Key Points
Haploidentical, unmanipulated, G-CSF–primed bone marrow transplantation.
Haploidentical hematopoietic stem cell transplantation for hematologic malignancies.
Abstract
Eighty patients with high-risk hematologic malignancies underwent unmanipulated, G-CSF–primed BM transplantation from an haploidentical family donor. Patients were transplanted in first or second complete remission (CR, standard-risk: n = 45) or in > second CR or active disease (high-risk: n = 35). The same regimen for GVHD prophylaxis was used in all cases. The cumulative incidence (CI) of neutrophil engraftment was 93% ± 0.1%. The 100-day CIs for II-IV and III-IV grade of acute GVHD were 24% ± 0.2% and 5% ± 0.6%, respectively. The 2-year CI of extensive chronic GVHD was 6% ± 0.1%. The 1-year CI of treatment-related mortality was 36% ± 0.3%. After a median follow-up of 18 months, 36 of 80 (45%) patients are alive in CR. The 3-year probability of overall and disease-free survival for standard-risk and high-risk patients was 54% ± 8% and 33% ± 9% and 44% ± 8% and 30% ± 9%, respectively. In multivariate analysis, disease-free survival was significantly better for patients who had standard-risk disease and received transplantations after 2007. We conclude that unmanipulated, G-CSF–primed BM transplantation from haploidentical family donor provides very encouraging results in terms of engraftment rate, incidence of GVHD and survival and represents a feasible, valid alternative for patients with high-risk malignant hematologic diseases, lacking an HLA identical sibling and in need to be urgently transplanted.
Introduction
Although the best results with allogeneic hematopoietic BM transplantation (BMT) are obtained in patients receiving the graft from an HLA genotypically identical sibling, this type of donor is only available for less than 30% of patients. In the last 3 decades, for patients lacking an HLA-matched family donor the main alternative was an 8/8 HLA antigens matched unrelated donor (MUD) allocated mainly through the international registries of volunteer donors.1,2 As of June 2012, the number of such volunteer donors exceeded 19 million,3 but almost two-thirds of patients do not reach transplantation. The enormous variability of HLA polymorphisms and the time required for identifying a suitable donor are the 2 most important factors limiting the use of MUD transplantation, especially for patients at high risk of disease progression in whom there is urgency for transplantation. For these patients, 2 potential alternative sources of graft remain available: umbilical cord blood (UCB) and HLA haploidentical related donors.4,5 UCB offers the advantages of ready availability and faster procurement of cryopreserved stem cells, no risk for the donors, low risk of transmissible infection, potentially reduced risk of GVHD, and less stringent criteria for donor-recipient HLA matching.6 The results of UCB transplantation in children and adults are well established7-10 and comparable with those reported for MUD transplantations.11,12 However, the low number of hematopoietic stem cells contained in a single UCB unit limits its use for transplantation and, despite several alternative strategies that are currently being explored,13-16 it remains the main obstacle for successful UCB transplantation, particularly in adults. Considering that virtually all patients have at least 1 HLA-haploidentical family member, when neither matched sibling donor nor MUD nor UCB are available, transplantation from a haploidentical family donor represents a valid alternative for patients with high-risk hematologic malignancies.5,17 Together with immediate donor availability, the more adjustable management of graft procurement, the cost savings for the donor search and, if indicated, the possibility of posttransplantation cellular therapies represent other important advantages of haploidentical transplantation. Historically, in haploidentical transplantation, the high risk of both graft failure and, conversely, of acute GVHD has been overcome by infusing megadoses of ex vivo T cell–depleted CD34+ purified peripheral blood stem cells (PBSCs).18,19 However, because of the almost complete T-cell depletion, immune reconstitution is slow, leading to a high frequency of mainly viral and fungal infection complications and a high relapse rate. Recently, encouraging results have been reported with an alternative approach enabling haploidentical transplantations with an unmanipulated non-T cell–depleted graft using a vigorous pre- and posttransplantation pharmacologic GVHD prophylaxis.20-24 In the present study, we report the results of 80 patients with high-risk hematologic malignancies who underwent an unmanipulated BM graft from an haploidentical family donor primed with low-dose G-CSF and received an identical regimen for GVHD prophylaxis.
