Key Points
Patients with VT have an increased risk of subsequent CVD compared with control participants.
The increased risk of CVD in these patients can be explained by etiologic factors leading to both diseases.
Abstract
Patients with venous thrombosis (VT) have an increased risk of subsequent CVD (CVD), but the underlying pathophysiology is unclear. Using data from the Multiple Environmental and Genetic Assessment of risk factors for venous thrombosis follow-up study, 4480 patients with VT, 2926 partner control participants, and 2638 random digit dialing (RDD) control participants were followed-up between 1999 and 2008. Incidence rates and hazard ratios with 95% confidence intervals (95% CIs) of CVD (defined as myocardial infarction or ischemic stroke) were calculated for patients vs controls. Measurable confounders (age, sex, body mass index, smoking, chronic disease, malignancy, genetic thrombophilia, and procoagulant markers) were adjusted for when comparing patients with RDD controls. Unmeasured lifestyle-related factors were also considered by comparing patients with their partners. During a median follow-up time of 5 years, 124 CVD events occurred. Incidence of CVD per 1000 person-years was 3.2 (95% CI, 2.5-4.0) in patients, 2.2 (95% CI, 1.5-3.0) in partners, and 1.6 (95% CI, 0.9-2.6) in RDD controls. Crude hazard ratio was 2.2 (95% CI, 1.2-3.8) in patients compared with RDD controls and 1.5 (95% CI, 1.0-2.3) in patients compared with partners. After adjustment for all confounders, these risks attenuated to 1.8 (95% CI, 0.8-4.2) and 1.3 (95% CI, 0.7-2.5) for patients compared with RDD control participants and partners, respectively. In conclusion, individuals with VT had an increased risk of CVD. This could be explained by common etiologic factors.
Introduction
Venous thrombosis (VT) and arterial cardiovascular disease (CVD) have traditionally been seen as 2 separate diseases, each with their own risk factors and pathophysiological mechanisms.1 In the past decade, however, this notion has been contested. In 2003, it was shown that patients admitted to hospital with unprovoked VT had a higher prevalence of atherosclerosis than age- and sex-matched hospital control participants.2 A large population-based study in Denmark also found individuals with VT to be at a 2-fold increased risk of CVD compared with controls.3 These findings have since been confirmed for individuals with unprovoked venous thrombotic events.4,5
Whether VT is causally associated with CVD is under debate.6 Two prospective population-based cohort studies, the Atherosclerosis Risk in Communities and the Cardiovascular Health Study,7,8 failed to confirm the association found earlier between atherosclerosis and VT. Furthermore, the increased risk of CVD after VT could be confounded by shared risk factors. Age, sex, an increased body mass index (BMI), smoking, chronic disease, malignancy, and (genetic) thrombophilia have all been found to increase the risk of both VT and CVD.6,9,10 However, either because of a lack of information on classical cardiovascular risk factors (apart from age and sex) or because of a broad definition of CVD (eg, otherwise unexplained death, angina, transient ischemic attack, and angioplasty), no study has yet provided estimates of the risk of myocardial infarction and ischemic stroke after VT adjusted for shared risk factors.3-5,11 In addition, other health determinants that may affect the risk of both VT and CVD, such as socioeconomic status, diet, and alcohol consumption, have not previously been taken into account.12-15
In a large prospective cohort study (n > 10 000), we aimed to assess the risk of CVD in patients with VT compared with 2 separate control groups without a history of VT: random digit dialing (RDD) control participants and partners of patients. When comparing patients with RDD controls, we adjusted for the measurable confounders age, sex, BMI, smoking, chronic disease, malignancy, and thrombophilia by multivariate analysis. By comparing patients with their partners, some unmeasured health determinants also were taken into account, as partners generally share a similar lifestyle and socioeconomic status.16
Methods
Study design
Between March 1999 and September 2004, consecutive patients aged 18 to 70 years with a first deep vein thrombosis (DVT) or pulmonary embolism were included in the Multiple Environmental and Genetic Assessment of risk factors for venous thrombosis (MEGA) study from 6 anticoagulation clinics. In the Netherlands, anticoagulation clinics are regionally organized, and all patients with VT living in a certain area are monitored by the same clinic, regardless of the hospital they are admitted to or the physician who starts the treatment. Eighty-six percent of eligible patients were willing to participate in the MEGA study. Partners of patients were invited to participate as controls if they were aged 18 to 70 years and had no history of VT. From January 2002 to September 2004, additional controls were recruited by RDD. All participants provided informed consent in accordance with the Declaration of Helsinki. The MEGA study was approved by the medical ethics committee of the Leiden University Medical Center and has been described in detail elsewhere.17,18
Data collection
Participants completed a detailed questionnaire on risk factors for VT. Items covered in the questionnaire included surgery, pregnancy, plaster cast immobilization and hospitalization in the 3 months before the index date, oral contraception or postmenopausal hormone therapy use in the month before the index date, and chronic disease (defined as liver disease, kidney disease, rheumatoid arthritis, or multiple sclerosis) and malignancy in the 5 years before the index date. The index date was defined as the date of diagnosis of VT for patients and their partners, and the date of completing the questionnaire for RDD controls. Self-reported information was also obtained on weight, height, and smoking status. BMI was calculated by dividing weight (in kilograms) by height (in meters) squared.
