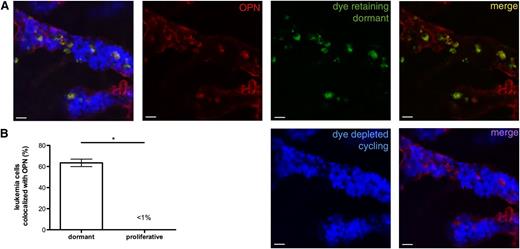
Dormant Nalm-6 GFP localize to areas of high OPN expression. (A) Nalm-6 GFP were labeled with DiR (green), a lipophilic membrane dye that is retained by noncycling cells. At 42 days after engraftment, mice were injected with AF647-labeled isotype control or anti-OPN (red) antibodies to visualize extracellular OPN. The calvarial BM was imaged using intravital confocal microscopic study ∼20 hours after antibody injection. Cycling Nalm-6 GFP cells that have diluted the membrane dye to undetectable levels are represented in blue. (B) Proportion of dye-retaining (dormant) and dye-depleted (cycling) leukemia cells that co-localize with BM extracellular OPN, *P < .0001. Whereas few proliferating Nalm-6 GFP are directly adjacent to extracellular OPN, the majority of dormant Nalm-6 GFP co-localize with the OPN signal, suggesting a functional relationship between stromal OPN and ALL dormancy.
Dormant Nalm-6 GFP localize to areas of high OPN expression. (A) Nalm-6 GFP were labeled with DiR (green), a lipophilic membrane dye that is retained by noncycling cells. At 42 days after engraftment, mice were injected with AF647-labeled isotype control or anti-OPN (red) antibodies to visualize extracellular OPN. The calvarial BM was imaged using intravital confocal microscopic study ∼20 hours after antibody injection. Cycling Nalm-6 GFP cells that have diluted the membrane dye to undetectable levels are represented in blue. (B) Proportion of dye-retaining (dormant) and dye-depleted (cycling) leukemia cells that co-localize with BM extracellular OPN, *P < .0001. Whereas few proliferating Nalm-6 GFP are directly adjacent to extracellular OPN, the majority of dormant Nalm-6 GFP co-localize with the OPN signal, suggesting a functional relationship between stromal OPN and ALL dormancy.
We next used in vivo confocal microscopic studies to determine the relationship of OPN expression to leukemic growth in the BM. As shown in Figure 3E-H, the OPN signal is intense and is highest in the ECM adjacent to areas of significant tumor burden. These data confirm that OPN expression is increased in the tumor microenvironment and that it is highly expressed in specific niches that are invaded by leukemia.
Dormant, but not proliferative, Nalm-6 cells localize to areas of high OPN expression in vivo
We next performed studies to determine whether quiescent Nalm-6 cells, like dormant primary ALL cells, co-localized with ECM OPN in vivo. By engineering Nalm-6 to constitutively express GFP, we were also able to determine whether proliferating cells that had diluted fluorescent membrane dye label to undetectable levels co-localized with or were distant from extracellular OPN. At 42 days after engraftment of dye-labeled Nalm-6 GFP, mice were injected with AF647-labeled anti-OPN antibodies, and imaging studies were performed on the calvarial BM as described previously. As shown in Figure 4A-B, the majority of dormant, dye-retaining cells co-localize with areas of high OPN expression in the marrow, whereas few proliferating cells (GFP+, dye-negative) co-localize with OPN. These data suggested the possibility of a functional connection between dormant leukemic cells and BM OPN.
Neutralization of OPN in vivo induces leukemia cell cycle entry and increases disease burden
To further test the relationship between OPN and tumor dormancy, we developed an in vivo OPN neutralization strategy. Because our data suggested that tumor and host both contribute to the increased OPN levels in leukemic BM, we used a cocktail of anti-mouse and anti-human OPN-neutralizing antibodies to inhibit Nalm-6 interaction with extracellular OPN. As shown in Figure 5A, both neutralizing antibodies effectively reduce Nalm-6 binding to murine or human thrombin-cleaved OPN in vitro, and each display some degree of cross-species neutralization. Because OPN is a chemotactic cytokine, we also assessed whether treatment of mice with the neutralizing antibody cocktail could inhibit leukemia cell homing to the BM. Treatment of mice with the neutralizing cocktail before leukemic cell engraftment did not affect Nalm-6 GFP BM homing (Figure 5B).