Methods
Eligibility criteria
All patients affected by malignant hematologic disease in active status or in complete remission (CR) but at high risk of progression were offered the option of haploidentical transplantation if they fulfilled the following criteria: (1) no available ≥ 8/10 HLA antigen MUD in the preliminary search through the international volunteer donor registry; (2) no available cord blood unit suitable for transplantation on the base of cellularity (nucleated cells > 3 × 107/kg recipient body weight) and HLA compatibility (at least 4/6 class 1 or 2 identical HLA antigens by molecular typing); and (3) expected interval time to transplantation of less than 3 months. This study includes data from 80 patients with hematologic malignancies who received an allogeneic BMT from a haploidentical related donor in 4 transplantation centers (Rome, Italy, n = 38; Pescara, Italy, n = 36; Ancona, Italy, n = 4; and Tel-Hashomer, Israel, n = 2) between August 2005 and October 2010. The primary end points of the study were engraftment, acute GVHD, and 1-year treatment-related mortality (TRM); the secondary end points were chronic GVHD, relapse, overall survival (OS) and disease-free survival (DFS). The study was approved by the institutional review board of each participating institution. Informed consent for the treatment was obtained from all patients and donors or their legal guardians in accordance with the Declaration of Helsinki.
Patients
Patients (male, 65%) had a median age of 37 years (range, 5-71) with 29% more than 50 years of age (Table 1). The patients had the following range of underlying diseases: acute myeloid leukemia (AML, n = 45), acute lymphoblastic leukemia (ALL, n = 15), chronic myeloid leukemia (CML, n = 5), Hodgkin lymphoma (n = 5), myelodysplastic syndrome (n = 3), plasma cell leukemia (n = 3), myelofibrosis (n = 2), or non-Hodgkin lymphoma (n = 2). Patients transplanted in first (n = 30) or second (n = 15) CR were considered as standard-risk. The remaining 35 patients (44%) were considered as high-risk if they received transplantation in ≥ CR3 (n = 3), in second chronic phase of CML (n = 4), or in refractory or recurrent active disease (n = 28). For the 21 patients with AML transplanted in CR1, the risk factors were: refractoriness to first-line chemotherapy (n = 9), secondary leukemia (n = 4), hyperleukocytosis with complex karyotype (n = 4), FLT-3/ITD positivity (n = 3), and engraftment failure following autologous PBSC transplantation (n = 1). For the 8 patients with ALL transplanted in CR1, the risk factors were: Philadelphia chromosome positivity (n = 4), refractoriness to first-line chemotherapy (n = 2), and hyperleukocytosis with T-cell phenotype (n = 2). Nineteen patients (23%) had been previously transplanted: 16 with autologous PBSCs and 3 with allogeneic BM (2 from an HLA-identical sibling and 1 from an MUD). The median interval from date of diagnosis to BMT for all patients was 11 months (range, 3-442).
Donors
Donors (male, 54%) had a median age of 39 years (range, 16-72) and were represented by (in order): siblings (47%), mothers (25%), children (22%), fathers (5%), and cousins (1%). In the case of multiple available donors, the mother had priority, followed by the youngest male adult donor within the family. Donor-recipient CMV status and ABO matching were also considered for donor selection. The donor-recipient combinations were female to male in 30%, negative to CMV positive in 17%, with a donor-recipient CMV negativity occurring in only 4% of cases and ABO minor and major incompatibility in 15% and 38%, respectively. Patients and their donors were typed for HLA-A, HLA-B, HLA-DR, and HLA-C loci by at least intermediate-resolution DNA typing, and HLA-DRB1, HLA-DQB1, and HLA-DPB1 typing by high-resolution techniques. All donors were HLA identical for 1 haplotype and mismatched for 2 (n = 28, 35%) or 3 (n = 52, 65%) A, B, or DR loci on the unshared haplotype (Table 1).
Conditioning regimen
A myeloablative conditioning (MAC) regimen was used in 64 (80%) patients and a reduced intensity conditioning (RIC) in 16 (20%) patients. From April 2005 to April 2008, for the first 29 patients, the MAC regimen included cytarabine 3 g/m2/d IV in 2 divided doses for 3 days and cyclophosphamide 45 mg/kg/d for 2 days associated with 10 Gy of total body irradiation (TBI) in 4 fractions over 2 days (n = 7) or treosulfan 14 g/m2/d for 3 days (n = 11) or oral busulfan 16 mg/kg for 4 days (n = 11). In the same period of time, 3 patients received a RIC regimen including fludarabine alone at 160 mg/m2 over 4 days because of persistent engraftment failure following autologous PBSC transplantation (n = 1) or thiotepa 5 mg/kg/d for 1 day, followed by fludarabine 150 mg/m2 over 3 days and melphalan 140 mg/m2 for 1 day (n = 2). Since May 2008, the 2 centers of Rome and Pescara, contributing the highest number of patients in this study, changed the general strategy of allogeneic transplantation by adopting an identical conditioning regimen not including TBI for the different sources of hematopoietic stem cells: HLA identical sibling, MUD, UCB, or haploidentical donor. According to this new transplantation policy, a uniform chemotherapy-based conditioning regimen was used in the MAC and RIC version consisting, respectively, of thiotepa 5 mg/kg/d at days −7 and −6, busulfan 3.2 mg/kg/d in a single IV infusion over 3 hours combined with fludarabine 50 mg/m2/d IV in 1 hour at days −5, −4, and −3 (TBF-MAC), as described recently by the Spanish group for cord blood transplantation,25 or thiotepa 5 mg/kg on day −6, Busulfan 3.2 mg/kg/d in a single IV infusion over 3 hours at days −5 and −4 and fludarabine 50 mg/m2/d IV in 1 hour at days −5, −4, and −3 (TBF-RIC). Thirty-five patients were conditioned with TBF-MAC and 13 received TBF-RIC.