At least 3 months after discontinuation of anticoagulation, or during anticoagulant therapy in patients who continued this therapy for more than 1 year, patients and controls visited the anticoagulation clinic for an interview and a blood sample to obtain DNA. The blood samples were used to assess levels of coagulation factors and the presence or absence of common genetic risk factors. All assays were performed in automated machines by laboratory technicians who were unaware of the case–control status of the samples. A detailed description of the assays can be found elsewhere.19-23
Follow-up
Between February 2007 and May 2009, the vital status of all MEGA participants was acquired from the Dutch population register, as has been described previously.24 For participants who had died, both primary and secondary causes of death were retrieved from the national registry of death certificates. Causes of death were encoded according to the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM). In 2011, participants of the MEGA study were linked to the Dutch Hospital Data registry to identify individuals with an arterial cardiovascular event. This registry has provided nationwide electronic coverage of data on all hospital admissions since 1995. Data are collected in virtually all general and university hospitals and most specialized clinics.
For each hospital admission, information on the date of admission and discharge, diagnoses, and surgical procedures is available. These diagnoses are encoded according to ICD-9-CM. A previous study comparing a random sample of hospital admissions in the Dutch Hospital Data registry to information from hospital records showed that 99% of the personal, admission, and discharge data and 84% of principal diagnoses were correctly encoded.25 The percentage of correctly encoded myocardial infarctions has since been found to be almost 100%.26 Participants of the MEGA study were linked to this registry through date of birth, sex, and postal code. Individuals with information leading to more than 1 person (eg, twins or individuals with the same date of birth in the same postal area) or to nobody at all (immigrants, visitors) were excluded. Of the 11 253 MEGA participants, 10 178 (4539 patients, 2967 partners, and 2672 RDD controls) could be uniquely linked to the registry.
After linkage to the Dutch Hospital Data registry, all MEGA participants with an acute arterial cardiovascular event were identified. Acute CVD was defined as myocardial infarction (ICD-9-CM codes 4100-4109; ICD-10-CM code I21) or ischemic stroke (ICD-9-CM codes 433, 4330-4333, 4338, 4339, 434, 4340, 4341, and 4349; ICD-10-CM codes I63 and I64). We specifically chose to include these 2 cardiovascular diseases only, as patients with myocardial infarction or ischemic stroke will always have been hospitalized because of the disease severity and were therefore definitely captured by the Dutch Hospital Data registry (in contrast to, for example, patients with transient ischemic attacks). Individuals with CVD diagnosed between 1995 and the index date and individuals for whom either the date of inclusion in the study or the date of diagnosis was unknown were excluded.
Statistical analysis
We estimated the absolute risk of incident CVD in patients with VT vs controls by dividing the number of arterial cardiovascular events by the observation time. The observation time was defined as the time between the index date and the end of follow-up. Follow-up ended on the date of a first arterial cardiovascular event, the date of recurrent VT, the date of death, or the date on which the vital status was retrieved (between February 2007 and May 2009), whichever came first. The 95% confidence intervals (95% CIs) around the incidence rates were based on a Poisson distribution.