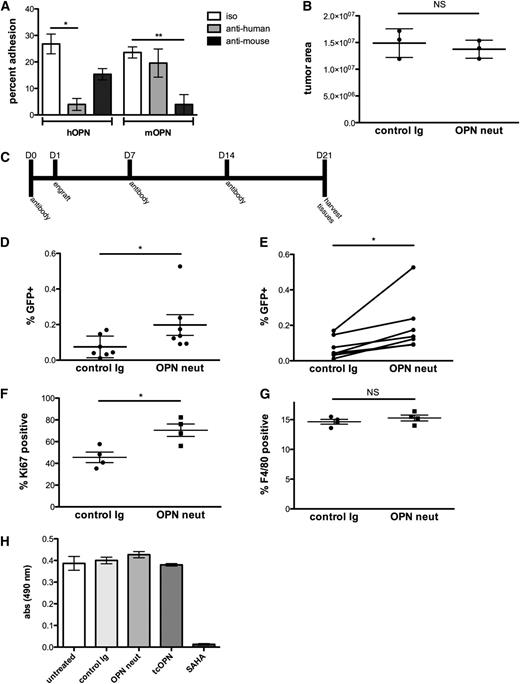
OPN neutralization increases leukemia burden by decreasing the proportion of dormant tumor cells. (A) In vitro adhesion of Nalm-6 to thrombin-cleaved human and murine OPN is blocked by anti-OPN neutralizing antibodies, n = 3, *P = .0008, **P = .0014. OPN-coated dishes were pretreated with OPN-neutralizing antibodies before plating cells. (B) Nalm-6 homing in anti-OPN– vs isotype control-treated mice is equivalent. Intravital confocal microscopic study was used to examine the number of Nalm-6 leukemia cells in the calvarial BM of control vs anti-OPN–treated mice at 24 hours after engraftment; n = 3, *P = .5. (C) The antibody treatment scheme used in the OPN-neutralizing studies. (D-E) Flow cytometric quantification of GFP+ leukemia cells in BM aspirates from control and OPN-neutralized mice demonstrates increased disease burden in anti-OPN–treated mice. The paired analysis of the data is shown on the right; n = 7, *P = .02. (F) The proportion of Ki-67–positive leukemia cells is significantly higher in the BM of OPN-neutralized mice compared with isotype control-treated mice; n = 4, *P = .0005. (G) The percentage of F4/80-positive macrophages is equivalent in OPN-neutralized and isotype control-treated leukemic mouse BM; n = 4, *P = .5. (H) To determine whether OPN directly alters Nalm-6 proliferation, cells were incubated with tcOPN, an OPN neutralizing Ab, control immunoglobulin, or the chemotherapeutic agent SAHA. SAHA induced a dramatic decrease in proliferation, but neither incubation with tcOPN nor OPN-neutralizing Ab (to block autocrine binding of OPN secreted by Nalm-6 in culture) affected proliferation (n = 3). These data demonstrate that soluble OPN does not directly induce Nalm-6 dormancy.
OPN neutralization increases leukemia burden by decreasing the proportion of dormant tumor cells. (A) In vitro adhesion of Nalm-6 to thrombin-cleaved human and murine OPN is blocked by anti-OPN neutralizing antibodies, n = 3, *P = .0008, **P = .0014. OPN-coated dishes were pretreated with OPN-neutralizing antibodies before plating cells. (B) Nalm-6 homing in anti-OPN– vs isotype control-treated mice is equivalent. Intravital confocal microscopic study was used to examine the number of Nalm-6 leukemia cells in the calvarial BM of control vs anti-OPN–treated mice at 24 hours after engraftment; n = 3, *P = .5. (C) The antibody treatment scheme used in the OPN-neutralizing studies. (D-E) Flow cytometric quantification of GFP+ leukemia cells in BM aspirates from control and OPN-neutralized mice demonstrates increased disease burden in anti-OPN–treated mice. The paired analysis of the data is shown on the right; n = 7, *P = .02. (F) The proportion of Ki-67–positive leukemia cells is significantly higher in the BM of OPN-neutralized mice compared with isotype control-treated mice; n = 4, *P = .0005. (G) The percentage of F4/80-positive macrophages is equivalent in OPN-neutralized and isotype control-treated leukemic mouse BM; n = 4, *P = .5. (H) To determine whether OPN directly alters Nalm-6 proliferation, cells were incubated with tcOPN, an OPN neutralizing Ab, control immunoglobulin, or the chemotherapeutic agent SAHA. SAHA induced a dramatic decrease in proliferation, but neither incubation with tcOPN nor OPN-neutralizing Ab (to block autocrine binding of OPN secreted by Nalm-6 in culture) affected proliferation (n = 3). These data demonstrate that soluble OPN does not directly induce Nalm-6 dormancy.