GVHD prophylaxis
All patients received the same GVHD prophylaxis described previously by Ji et al20 and consisting of a combination of 5 drugs with different points of attack: (1) antithymocyte globulin (Fresenius AT) infused intravenously at 5 mg/kg from day −4 to −1; (2) cyclosporine (CsA) given as continuous IV 12-hour infusion at 1.5 mg/kg/d from day −7 to −2 and at 3 mg/kg/d from day −1 until patients were able to tolerate oral medication; then, CsA was given orally at 5-6 mg/kg/d in 2 divided doses (the CsA doses were adjusted on the basis of plasma levels (150 to 350 ng/mL) and hepatic and renal toxicity; starting from day +180 and in the absence of chronic GVHD, the CsA dose was progressively tapered by 5% every week and stopped by day +365); (3) methotrexate (MTX) was given intravenously at 15 mg/m2 on day 1 and at 10 mg/m2 at day 3, 6, and 11; (4) mycophenolate mofetil (MMF) administered orally at 15 mg/kg/d in 2 divided doses from day 7 to 100 (the MMF doses were adjusted on the basis of BM toxicity; and (5) basiliximab (Simulect; Novartis Pharma), an anti-CD25 mAb given at fixed dose of 20 or 10 mg to patients with a body weight, respectively, exceeding or inferior to 35 kg as a 30-minute IV infusion on day 0 (2 hours before graft infusion) and on day 4. Diagnosis and clinical grading of acute and chronic GVHD were established according to the standard criteria.26-28
Collection of hematopoietic cells
The day of transplantation was designated as day 0. All donors were primed with 4 μg/kg/d of G-CSF (Filgrastim; Granulokine) in a single daily subcutaneous injection for 7 consecutive days from days −7 through −1. BM cells were harvested from the posterior iliac crests on day 0 with a target volume collection of 15 mL/kg recipient body weight. Fresh and unmanipulated BM cells were infused into the recipient on the same day.
Evaluation of engraftment and donor chimerism
Neutrophil and platelet engraftment was defined as the first of 3 consecutive days with an absolute neutrophil count of ≥ 0.5 × 109/L and an absolute platelet count of ≥ 25 × 109/L, respectively. Chimerism was evaluated either on BM cells or PBSCs on days 30, 90, 180, and 365. Sex-mismatched donor-recipient chimerism was evaluated by cytogenetic G-banding or FISH. Sex-matched donor-recipient chimerism was assessed by PCR-based analyses of polymorphic microsatellite regions by short number tandem repeats. HLA typing was performed after transplantation as confirmation of engraftment.
Supportive therapy and infection prophylaxis
All patients were hospitalized in HEPA-filtered rooms and received anti-infectious prophylaxis with oral trimethoprim-sulfamethoxazole (days −10 to −2), fluconazole (from day −10), acyclovir (from day −1), and ciprofloxacin (from day −1). All blood products were irradiated with 2500 cGy. CMV and EBV were regularly monitored in the blood by PCR assays.
Definitions
Primary failure of engraftment was defined as no evidence of myeloid donor cells in the recipient BM at day 28 after transplantation. The incidence of acute GVHD was evaluated in all patients with evidence of engraftment. The incidence of chronic GVHD was evaluated in patients surviving more than 100 days after transplantation with allogeneic engraftment. TRM was defined as death from any cause except relapse. Relapse was defined by molecular, cytogenetic, or morphologic evidence of the original hematologic disease in the peripheral blood, BM, or any extramedullary site. Overall survival was defined as time to death from all causes. Disease-free survival was defined as time to relapse or death in remission.
Statistical analysis
A descriptive analysis of all variables was performed including mean, median, standard deviation, range, minimum and maximum value for continuous variables, absolute and relative frequencies for categorical variables. Using parametric and nonparametric statistical procedures (Chi-square test, Fisher exact test and rank correlation coefficient of Spearman), the possible interdependence between 2 or more variables was evaluated and a P value of < .05 was considered significant. OS and DFS were estimated by the product-limit method of Kaplan-Meier29 and the curves of various subgroups were compared using the log-rank test.30
Taking into consideration the corresponding competing risks, the probabilities of neutrophil and platelet engraftment, acute and chronic GVHD, TRM and disease relapse were estimated with the CI method31 and the curves of various subgroups were compared using the Gray test.32 Using the stepwise selection procedure for all of the variables of interest (diagnosis, age, disease stage at transplantation, donor type, conditioning, and year of transplantation), the Cox proportional hazard model33 was used for multivariate analyses of OS and DFS. The joint effect of variables on TRM and relapse was evaluated using the multivariate model of Fine and Gray.34 The analyzed data are from patients transplanted from August 2005 to October 2010. The analysis was conducted using 2 statistical software packages: SAS Version 9.1.3 and R Version 2.11.0.
