In this issue of Blood, Momi et al demonstrate that inhibition of the Glycoprotein Ib (GPIb)–von Willebrand factor (vWF) interaction dissolves newly formed intracranial vessel-occluding thrombi and reduces brain infarct size without increasing intracerebral hemorrhage in an experimental stroke model in guinea pigs.1
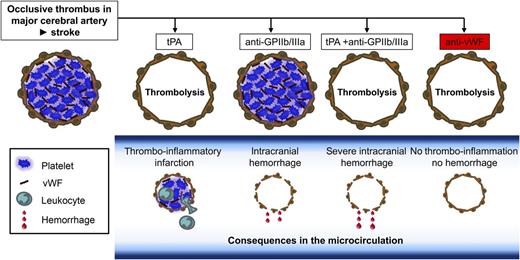
Inhibition of the GPIb-vWF axis exerts thrombolytic effects and prevents stroke progression in the absence of intracranial bleeding. Ischemic stroke is mainly caused by thromboembolic occlusion of a major cerebral artery (top panel, left). Treatment with tPA leads to recanalization of the occluded macrovessel, but in the study by Momi et al,1 it did not prevent thrombo-inflammation in the microcirculation and further stroke progression. Inhibition of platelet GPIIb/IIIa (top panel, anti-GPIIb/IIIa) has no thrombolytic effect but causes intracranial hemorrhage, which is even worse when given in combination with tPA (tPA + anti-GPIIb/IIIa). In sharp contrast, inhibition of the GPIb-vWF axis induces thrombolysis at early time points after thrombus formation and prevents thrombo-inflammatory damage of the microcirculation and infarct progression (top panel, right). The latter effect is even seen at later time points when the thrombolytic effect is lost due to thrombus stabilization (not shown).
Inhibition of the GPIb-vWF axis exerts thrombolytic effects and prevents stroke progression in the absence of intracranial bleeding. Ischemic stroke is mainly caused by thromboembolic occlusion of a major cerebral artery (top panel, left). Treatment with tPA leads to recanalization of the occluded macrovessel, but in the study by Momi et al,1 it did not prevent thrombo-inflammation in the microcirculation and further stroke progression. Inhibition of platelet GPIIb/IIIa (top panel, anti-GPIIb/IIIa) has no thrombolytic effect but causes intracranial hemorrhage, which is even worse when given in combination with tPA (tPA + anti-GPIIb/IIIa). In sharp contrast, inhibition of the GPIb-vWF axis induces thrombolysis at early time points after thrombus formation and prevents thrombo-inflammatory damage of the microcirculation and infarct progression (top panel, right). The latter effect is even seen at later time points when the thrombolytic effect is lost due to thrombus stabilization (not shown).
Stroke is the second leading cause of death and disability worldwide.2 Since most ischemic strokes are caused by an embolic or thrombotic occlusion of an intracranial artery, recanalization of the occluded artery with reperfusion of the ischemic brain areas is the primary therapeutic goal. Intravenous application of tissue plasminogen activator (tPA) within 4.5 hours after stroke onset is currently the only approved acute stroke treatment. Additional endovascular mechanical thrombectomy achieved significantly higher recanalization rates but, surprisingly, did not improve outcome.3 As a consequence, the majority of patients treated by thrombolysis or thrombectomy still have substantial disability. This indicates that brain infarction can progress in reperfused brain territories, a phenomenon called reperfusion injury that can also be reproduced in animal models of stroke.4 Thus, the development of more effective means to treat patients with acute ischemic stroke aimed at recanalization and simultaneous prevention of reperfusion injury is mandatory.