On the basis of these data, we then undertook long-term studies of in vivo OPN blockade in leukemic mice. Mice were injected with the OPN-neutralizing antibody or isotype control antibody cocktail following the schedule outlined in Figure 5C. At 21 days after tumor engraftment, the mice were euthanized, and femur and iliac crest BM were harvested for flow cytometric analysis of GFP+ leukemia cell burden. In marked contrast to the antiproliferative effect of OPN inhibition that has been observed in solid tumor models,16,23-25,32 OPN neutralization in our ALL model led to an approximately twofold increase in tumor burden (Figure 5D-E). Moreover, OPN neutralization increased the Ki-67 proliferative index in leukemic cells, suggesting that interaction with OPN in the BM microenvironment leads to cell cycle withdrawal in leukemic blasts (Figure 5F).
Multiple groups have demonstrated an in vivo role for OPN in the chemoattraction of macrophages.33,34 To confirm that the observed increase in tumor burden was not the result of failure to recruit macrophages into the tumor bed, we stained BM aspirates from control and OPN-neutralized mice for the murine macrophage marker, F4/80 antigen. Flow cytometric analysis showed that OPN neutralization did not affect macrophage residency in the marrow (Figure 5G).
To test whether leukemic cell engagement with OPN directly altered cell proliferation, Nalm-6 cells were incubated with either tcOPN or OPN-neutralizing Abs, and a 72-hour MTT proliferation assay was performed (Figure 5H). Neither treatment had a direct effect on cell proliferation. We next examined whether blockade of the α4β1 receptor, which abrogated Nalm-6 binding to OPN in vitro, would also increase Nalm-6 tumor burden. Consistent with the previously defined role of integrins in activating cell cycle progression, blockade of Nalm-6 VLA-4 with anti-human VLA-4–blocking antibodies decreased tumor burden in treated vs control mice (data not shown). These data suggest that the dormancy-inducing effect of ECM OPN on Nalm-6 is indirect and is likely related to its role in anchoring Nalm-6 within specific microenvironments where additional factors act to induce cell cycle arrest.
OPN neutralization synergizes with Ara-C–based chemotherapy to reduce MRD
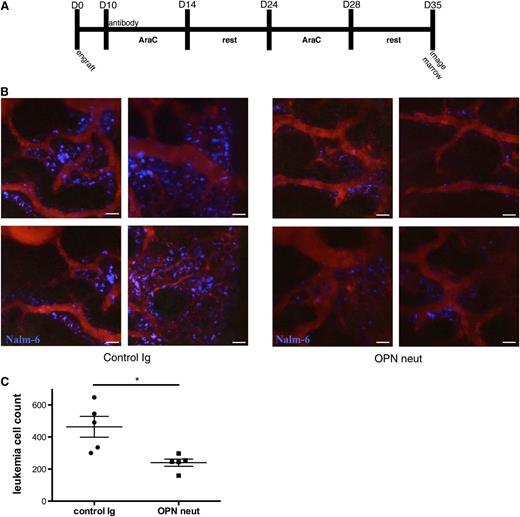
Current frontline therapies for ALL revolve around cytotoxic agents that target actively cycling cells. MRD that persists after chemotherapy, however, can lead to disease relapse, and the degree of MRD is an independent prognostic indicator in humans.2 A contributing factor to MRD may be the inability of cytotoxic agents to eradicate noncycling, or dormant, cells. Our results suggested that a significant number of leukemic blasts enter a dormant state in the BM via their interaction with extracellular OPN. Therefore, we wished to test whether OPN neutralization could sensitize leukemic blasts to an Ara-C–based chemotherapy regimen and thereby reduce MRD. To test this hypothesis, we developed an Ara-C dosing strategy involving multiple rounds of chemotherapy designed to eliminate all but the most highly resistant disease (Figure 6A). MRD after treatment could only be detected by high-resolution imaging of the calvarial BM yet produced disease relapse measurable by flow cytometry in mice as time progressed, thus modeling disease recurrence in patients who appear to have an initial cure. Mice were given chemotherapy with or without OPN neutralization as pretreatment. At the end of therapy, we performed imaging studies of the calvarial BM to sensitively identify rare tumor cells. As demonstrated in Figure 6B-C, the combination of a single OPN neutralization treatment before Ara-C significantly reduces MRD compared with Ara-C treatment alone.
OPN neutralization synergizes with Ara-C to reduce MRD. (A) Ara-C and OPN neutralization treatment protocol. (B-C) MRD in leukemic mice treated with OPN neutralization and Ara-C or Ara-C chemotherapy alone, as detected by intravital microscopic study. Imaging was performed on the entirety of the calvarial BM, and individual fluorescent cells were counted by hand. Representative images are shown in (B), and quantification of data is shown in (C). MRD burden is significantly decreased in anti-OPN–treated mice, demonstrating that OPN neutralization synergizes with Ara-C to sensitize cells to chemotherapy; n = 5, *P = .01. Scale bars equal 50 µm.