Results
BM cell composition
A median of 7.4 × 108/kg (range, 1-29) total nucleated cells, 0.6 × 108/kg (range, 0.3-5) mononucleated cells, 2 × 106/kg (range, 0.7-11) CD34+ cells, and 2.9 × 107/kg (range, 1-9.8) CD3+ cells were infused into the recipients. No untoward side effect related to G-CSF administration or BM harvest was reported by donors.
Engraftment
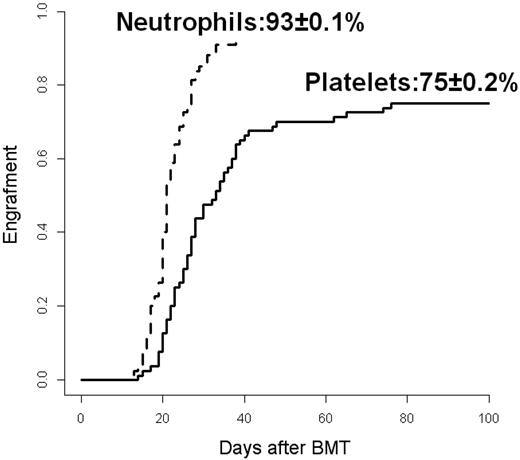
Six patients died of transplantation-related complications before day 21 after transplantation without myeloid recovery. One patient showed primary engraftment failure and died of infection on day 29. Seventy-three (91%) patients achieved a trilineage engraftment with a median time of 21 days (range, 12-38) for absolute neutrophil count and 28 days (range, 14-185) for platelets. The 100-day CI for neutrophil and platelet engraftment was 93% ± 0.1% and 75% ± 0.2%, respectively (Figure 1). For all engrafted patients, the chimerism at 60 days was of full donor origin. The median number of RBC and platelet units from single donor transfused over the first 100 days after transplantation was 9 (range, 1-52) and 14 (range, 2-70), respectively.
Engraftment. CI for neutrophils (continuous line) and platelets (dotted line).
Acute and chronic GVHD incidence and severity
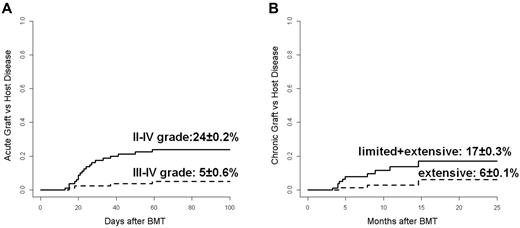
Among 73 evaluable cases, no signs of acute GVHD were observed in 38 patients (52%). Acute GVHD occurred at a median time of 26 days (range, 12-130) after transplantation and was grade I in 14 patients (19%), grade II in 16 (22%), grade III in 2 (3%), and grade IV in 3 (4%). The CI of grade II-IV and grade III-IV acute GVHD at 100 days was 24% ± 0.2% and 5% ± 0.6%, respectively (Figure 2A).
Acute and chronic GVHD. (A) CI of grade II-IV (continuous line) and grade III-IV (dotted line) acute GVHD. (B) CI of limited plus extensive (continuous line) and extensive alone (dotted line) chronic GVHD.
Acute and chronic GVHD. (A) CI of grade II-IV (continuous line) and grade III-IV (dotted line) acute GVHD. (B) CI of limited plus extensive (continuous line) and extensive alone (dotted line) chronic GVHD.
No chronic GVHD was observed in 49 of 59 evaluable patients (83%); 7 patients (12%) experienced a limited form and 3 (5%) an extensive form of chronic GVHD at a median time of 145 days (range, 100-445) after transplantation. The CI of overall (limited and extensive) and only extensive chronic GVHD at 2 years was 17% ± 0.3% and 6% ± 0.1%, respectively (Figure 2B). Complete resolution of the clinical manifestations of chronic GVHD with discontinuation of any immunosuppressive treatment was observed in all 7 patients with the limited form and in 2 of 3 patients with the extensive form, respectively. Only 1 patient continues to receive immunosuppressive therapy at time of the analysis.