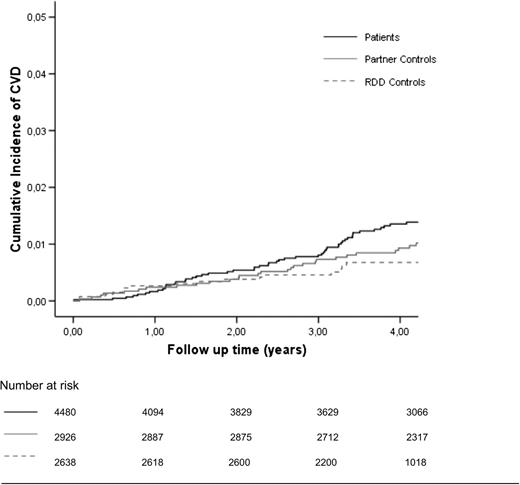
Kaplan-Meier curves were used to plot the survival for patients vs controls (Figure 1). As we observed that the Kaplan-Meier curves crossed at around 1 year of follow-up, the assumption for proportional hazards was not met during the entire observation period. Patients with acute VT are treated with oral anticoagulant therapy (ie, vitamin K antagonists) for at least some time to prevent a recurrent event.27 However, vitamin K antagonists are also highly effective for the prevention of CVD,28,29 and their use could explain this finding. We therefore used an extended Cox regression model to compare the risk of CVD in patients and controls and included anticoagulant therapy for the treatment of acute VT as a time-dependent covariate.30
Cumulative incidence of CVD in VT patients, their partners, and RDD controls. CVD, arterial cardiovascular disease.
Cumulative incidence of CVD in VT patients, their partners, and RDD controls. CVD, arterial cardiovascular disease.
The effect of measured confounders was taken into account through adjustments for age (categorical: deciles), sex, BMI (categorical: <25 kg/m2, 25-30 kg/m2, and >30 kg/m2), smoking history, chronic disease, malignancy, genetic thrombophilia (factor V Leiden, prothrombin G20210A, and blood group non-O), and procoagulant markers (fibrinogen, factor VIII, and C-reactive protein). As the distribution of C-reactive protein was skewed to the left, the log transformation of this variable was used. Fibrinogen and factor VIII were normally distributed and were added as continuous variables. Other cardiovascular risk factors (ie, diabetes mellitus, hyperlipidemia) were not considered, as these have not been found to be associated with VT.31 Unmeasured lifestyle-related confounders were also taken into account by comparing patients with the partner controls, as partners generally share the patients’ lifestyle.16
Patients with DVT and pulmonary embolism were studied separately. In addition, we analyzed myocardial infarction and ischemic stroke separately. Participants with ischemic stroke were excluded from the analysis of myocardial infarction, and vice versa. Two participants (a patient and a partner control) experienced both myocardial infarction and ischemic stroke. They were included in both analyses, with follow-up time ending on the date of the event of interest.
It has been suggested that the risk of CVD is higher after an unprovoked VT than after a provoked VT.3 Therefore, we also analyzed individuals with unprovoked and provoked VT separately. In addition, as the many provoking risk factors have varying levels of severity, the relation between arterial and venous disease was considered separately for participants who reported exogenous hormone use (defined as oral contraception or postmenopausal hormone therapy), pregnancy, surgery, hospitalization, and malignancy at the time of their venous event.
The diagnosis of a fatal arterial cardiovascular event may not always have been classified by objective methods. To explore whether fatal arterial cardiovascular events influenced our results, we performed a sensitivity analysis in which fatal arterial cardiovascular events were censored.
Our study hypothesis and alternative explanations, as discussed earlier, are graphically summarized in supplemental Figure 1, available on the Blood Web site. All statistical analyses were performed with SPSS for Windows, release 14.0 (SPSS Inc).