OPN neutralization synergizes with Ara-C to reduce MRD. (A) Ara-C and OPN neutralization treatment protocol. (B-C) MRD in leukemic mice treated with OPN neutralization and Ara-C or Ara-C chemotherapy alone, as detected by intravital microscopic study. Imaging was performed on the entirety of the calvarial BM, and individual fluorescent cells were counted by hand. Representative images are shown in (B), and quantification of data is shown in (C). MRD burden is significantly decreased in anti-OPN–treated mice, demonstrating that OPN neutralization synergizes with Ara-C to sensitize cells to chemotherapy; n = 5, *P = .01. Scale bars equal 50 µm.
Discussion
Clinical studies have demonstrated that even when MRD is not completely eradicated, decreases in MRD burden correlate with significant improvements in overall survival duration.2,35,36 New methods to diminish post-therapy MRD are therefore critically important. Given that quiescent cancer cells are resistant to standard cytotoxic therapies, one approach to improve chemotherapy efficacy and reduce MRD would be to force quiescent leukemic cells into active phases of the cell cycle.
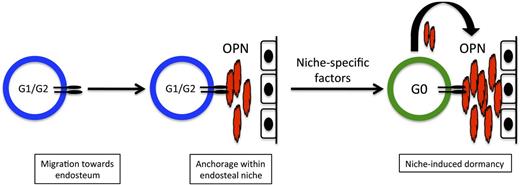
The BM microenvironment contains niches that regulate the dormancy of HSCs and shelter them from chemotherapeutic insult. Moreover, several HSC niche components, including OPN, stromal cell-derived factor-1, angiopoietin, and thrombopoietin, have been implicated in maintaining HSC quiescence.11-14,20,21,37 We have previously demonstrated that leukemic cells hijack normal HSC-niche homing signals to metastasize within the BM, and that leukemia engraftment in HSC niches induces significant alterations in the niche that favor leukemic cell growth over HSC survival.3,28 How unique niches regulate leukemic cell proliferation or dormancy is not, however, well understood. Here we provide evidence that specific OPN+ niches within the marrow function to reversibly inhibit the proliferation of leukemic blasts, and that blocking this interaction increases the efficacy of cytotoxic chemotherapy aimed at actively cycling cells. A model for this relationship is presented in Figure 7. These data provide the first proof-of-principle that specific BM niches can function to maintain leukemia cell dormancy.
Model for OPN-induced dormancy at the endosteum. Cycling ALL blasts migrate to the endosteum in response to niche-specific chemokine expression. Once within the niche, blasts use integrin receptors such as VLA-4 to adhere to extracellular OPN produced locally by osteoblasts. This interaction confines them to the endosteal niche, where blast-derived OPN is also incorporated into the ECM. Additional local niche-specific factors induce cell cycle exit and leukemia dormancy. Dormant blasts that are insensitive to chemotherapy agents that target dividing cells contribute to MRD.
Model for OPN-induced dormancy at the endosteum. Cycling ALL blasts migrate to the endosteum in response to niche-specific chemokine expression. Once within the niche, blasts use integrin receptors such as VLA-4 to adhere to extracellular OPN produced locally by osteoblasts. This interaction confines them to the endosteal niche, where blast-derived OPN is also incorporated into the ECM. Additional local niche-specific factors induce cell cycle exit and leukemia dormancy. Dormant blasts that are insensitive to chemotherapy agents that target dividing cells contribute to MRD.
Although OPN adherence is a key mechanism to retain leukemic cells in prodormancy niches, ECM OPN does not appear to directly induce quiescence in blasts. Our data suggest that OPN exerts its effects in the Nalm-6 ALL model via engagement of α4β1 integrin receptors, as inhibition of α4β1 (VLA-4) completely abrogated Nalm-6 binding to OPN in vitro. VLA-4, however, interacts with multiple binding partners present in the BM microenvironment, including VCAM-1 and fibronectin, and activation of integrin receptors by these ligands typically stimulates cell cycle progression.38 Indeed, in vivo VLA-4 blockade in Nalm-6 ALL mice inhibits rather than promotes ALL proliferation.39 These findings suggest that OPN-induced quiescence is the result of its critical role as an anchor for leukemic cells within prodormancy niches, where other factors in the local microenvironment then promote cell cycle arrest. Such secondary factors might include hypoxia; specific cytokines; direct interaction with stromal cells; or, potentially, a complex combination of these metabolic, stromal, and cytokine cues.
