Key Points
We have created a new highly active chimeric antigen receptor (CAR) specific for CD22.
The design of new CARs may benefit more from target antigen epitope selection than from optimizing affinity.
Abstract
Immune targeting of B-cell malignancies using chimeric antigen receptors (CARs) is a promising new approach, but critical factors impacting CAR efficacy remain unclear. To test the suitability of targeting CD22 on precursor B-cell acute lymphoblastic leukemia (BCP-ALL), lymphoblasts from 111 patients with BCP-ALL were assayed for CD22 expression and all were found to be CD22-positive, with median CD22 expression levels of 3500 sites/cell. Three distinct binding domains targeting CD22 were fused to various TCR signaling domains ± an IgG heavy chain constant domain (CH2CH3) to create a series of vector constructs suitable to delineate optimal CAR configuration. CARs derived from the m971 anti-CD22 mAb, which targets a proximal CD22 epitope demonstrated superior antileukemic activity compared with those incorporating other binding domains, and addition of a 4-1BB signaling domain to CD28.CD3ζ constructs diminished potency, whereas increasing affinity of the anti-CD22 binding motif, and extending the CD22 binding domain away from the membrane via CH2CH3 had no effect. We conclude that second-generation m971 mAb-derived anti-CD22 CARs are promising novel therapeutics that should be tested in BCP-ALL.
Introduction
Despite great progress in the treatment of children and adults with acute lymphoblastic leukemia (ALL), substantial numbers of patients continue to die of this disease and the short and long-term toxicities of standard therapy are substantial.1-3 Monoclonal antibody-based therapies offer promise for overcoming chemoresistance and potentially diminishing the toxicities associated with therapy.4 Among the most promising of these therapies involve the engineering of mature T lymphocytes to recognize MHC nonrestricted tumor antigens by transducing chimeric antigen receptors (CARs), reviewed by Lee at al.5 CARs incorporate an extracellular binding domain (often derived from the antigen binding region of an antibody) with transmembrane and signaling motifs to render T cells capable of targeting any surface antigen that is amenable to antibody-like recognition. Early clinical results have demonstrated impressive antitumor effects in patients with leukemia,6-10 although the ideal CAR design with respect to structural and signaling features remains unclear and has been the topic of intense inquiry.
B-cell antigens are compelling targets for CAR-based therapies because normal tissue expression of these antigens is restricted to the B-cell lineage and clinical tolerance for B-cell ablation is high using modern supportive care. Indeed, CARs targeting CD19 have demonstrated activity against B-cell malignancies with acceptable toxicity6-8 as have anti-CD20 antibodies in CD20+ malignancies, including CD20-expressing ALL.11 CD22 is another member of the B-cell antigen family with a tissue distribution that is similar to CD19. A Siglec-family lectin, CD22 consists of 7 extracellular IgG-like domains and is expressed on the B-cell surface starting at the pre-B cell stage, persists on mature B cells, and is lost on plasma cells, reviewed by Nitschke.12 CD22 has been validated as a successful target for B-cell leukemias and lymphomas using an immunotoxin approach.13 BL22 is a recombinant immunotoxin that consists of the scFv portion of an anti-CD22 antibody fused to PE38, a 38-kDa portion of Pseudomonas exotoxin A.14 A higher affinity mutant of the scFv portion (HA22) was generated to improve therapeutic response, has undergone testing, and has produced complete remissions in patients with drug-resistant hairy-cell leukemia.15 Both BL22 and HA22 mediate antitumor activity in B-cell precursor acute lymphocytic leukemia and therefore we tested their antigen binding domains for efficacy in the context of CAR therapy.16,17 We also developed a CAR incorporating an alternative fully human scFv, derived from mAb m971,18 which binds a more membrane proximal epitope on CD22, to investigate the impact of epitope selection on the efficacy of CAR-based therapy. In this report, we demonstrate that epitope specificity has a major impact on CAR efficacy because CD22-CARs incorporating the m971 binding domain mediate much more potent antileukemic activity in preclinical models than CD22-CARs of similar affinity targeting distinct epitopes. We further demonstrate that CD22 is essentially universally expressed on precursor B-ALL, and that second-generation CD22-CARs are more potent in preclinical models than those incorporating 2 costimulatory domains.