Results
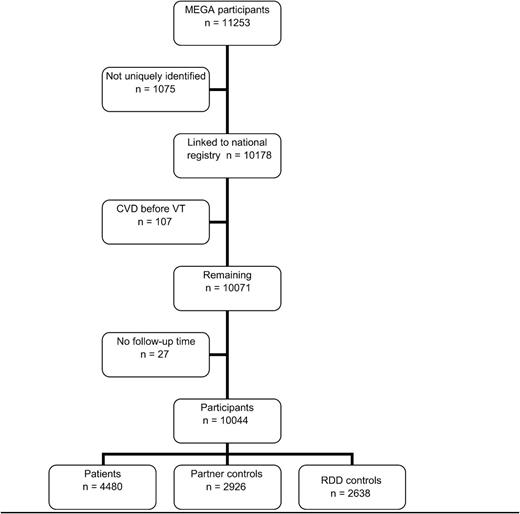
In total, 11 253 individuals were included in the MEGA study (Figure 2). Of these individuals, 1075 could not be linked to the Dutch Hospital Data registry. Of the 10 178 individuals remaining, 107 were excluded because their arterial event occurred before the index date, and 27 were excluded because either their index date or the date of diagnosis of CVD or recurrent VT was unknown. A total of 4480 patients, 2926 partner controls, and 2638 RDD controls were available for analysis. Their clinical characteristics at enrollment are depicted in Table 1. In all groups there were slightly more women than men. RDD controls were slightly younger (46 years; range, 18-70 years) than patients and their partners (both 49 years; range, 18-70 years). Mean follow-up time was 5.5 years in patients, 5.6 years in partner controls, and 3.9 years in RDD controls. During follow-up, 557 (12%) patients, 76 (3%) partner controls, and 46 (2%) RDD controls died. CVD was the cause of death in 24 patients, 6 partner controls, and 1 RDD control.
Flowchart of number of individuals included. CVD, arterial cardiovascular disease; VT, venous thrombosis.
Flowchart of number of individuals included. CVD, arterial cardiovascular disease; VT, venous thrombosis.
In the patients, 72 arterial cardiovascular events occurred during a follow-up time of 22 423 person-years, which led to an incidence rate of 3.2 per 1000 person-years (95% CI, 2.5-4.0) (Table 2). The incidence rate of CVD was similar after DVT, at 3.4 per 1000 person-years (95% CI, 2.5-4.5), and after a pulmonary embolism, at 3.0 per 1000 person-years (95% CI, 2.0-4.3). The incidence rate of myocardial infarction was higher, at 2.4 per 1000 person-years (95% CI, 1.8-3.2), than the incidence rate of ischemic stroke, at 0.9 per 1000 person-years (95% CI, 0.5-1.3). RDD controls had 16 arterial cardiovascular events during a follow-up time of 10 166 person-years, corresponding to an incidence rate of 1.6 per 1000 person-years (95% CI, 0.9-2.6). Partner controls had 36 arterial cardiovascular events over the course of 16 402 person-years, yielding an incidence rate of 2.2 per 1000 person-years (95% CI, 1.5-3.0).
The hazard ratio (HR) of CVD during anticoagulation therapy was 0.6 (95% CI, 0.3-1.7) for patients compared with RDD controls and 0.7 (95% CI, 0.3-1.8) for patients compared with partner controls. The anticoagulation-adjusted HR for arterial events compared with RDD controls was 2.2 (95% CI, 1.2-3.8). This risk estimate was not affected by adjustments for age, sex, BMI, and smoking (2.3; 95% CI, 1.2-4.3) or by additional adjustments for chronic disease and malignancy (2.2; 95% CI, 1.2-4.2). When compared with partner controls, the HR of CVD adjusted for anticoagulation therapy was 1.5 (95% CI, 1.0-2.3). Again, adjustments for age, sex, BMI, and smoking (1.6; 95% CI, 1.0-2.4) and additional adjustment for chronic disease and malignancy (1.5; 95% CI, 1.0-2.4) did not influence the risk estimate. The analysis restricted to patients with unprovoked VT and the analyses for the various provoking risk factors both revealed results that pointed into the same direction, except with regard to patients who were pregnant (Table 3). The sensitivity analysis in which fatal arterial cardiovascular events were censored resulted in slightly lower relative risk estimates compared with RDD controls (1.7; 95% CI, 0.8-3.4) and compared with partner controls (1.3; 95% CI, 0.8-2.2; Table 4).