Our study is the first to address the role of OPN in ALL, although some translational data suggest that OPN BM transcript or serum levels in AML correlate negatively with disease prognosis.26,27 In contrast to the paucity of research on OPN and leukemia, there is a well-defined association between OPN and solid tumor progression, with multiple reports linking OPN expression to tumor growth and metastasis in breast, gastrointestinal, prostate, and lung cancers, among others.16,22-25 In contrast to our findings, these studies have shown OPN to promote tumorigenesis, in some cases through autocrine signaling mechanisms.32 One potential explanation for these dissimilar results is that leukemic cells, in contrast to solid tumors, bind poorly to full-length OPN. Rather, ALL binds strongly to thrombin-cleaved OPN, the predominant form of OPN in the BM of mice and humans.20 Cleavage of OPN by thrombin reveals a cryptic epitope that is recognized specifically by the integrin receptors α4β1 and α9β1 that are preferentially expressed by leukocytes and HSCs.20 This mechanism suggests that control of tumor cell dormancy is a novel function of OPN that is contextual to the BM microenvironment.
Another intriguing finding from our study is that OPN expression in the niche is influenced by dynamic cross-talk between leukemia and host. Our data reveal that the niche is “remodeled” during leukemia progression as host and tumor react to each other. In response to leukemic infiltration, host OPN production increases. At the same time, leukemia cells in OPN-rich BM regions secrete high levels of OPN that are incorporated locally into the ECM. These results suggest a positive feedback loop in which tumor-derived OPN can expand the quiescent niche and reinforce the dormant phenotype.
Our investigation of OPN expression in primary human pre-B ALL samples revealed blast-specific OPN expression and expression of OPN cell surface receptors in all subjects analyzed, suggesting that OPN expression is a common feature of human ALL. The ability of primary blasts to adhere to OPN in vitro, however, was highly variable. Determining whether the capacity of blasts to adhere to OPN correlates with clinical outcomes in ALL is thus an interesting subject for future investigations.
Before a therapy based on OPN neutralization can be translated to the clinic, potential toxic adverse effects on benign HSCs need to be assessed to determine the effect of combined OPN blockade and chemotherapy on the viability and function of the HSC pool. The relatively mild phenotype in the HSC compartment of the OPN knockout mouse, however, suggests that additional mechanisms function to prevent exhaustion of the HSC pool in the absence of OPN.37 Moreover, although it may be necessary to combine multiple cycles of OPN blockade with chemotherapy to achieve maximal therapeutic benefit, the temporary nature of the OPN inhibition is likely to make this approach feasible. Alternatively, therapies could be designed to reinforce the interaction between BM OPN and leukemia cells to induce quiescence. Such an approach could be used to enforce a dormant state on leukemia in the BM, thus preventing or slowing disease progression.
In summary, we have demonstrated a unique mechanism by which leukemic blasts hijack a HSC BM niche to gain indirect protection from cytotoxic chemotherapy. These data demonstrate for the first time how BM niches can play a functional role in controlling ALL cell dormancy: Leukemic cells use stromal-derived OPN to anchor themselves in microdomains that support quiescence. As tumor growth progresses, blasts secrete OPN into the local microenvironment, where it is incorporated into the ECM and may function to reinforce the dormant phenotype. OPN neutralization may, in turn, be an effective strategy to reduce blast dormancy and increase the efficacy of currently used cytotoxic chemotherapeutic regimens in patients with ALL.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Janet Rowley and Anjen Chenn for their careful review of the manuscript.
This work was supported in part by a Gabrielle’s Angel Foundation grant award, a National Institutes of Health Director’s DP2 award (1DP2OD002160), and a University of Chicago Cancer Research Center Pilot Project award. B.B. was supported by The University of Chicago Department of Cancer Biology Postdoctoral Fellowship (NRSA, T32).
Authorship
Contribution: B.B. performed all experiments except for those presented in Figure 1C, Figure 2B, and Figure 6; M.Z. performed the flow cytometry presented in Figure 1C and quantified data presented in Figure 2D-F, Figure 4A, and Figure 5B; M.Z. performed the tail vein engraftments for all animal studies; A.E.Y. performed the IHC presented in Figure 2B and Figure 6, and E.M.H. helped optimize IHC protocols; T.T.P. performed the RT-PCR presented in supplemental Figure 3; and B.B. and D.A.S. designed the experiments, interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dorothy A. Sipkins, University of Chicago, 5841 South Maryland Ave, MC 2115, Chicago, IL 60637; e-mail: dsipkins@medicine.bsd.uchicago.edu.