TRM and complications
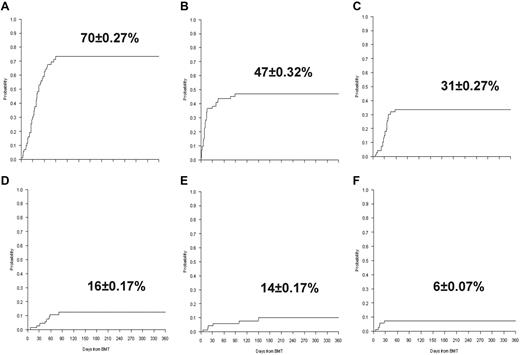
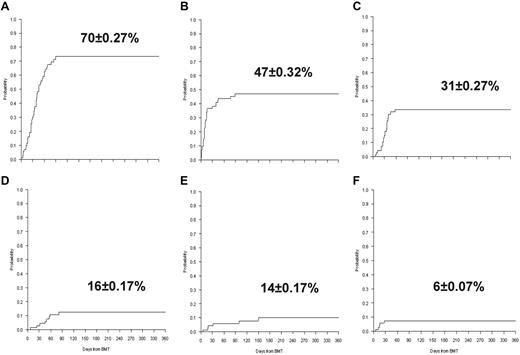
Twenty-seven patients (34%), 13 in the standard-risk group and 14 in the high-risk group, respectively, died from transplantation-related complications at a median time of 76 days (range, 6-369). Causes of death included infections in 11 patients (14%), pneumonia in 5 (6%), multiorgan failure in 5 (6%), acute GVHD in 3 (4%), liver failure in 1 (1%), venoocclusive disease in 1 (1%), and CNS disease complications in 1 (1%). The CI of TRM was 32% ± 0.3% at 6 months and 36% ± 0.3% at 1 and 3 years. Although the TRM was higher in the high-risk group compared with the standard-risk group (45% ± 0.1% vs 30% ± 0.5% at 3 years), the difference was not statistically different. Furthermore, no difference was found for the 3-year CI of TRM between patients (n = 35) prepared with TBF-MAC and patients (n = 29) conditioned with other MAC regimens (39% ± 01% vs 32% ± 0.7%; P = .58). In multivariate analysis, no significant factor was found to affect TRM. In the first 6 months after transplantation, 56 patients developed CMV reactivation (CI, 70% ± 0.27%), 38 bacterial septicemia (CI, 47% ± 0.32%), 25 hemorrhagic cystitis (CI, 31% ± 0.27%), 13 CNS complications (CI, 16% ± 0.17%), 10 fungal infections (CI, 14% ± 0.17%), and 5 venoocclusive disease (CI, 6% ± 0.07%; Figure 3).
Transplantation-related complications. (A) CI of CMV. (B) CI of bacterial infections. (C) CI of hemorrhagic cystitis. (D) CI of CNS complications. (E) CI of fungal infections. (F) CI of venoocclusive disease.
Transplantation-related complications. (A) CI of CMV. (B) CI of bacterial infections. (C) CI of hemorrhagic cystitis. (D) CI of CNS complications. (E) CI of fungal infections. (F) CI of venoocclusive disease.
Relapse
Eighteen patients (10 in the standard-risk group and 8 in the high-risk group) relapsed after a median time of 180 days (range, 56-467) after transplantation. Ten patients had AML, 7 ALL, and 1 plasma cell leukemia. The overall CI of relapse was 21% ± 0.2% at 1 year and 28% ± 0.3% at 5 years, respectively. The CI of relapse was not significantly different between patients in the standard-risk group and those in the high-risk group at 1 year (17% ± 0.2% vs 28% ± 0.7%; P = .19) and 3 years (26% ± 0.5% vs 28% ± 0.7%; P = .38), respectively. Although the difference was not statistically significant, the 3-year CI of relapse for the 35 patients conditioned with TBF-MAC regimen was remarkably lower than for the 31 patients receiving other MAC regimens (24% ± 07% vs 39% ± 0.8%; P = .19). All leukemia relapses occurred in the BM, with no extramedullary relapses. Among the 18 relapsed patients, 14 died of leukemia progression or chemotherapy-related complications and 4 patients are currently surviving in CR, 2 following chemotherapy reinduction and 2 after a second haploidentical BMT from a different donor. In multivariate analysis, the TBF-MAC regimen was the only significant factor for lower relapse rate (Table 2).