Platelets are key mediators of hemostasis and thrombosis. Therefore, platelet inhibitors are effective in the prevention of acute cardio- and cerebrovascular events, but they also increase the risk of clinical hemorrhage. Among the multiple surface receptors expressed in platelets, two are of particular importance for platelet adhesion and aggregation at the damaged or activated vessel wall. (1) GPIb interacts with the plasma protein vWF immobilized on the injured vessel wall or on activated platelets and thereby recruits platelets from the blood stream to the reactive surface under conditions of elevated shear. (2) GPIIb/IIIa is a receptor for both fibrinogen and vWF. GPIIb/IIIa requires inside-out activation mediated by agonist receptors, contributes to firm shear-resistant platelet adhesion and is essential for aggregate formation. Although both receptors may critically contribute to the formation of vessel-occluding thrombi that trigger ischemic stroke (vessel occlusion), their pathophysiological roles in the reperfusion injury during acute stroke appear to be markedly divergent.4,5
Pharmacologic blockade of GPIIb/IIIa, which has proven beneficial in patients undergoing percutaneous coronary intervention, was not effective in the treatment of acute stroke in patients6 and mice,5 but it induced pronounced intracranial hemorrhage in both species, especially in combination with recanalization procedures such as mechanical thrombectomy.7 In sharp contrast, inhibition of the vWF binding site on GPIb by antibodies5 or the absence of vWF8 profoundly protected mice from brain infarction in the transient middle cerebral artery occlusion model of acute stroke while not increasing the incidence of intracranial hemorrhage. Remarkably, the GPIb-blocking antibodies were still protective when administered after ischemia, indicating that targeting of the GPIb-vWF interaction might be therapeutically beneficial in acute stroke.
The current study by Momi et al1 demonstrates that ALX-0081, a divalent humanized nanobody directed against the GPIb-binding site on vWF (A1 domain), effectively dissolved newly formed intracranial thrombi leading to reperfusion and reduced infarct size in guinea pigs (see figure). ALX-0081 treatment was as effective as tPA in re-establishing cerebral blood flow when given at early but not late time points after vessel occlusion, indicating that it only exerts its thrombolytic activity before the thrombus is stabilized by fibrin formation and possibly other interactions that are not established at early phases. This is the first demonstration that a newly formed thrombus within the cerebral circulation can be dissolved by targeting platelet adhesion mechanisms, indicating that platelet-platelet interactions in such early thrombi are principally reversible and therefore “druggable.” Moreover, ALX-0081 reduced microvascular thrombus formation in the ischemic brain parenchyma at a stage when it was no longer effective as a thrombolytic agent at the site of the major stroke-inducing middle cerebral artery clot. In contrast, the GPIIb/IIIa inhibitor tirofiban obviously had no effect on microvascular patency, which is in agreement with previous observations in humans and mice where GPIIb/IIIa inhibition had no beneficial effect on infarct progression.5,6
How can the divergent effects between GPIIb/IIIa and GPIb-vWF–targeted treatment strategies be explained? Thrombus formation requires both platelet tethering via GPIb-vWF and platelet aggregation via GPIIb/IIIa. However, increasing experimental evidence suggests that ischemic brain infarction is not simply the consequence of thrombotic occlusion of intracerebral vessels, but that it also has an acute inflammatory component that links GPIb-vWF interactions to immune cell recruitment and breakdown of the blood brain barrier by unknown mechanisms.4 An involvement of GPIb-vWF interaction in immune cell recruitment and inflammation has also been demonstrated in other disease models such as experimental peritonitis.9 Thus, some of the effects seen with ALX-0081 nanobodies in the microvasculature in the study by Momi et al1 may be due to anti-inflammatory activity of the compound. Another remarkable result presented by Momi et al is the observation that ALX-0081 could be combined with low-dose tPA to improve thrombolytic activity without causing major intracranial hemorrhage. This confirms the previous notion that GPIb-vWF interactions are not required for maintaining vascular integrity in the ischemic brain, whereas GPIIb/IIIa appears to be absolutely critical.5-7
Given the multiple failures in improving acute stroke outcome by treatment with conventional platelet aggregation inhibitors such as acetylsalicylic acid10 and the GPIIb/IIIa inhibitors abciximab6 or tirofiban,7 the findings by Momi et al1 may pave the way for novel therapeutic options by targeting both primary thrombus formation and secondary thrombo-inflammatory processes during reperfusion of brain tissue.
Conflict-of-interest disclosure: The authors declare no competing financial interests.