In a final analysis, we recalculated the risk of CVD disease in patients compared with controls, this time including genetic thrombophilia, procoagulant markers, or both as covariates (Table 5). Further adjusting the risk estimates for factor V Leiden, prothrombin G20210A, and blood group non-O yielded risk estimates of 1.8 (95% CI, 0.9-3.6) compared with RDD controls and 1.4 (95% CI, 0.9-2.3) compared with partner controls. Replacing the genetic factors with fibrinogen, factor VIII, and C-reactive protein yielded similar results: 1.9 (95% CI, 0.8-4.4) and 1.4 (95% CI, 0.7-2.6), respectively. These results also were virtually the same in a sensitivity analysis in which individuals who had been using anticoagulant therapy at the blood draw (n = 271) were excluded, with HRs of 1.8 (95% CI, 0.7-4.6) and 1.4 (95% CI, 0.7-2.6). Finally, after full adjustment for age, sex, BMI, smoking, chronic disease, malignancy, genetic thrombophilia, and procoagulant markers, the risk estimates were 1.8 (95% CI, 0.8-4.3) compared with RDD controls and 1.3 (95% CI, 0.7-2.5) compared with partner controls. Results were not different when continuous variables were included as categorical variables in the model, rather than as continuous variables.
Discussion
In this study, we found patients with VT to have a 2.2-fold (95% CI, 1.2-3.8) increased risk of CVD compared with control participants who did not have VT. This risk estimate was lowered (HR, 1.5; 95% CI, 1.0-2.3) when unmeasured lifestyle-related confounders were taken into account by comparing patients with their partners. After measured confounders (age, sex, BMI, smoking, malignancy, chronic disease, genetic thrombophilia, and procoagulant markers) were also considered, the risk estimate was no longer increased in patients compared with partner controls (HR, 1.3; 95% CI, 0.7-2.5). Thus, from our results, it seems that the relationship between VT and subsequent CVD is not causal but can be explained by common etiologic factors.
Our overall results are in line with findings from previous studies showing that patients with VT have an increased risk of subsequent CVD. In addition, the absolute risk of CVD after VT that was found in our study was the same as the absolute risk estimate previously shown by a systematic review, at 3.2 per 1000 person-years.5 However, to our knowledge, this is the first study to view the relationship between VT and CVD while adjusting for both measured and unmeasured shared risk factors. Unlike most control groups, partners generally share the patients’ lifestyle. They are therefore likely to have a similar socioeconomic status, diet, and level of alcohol consumption or other unknown factors that may affect the risk of both VT and CVD.12-15 The fact that our fully adjusted risk estimates were no longer increased when patients were compared with their partners indicates that VT and CVD are associated with the same risk factors but are not causally related.
Two previous studies found a higher risk of CVD in the first year after VT than in the years thereafter.3,11 However, in our study we found the opposite. A possible explanation for this difference could be that all of the patients who are included in the MEGA study had to survive long enough for a first visit to the anticoagulation clinic. Therefore, individuals who died shortly after the venous thrombotic event were not included in our study. If the cause of death in those individuals was CVD, we would have missed it. However, it is not likely that this can explain the discrepancy, as the largest published study on this issue, by Sørensen et al,3 did not include cardiovascular deaths in the analysis. Another explanation could be that the risk of CVD in the first year of follow-up is in fact lower. Indeed, a study performed by a different research group in our hospital (with an identical treatment program for VT) also found that the absolute risk of CVD after VT was low in the first year of follow-up.4 This is understandable, as most patients with VT are prescribed anticoagulant therapy to prevent a recurrent event.27 Considering that anticoagulant therapy is highly effective for the primary and secondary prevention of CVD,28,29 an increased risk in the first year after VT seems unlikely unless anticoagulation therapy was only given for a very short period. Furthermore, unlike in our study, the results of Sørensen et al were partly driven by hemorrhagic stroke.3 As hemorrhagic stroke is a common complication of anticoagulant treatment,32 including this as a type of CVD would overestimate the number of events in the first year after VT.
In a study by Van Schouwenburg et al,11 a broad definition of CVD was used that included subacute ischemic heart disease and angioplasty. As patients are often admitted to hospital for a short time after VT and are closely monitored thereafter, it is possible that increased medicalization can explain the high number of arterial cardiovascular diagnoses made in the first year after VT onset. This, too, would explain the difference in our findings compared with those from the study by Van Schouwenburg et al,11 as we only used hard endpoints.