OS and DFS
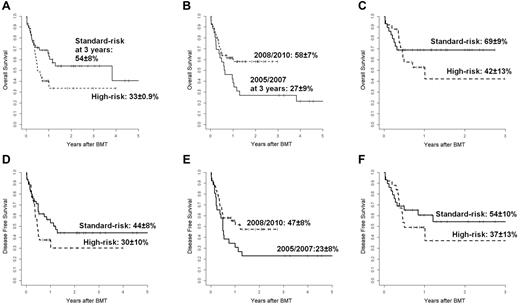
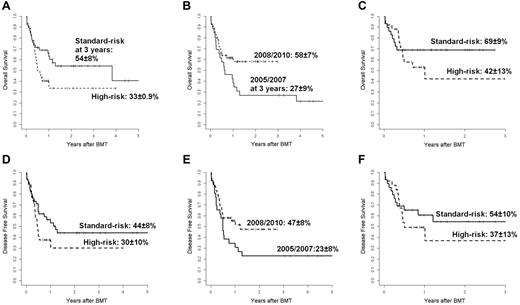
The 3-year probability of OS for all patients was 45% ± 6%: 54% ± 8% for patients in the standard-risk group and 33% ± 9% for patients in the high-risk group (P = .06; Figure 4A). Although patient and donor characteristics were not statistically different before and later than 2007 (data not shown), the 3-year probability of OS was significantly better for the 54 patients transplanted after 2007 (58% ± 7% vs 27% ± 9%; P = .04; Figure 4B). Of these patients, 29 were standard-risk and 25 high-risk at the time of transplantation and the 3-year OS was 69% ± 9% and 42% ± 13%, respectively (P = NS; Figure 4C). Most of these patients were conditioned with TBF regimen, MAC (n = 35), or RIC (n = 13), and 6 patients received other MAC (n = 4) or RIC (n = 2) regimens; 4 of these last patients are alive in continuous CR. The 1-year probability of OS was higher for 13 patients conditioned with TBF-RIC compared with 35 patients conditioned with TBF-MAC (77% ± 12% vs 51% ± 9%; P = NS). At a median follow-up of 18 months (range, 6-74), 36 patients (45%; 20 AML, 5 ALL, 3 CML, 3 Hodgkin lymphoma, 2 myelodysplastic syndrome, 2 myelofibrosis, and 1 non-Hodgkin lymphoma) are surviving in CR. Of these patients, 25 were standard-risk and 11 high-risk at the time of transplantation. The 3-year probability of DFS was 38% ± 6% for all patients: 44% ± 8% for the standard-risk patients and 30% ± 10% for the high-risk patients (P = NS; Figure 4D). Among 28 patients transplanted with active disease, 12 (43%) died of transplantation-related causes and 5 (18%) of relapse; 11 patients (39%) are alive and disease-free with a median follow-up of 14 months (range, 6-40). For the 54 patients transplanted later than 2007, the DFS was better than that of the 26 patients transplanted before 2007 (47% ± 8% vs 23% ± 8%, P = .059; Figure 4E). The DFS of the 29 standard-risk patients grafted after 2007 was higher, although not significantly different, than that of the 25 high-risk patients transplanted in the same period (54% ± 10% vs 37% ± 13%; (Figure 4F). In the Cox model, the risk category (standard-risk vs high-risk) and the year of transplantation (2005-2007 vs 2008-2010) were the only 2 variables significantly affecting both OS (P < .03 and P < .02, respectively) and DFS (P < .05 and P < .03, respectively; Table 2). Recipients of BM from haploidentical mothers showed an advantage in terms of both OS and DFS compared with patients transplanted from other related haploidentical donors, but this difference was not statistically significant, with a hazard ratio of 1.2 (95% confidence interval, 0.59-2.49) and 1.32 (95% confidence interval, 0.66-2.49), respectively.
Overall and disease-free survival. (A) Probability of OS according to disease risk. Shown are standard-risk (n = 45, continuous line) and high-risk (n = 35, dotted line) patients. (B) Probability of OS according to year of transplantation. Shown are 2005-2007 (n = 26, continuous line) and 2008-2010 (n = 54, dotted line). (C) Probably of OS for 54 patients transplanted over the years 2008-2010. Shown are standard-risk patients (n = 29, continuous line) and high-risk patients (n = 25, dotted line). (D) Probability of DFS according to disease risk. Shown are standard-risk patients (n = 45, continuous line) and high-risk patients (n = 35, dotted line). (E) Probably of DFS according to year of transplantation. Shown are 2005-2007 (n = 26, continuous line) and 2008-2010 (n = 54, dotted line). (F) Probability of DFS for 54 patients transplanted over the years 2008-2010. Shown are standard-risk patients (n = 29, continuous line) and high-risk patients (n = 25, dotted line).
Overall and disease-free survival. (A) Probability of OS according to disease risk. Shown are standard-risk (n = 45, continuous line) and high-risk (n = 35, dotted line) patients. (B) Probability of OS according to year of transplantation. Shown are 2005-2007 (n = 26, continuous line) and 2008-2010 (n = 54, dotted line). (C) Probably of OS for 54 patients transplanted over the years 2008-2010. Shown are standard-risk patients (n = 29, continuous line) and high-risk patients (n = 25, dotted line). (D) Probability of DFS according to disease risk. Shown are standard-risk patients (n = 45, continuous line) and high-risk patients (n = 35, dotted line). (E) Probably of DFS according to year of transplantation. Shown are 2005-2007 (n = 26, continuous line) and 2008-2010 (n = 54, dotted line). (F) Probability of DFS for 54 patients transplanted over the years 2008-2010. Shown are standard-risk patients (n = 29, continuous line) and high-risk patients (n = 25, dotted line).