Methods
Cells and culture conditions
The following CD22+ and CD19+ B cell acute lymphoblastic leukemia (ALL) cell lines were used: REH (DSMZ ACC 22), SEM (DSMZ ACC 546), NALM6 (DSMZ ACC 128), and KOPN8 (DSMZ ACC 552). The K562 (ATCC) cell line, negative for CD22 and CD19, was used as a control. We also used the Daudi cell line, provided by Dr Daniel Vallera (University of Minnesota), and Raji (ATCC). Cells were cultured in RPMI 1640 (Invitrogen). The 293GP retroviral vector packaging cell line (Clonetech) was cultured in DMEM (Invitrogen). A CD19 CAR supernatant producer cell clone, H3, was kindly provided by Dr James Kochenderfer (NCI Surgery Branch, NIH) and was cultured as previously described.19 Human PBMCs from healthy donors were obtained from the Department of Transfusion Medicine, NIH clinical center, under an NIH IRB approved protocol after informed consent in accordance with the Declaration of Helsinki, then cultured in AIM-V (Invitrogen). All media were supplemented with 10% (5% for AIMV) heat-inactivated FBS (Gemini Bioproducts), 10mM HEPES, 100 U/mL penicillin, 100 ug/mL streptomycin, 2mM l-glutamine (Invitrogen). Recombinant human IL-2 (teceleukin, rhIL-2; Roche) 300 IU/mL was added to AIMV for T-cell culture.
Construction of chimeric antigen receptors
CD22 binding single chain fragment variable (scFv) sequences were derived from the RFB4 hybridoma (BL22) and from a mutated higher affinity derivative of RFB4 (HA22), as previously described.20 The creation of the m971 scFv was also previously described.18 For integration into the CAR vector, BL22, and HA22 scFv sequences were modified by removal of the first 2 amino acids, and a huGM-CSFR leader sequence was added.21 The CH2CH3 domains from IgG1 (Gene ID: 3500 IGHG1, aa 176-407) were included where designated. The CAR-encoding amino acid sequences were reverse translated, codon optimized, synthesized as single constructs (Mr Gene or Gene Art/Life Technologies), then subcloned into a MSGV-1–based retroviral backbone plasmid that encoded the designated intracellular signaling motifs. The second-generation CAR plasmids encoded either CD28 and CD3-ζ chain (derived from MSGV-4D5-28Z) or CD28 and 4-1BB signaling domains. The third-generation CAR plasmid encoded CD28, 41BB (CD137), and CD3-ζ chain signaling domains (MSGV-4D5-CD8-28BBZ) as previously described.21 Where indicated, the backbone vectors were also modified to express a CD8 transmembrane domain linked to 4-1BB and CD3-ζ signaling domains, using sequences kindly provided by Dr Carl June (University of Pennsylvania).
Retroviral vector production and transduction of T cells
CD22 CAR-encoding retroviral vectors were produced by transient transfection of the 293GP cell line as previously described.22 Briefly, 6 × 106 293GP cells were plated into poly-D lysine coated 10 cm plates (BD Bioscience). The following day, 293GP cells were transfected using lipofectamine 2000 (Invitrogen) with plasmids encoding the CD22 CAR and the RD114 envelope protein.21 Supernatants were collected 48 to 72 hours posttransfection, centrifuged at 3000 RPM for 10 minutes to remove cell debris, then stored at − 80°C. CD19 CAR retroviral supernatant was obtained from the H3 producer cell clone.19 T cells were activated on plates coated with 10 μg/mL OKT3 (Ortho) in AIMV media containing 40 IU/mL IL-2 for 48 hours. Retroviral supernatants were diluted 1:2 or 1:4 in AIMV media and applied to nontissue culture–treated 6-well or 24-well plates (BD Bioscience) coated with retronectin (per the manufacturer, Takara). Plates were centrifuged at 2000g for 2 hours at 32°C. Vector-containing supernatant was removed and activated T cells (1 × 106 cells/well in 6-well plates and 0.25 × 106 cells/well in a 24-well plates) were added to the coated wells in media containing 300 IU/mL IL-2. A second transduction was performed the following day. On the third day after transduction, cells were cultured at 3 × 105 cells/mL in AIMV containing 300 IU/mL IL-2 and expanded by feeding with fresh IL2-containing media every 2 to 3 days.