The results of the analyses in which the outcomes myocardial infarction and ischemic stroke were analyzed separately seemed to yield different risk estimates according to whether the patient had experienced a DVT or a pulmonary embolism (Table 2). However, as numbers were small and confidence intervals were wide, these differential findings should be interpreted with caution.
As this is an observational study in which blood samples were collected after the venous thrombotic event, one could argue that levels of procoagulant markers in our study may have been affected by acute-phase reactions. For instance, it may be possible that the venous event itself may have led to increases in procoagulant markers, and subsequently increased the risk of CVD. However, we consider it unlikely that the effect of procoagulant markers was a result of VT, as a previous study has shown that C-reactive protein, factor VIII, and fibrinogen levels normalize within 3 months after the acute venous thrombotic event.33 In our study, procoagulant markers were obtained at least 3 months after the end of anticoagulant therapy or, in case of long-term treatment with vitamin K antagonists, 1 year after venous thrombotic event onset. By this time, the effect of the acute-phase reaction will have worn off.33-35 Furthermore, our results were not affected by individuals using anticoagulant therapy at the blood draw, as a sensitivity analysis in which these individuals were excluded yielded similar results as our overall analysis.
Strengths of our study are that data were collected in the same manner for all participants and that all venous thrombotic events were objectively diagnosed. It was therefore possible to compare the risk of CVD in patients vs all controls and vs the 2 control groups separately. Furthermore, the 2 control groups made it possible to take both measured and unmeasured lifestyle factors into account for the first time.
A possible limitation of our study is that arterial cardiovascular events were not objectively confirmed, but obtained from the Dutch Hospital Data registry. However, a previous study showed that the percentage of correctly encoded myocardial infarctions in this registry was almost 100%.26 In addition, although the exact percentage of correctly encoded ischemic strokes is unknown, it is unlikely that any misclassification in this diagnosis would have occurred differently in patients and controls. Another limitation is that numbers of arterial cardiovascular events in some subgroups were small. However, from the upper limits of the confidence intervals, it seems that even if numbers had been larger, it is unlikely that risk estimates would have been much higher. In addition, as the MEGA study is one of the largest of its kind, the low numbers indicate that CVD after VT is not a common occurrence, at least in this age group.
In our study we did not have information on medication use during the follow-up period. Patients with VT can nowadays be treated with aspirin or statin to prevent a recurrent event.36,37 As these medications are also effective against cardiovascular diseases, the risk of CVD could have been underestimated in our study. However, as the protective effect of aspirin and statin on recurrent VT has only been discovered in recent years, whereas the patients in our study experienced a first VT between 1999 and 2004, it is unlikely that patients would have received more aspirin or statin than control participants. Nevertheless, we cannot rule out a slight underestimation of our risk estimates, as some patients could have been prescribed cardioprotective drugs more often than controls through increased medical attention after VT. Finally, information on clinical characteristics and procoagulant markers was only obtained once. It is possible that characteristics such as weight and smoking status and levels of procoagulant markers could have changed over time. This may explain why the fully adjusted risk estimate did not reach 1.0 (residual confounding). Future studies with multiple measurements could be useful to investigate this further.
In conclusion, we found individuals with VT to have an increased risk of subsequent CVD. This could be explained by the presence of common etiologic factors.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Statistics Netherlands for making data from the Dutch Hospital Data registry available. This study was supported by grants from the Netherlands Heart Foundation (NHS 98.113), the Dutch Cancer Foundation (RUL 99/1992), and the Netherlands Organization for Scientific Research (912-03-033 2003). Dr Lijfering is a Postdoctoral Researcher of the Netherlands Heart Foundation (2011T012).
Authorship
Contribution: R.E.J.R. performed the statistical analyses and drafted the manuscript; W.M.L. designed the analyses and revised the manuscript; L.E.F. collected the data and revised the manuscript; F.R.R. was responsible for the MEGA study concept and design and revision of the manuscript; and S.C.C. designed the analyses and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Suzanne C. Cannegieter, Department of Clinical Epidemiology, Leiden University Medical Center, PO Box 9600, 2300 RC Leiden, The Netherlands; e-mail: s.c.cannegieter@lumc.nl.