Discussion
After myeloablative conditioning and standard (MTX-CsA) GVHD prophylaxis, the outcome of patients undergoing allogeneic transplantation from a 2 or 3 HLA antigen-mismatched family donor is particularly dismal because they have the highest risk of graft rejection, acute GVHD and, therefore, TRM.35,36 The incidence of primary graft failure is correlated significantly with the degree of HLA incompatibility in the host-versus-graft direction, being 12.3% among BM transplants from HLA partially matched donors compared with 2.0% among recipients from an HLA-identical sibling.37 To favor engraftment and prevent GVHD, several different procedures have been adopted for patients undergoing an haploidentical transplantation after either an MAC or RIC regimen and using either manipulated (T cell–depleted) or unmanipulated (non-T cell–depleted) graft with more intensive in vivo GVHD prophylaxis.19-24,38-42 However, in patients receiving a T cell–depleted graft, the risk of graft failure remains in the range of 9%-15% and the incidence of ≥ grade II acute and chronic GVHD ranges from 8%-59% and 7%-14%, respectively. Conversely, among recipients of unmanipulated grafts, the risk of graft failure is 0.4%-13%, whereas the incidence of ≥ grade II acute and chronic GVHD is 16%-55% and 14%-74%, respectively.
In the present study, we analyzed the clinical outcome of 80 patients with high-risk hematologic malignancies who underwent allogeneic BMT from a haploidentical donor. Our transplantation strategy was based on the use of unmanipulated BM cells harvested from donors primed with low-dose G-CSF and on the administration of an intensive GVHD prophylaxis. Primary end points of our study were engraftment, acute-GVHD, and 1-year TRM. Several studies—in vitro studies, experimental models, and, most importantly, in the clinical setting—have demonstrated that relevant quantitative and qualitative modifications in the BM cell composition and function are induced by G-CSF priming. After G-CSF stimulation, the number of BM CD34+ cells increases 1.4- to 1.7-fold, the number of colony-forming cells 3-fold and the number of long-term culture-initiating cells 50- to 90-fold.43 Furthermore, G-CSF exerts an intense immunoregulatory effect on BM T cells by down-regulating the expression of adhesion and CD28/B7 molecules and by increasing the absolute number of DC2 APCs favoring a T-cell shift from Th1- to Th2-type cells and inducing an higher production of IL-4 and IL-10 anti-inflammatory cytokines.44-47 In transplantations from HLA identical siblings, engraftment of G-CSF–primed BM cells is faster, with an incidence of ≥ grade II acute GVHD of only 6.3%.48
In our study, the cumulative incidence of myeloid engraftment has been very high (93% ± 0.1%), with a median time to engraftment in the range of that usually observed in patients transplanted from an HLA-identical sibling. Only 1 evaluable patient did not achieve engraftment and died in aplasia at day 29. After an unmanipulated BMT from partially matched or haploidentical relatives, it is mandatory to use a highly effective regimen of GVHD prophylaxis to reduce the incidence and severity of acute GVHD. For all of our patients, an identical regimen of GVHD prophylaxis was adopted according to the protocol reported by Ji et al20 and based on the classic CsA and MTX combination with the addition of sequential immunosuppressive drugs given before (antithymocyte globulin), at the time of (anti-CD25 mAb basiliximab), and after (MMF) the G-CSF–primed BM graft infusion. However, with respect to the experience of the Chinese group, the higher number of patients, the multicentric nature of our study, and the use of the TBF conditioning regimen not including TBI provide relevant novelty to the present report. Indeed, the cumulative incidence of advanced grades acute and chronic GVHD was only 5% and 6%, respectively. Acute GVHD was the main cause of death in only 3 patients and all but 1 evaluable patient had complete resolution of the clinical manifestations of chronic GVHD and are free of immunosuppressive therapy. Most patients surviving 1 year after BMT returned to their full social and work activity. All of the patients in our series had underlying diseases with very poor prognosis and were at high risk of developing clinical complication. Of them, 35 were transplanted in active disease phase and most were heavily pretreated, with 23% having received a previous transplantation and 29% over the age of 50 years, including 7 patients older than 60 years. In this high-risk group of patients, a TRM occurring in one-third of patients with most events observed within the first 6 months after transplantation is not surprising. No fatal infection occurred after the first year from transplantation and no patient developed a after transplantation lymphoproliferative disease. In multivariate analysis, no factor was found to be significantly associated with TRM.
Using the combination of G-CSF–primed unmanipulated BM and peripheral blood as a source of hematopoietic stem cells for 250 patients transplanted from mismatched/haploidentical donors, Huang et al23 have reported an excellent rate of engraftment of 99.6%, a CI of II-IV and grade III-IV acute GVHD of 45.8% and 13.4%, respectively, and a CI of overall and extensive chronic GVHD of 53.9% and 22.6%, respectively. In that same study, TRM at 3 years ranged from 19.4%-59.8% depending on the diagnosis and the disease risk. The Chinese study included only acute leukemia patients, 16% of whom had only 1 HLA antigen disparity, with a median age of 25 years (range, 2-56) and higher proportion of patients receiving maternal grafts. Despite these differences in patient risk between the 2 cohorts, the primary end points achieved in the present study (engraftment, incidence of GVHD, and TRM) are comparable to the Chinese results. In particular, a clear reduction of the acute and chronic GVHD incidence can be observed in our patients with respect to that reported by the Chinese group (grade II-IV and III-IV acute GVHD: CI 24% vs 45.8% and 5% vs 13.4%, respectively; overall and extensive chronic GVHD: CI 17% vs 53.9% and 6% vs 22.6%, respectively).
In our study, the graft composition consisting of only G-CSF–primed BM by excluding peripheral blood and the use of the anti-CD25 mAb might have contributed to a better prevention of the GVHD. The number of patients and the heterogeneity of diagnosis do not allow evaluation of the impact of our transplantation procedure in preventing relapse for each single disease category, which was 21% ± 0.2% at 1 year and 28% ± 0.3% at 5 years for the whole group without significant difference between standard- and high-risk patients. Interestingly, 11 of 28 patients transplanted with active disease are disease free at 2 years after transplantation. The use of TBF-MAC was associated with a lower, although not statistically significant, risk of relapse and in multivariate analysis it was the only factor found to be significant in preventing relapse. This regimen initially proposed by Sanz et al25 in the setting of UCB transplantation seems particularly effective for recipients of an haploidentical unmanipulated BM transplantation. Indeed, in multivariate analysis, patients transplanted later than 2007, who were mostly conditioned with the TBF regimen and who had characteristics not substantially different from those of patients transplanted before 2007 (data not shown) had a significantly better outcome. Moreover, despite the relatively low number of patients and the rather short follow-up time, the observed 77% ± 12% OS at 1-year for patients conditioned with the RIC version of the TBF regimen is particularly encouraging.
Overall, the results of this study are comparable with those reported for similar series of high-risk patients lacking an HLA identical sibling and undergoing an allogeneic transplantation from a UCB or MUD.7-12 Several considerations can be drawn from this experience. First, the transplantation procedure consisting of a chemotherapy-based regimen not including TBI, combined with an intensive in vivo GVHD prophylaxis requiring neither expensive laboratory facilities nor personnel with high expertise in cell manipulation, allowed us to extend the practice of haploidentical transplantation to all centers involved in an allogeneic transplantation program. Furthermore, compared with UCB or MUD, haploidentical transplantation provides an easier management of the transplantation work-up and saves the relevant costs related to the search for the graft acquisition. Finally, because of the prompt donor availability, the potential use of donor lymphocyte infusion after transplantation for prevention or treatment of relapse is more guaranteed. To date, because no substantial differences in terms of patient outcomes are emerging from the retrospective studies of UCB, MUD, and haploidentical transplantations, and in absence of prospective randomized trials, all 3 options should be considered in the algorithm of search for an alternative donor. In this context, the G-CSF–primed, unmanipulated BM transplantation from an haploidentical family donor represents a valid alternative for patients with high-risk malignant hematologic diseases lacking an HLA-identical sibling donor and urgently requiring transplantation.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by grants from the Agenzia Regionale del Lazio per i Trapianti e le Patologie Connesse and from the Matteo Fabrizio Onlus Association.
Authorship
Contribution: P.D.B. and W.A. contributed patients, designed the study, analyzed the data, and wrote the manuscript; S.S., G.D.A., A.P., L.C., R.C., P.B., G.P., M.M., and A.N. contributed patients and critically reviewed the manuscript; G.A. provided transfusional and supportive care; S.A. and M.D.N. performed the statistical analysis; M.A. and F.P. provided the HLA typing; L.D.F. and M.C.R. provided the cell culture and molecular data; L.S. provided laboratory data and guidance in infectious complications; and all authors read and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: William Arcese, Department of Hematology, Stem Cell Transplant Unit, Rome Transplant Network, Tor Vergata University Hospital, 00133 Rome, Italy; e-mail: william.arcese@uniroma2.it.