Flow cytometry and antigen binding site determination
Surface expression of CAR-transduced T cells was determined by flow cytometry. One × 106 CAR-transduced T cells were incubated with 0.2 μg CD22-Fc (R&D Systems) in 0.1-mL volume followed by 0.2 μg PE or FITC-F(ab′)2 specific for human IgG-Fc (Jackson ImmunoResearch Laboratories). To detect CAR expressing cells by virtue of the CH2CH3 domain, 0.2 μg (goat anti–human IgG (H&L; Invitrogen) was used. The CD19-CAR contains no Ig regions and was detected using Protein L.23 Cells were incubated in 0.15 μg biotinylated protein L (Thermo Scientific) in a 0.1-mL volume for 40 minutes, washed twice, then detected with 2 μg SA-FITC (BD Bioscience). Expression levels of CD19 and CD22 on leukemia lines were measured using Quanti-Brite PE beads (BD Bioscience) and PE-labeled anti-CD19 and anti-CD22 antibodies (BD Bioscience). MFI signal per each calibrated bead population was determined according to manufacturer's protocol. To determine antigen copy number per tumor cell, cellular MFI was compared with a linear plot of bead MFI versus the number of PE molecules per bead.
Patient samples
Primary blast samples were obtained from patients with BCP-ALL. All samples were obtained and used in accordance with research protocols approved by the NCI institutional review board. Antigen site density on patient blasts was quantified by determining the anti-CD22 antibody binding capacity per cell using the BD Bioscience QuantiBRITE system for fluorescence measurement.24 In brief, cell-surface antigen expression was performed by determining the ABC (antibodies bound per cell, mean value of the maximum capacity of each cell to bind the monoclonal antibody) of the lymphoblasts for anti-CD22 (clone S-HCL-1, BDIS), using saturating concentrations of antibody and the BD Bioscience QuantiBRITE system (QuantiBRITE standard beads and QuantiCALC software) for fluorescence quantification. QuantiBRITE beads were acquired on a FACSCANTO II flow cytometer on the same day at the same instrument settings as the individual specimens. A standard curve comparing the geometric mean of fluorescence to known PE content of the QuantiBRITE beads was constructed using QuantiCALC software (BD Bioscience). The regression analysis, slope, intercept, and correlation coefficient were determined. Analysis gates were drawn based on CD10, CD19, and CD45 copositivity to include only the malignant cells for determination of the geometric mean fluorescence of CD22 staining. The ABC values were generated from the measured geometric mean fluorescence of only the malignant cells using the QuantiBRITE standard curve.
For in in vitro studies of CD22-CAR–mediated lysis of patient blasts, blood, and bone marrow samples were obtained from patients with multiply relapsed B-precursor ALL treated at the National Cancer Institute with informed consent. Cells were cryopreserved in RPMI-1640 medium with 10% fetal bovine serum (FBS) and 10% dimethyl sulfoxide.
Cellular cytotoxicity and cytokine assays
Standard 51Cr release assays were performed to determine cytotoxic activity of CD22-CAR T cells against CD22+ leukemia cell targets. One × 106 target cells were labeled with 100 uCi 51Cr (Perkin Elmer) for 1 hour. On washing, 1 × 104 targets per well were coincubated for 4 to 5 hours with effector T cells at various effector to target (E:T) ratios. Nontransduced cells served as a negative control. Assay supernatants were counted for 51Cr release using LumaPlates (Perkin Elmer) and a Top Count Reader (Packard). Specific lysis was calculated as follows: % Lysis = (experimental lysis−spontaneous lysis)/(maximum lysis−spontaneous lysis)× 100. T cells were purified before analysis using the Pan T Cell II isolation kit (Miltenyi Biotec). For direct comparison of killing by different CAR constructs, normalization for transduction efficiency was accomplished by the calculation of lytic units. For lytic unit determination, the E/T ratio was corrected for the actual number of CAR-expressing effectors per well (ie, the E/T ratio is changed from 10:1 to 5:1 when the transduction percentage is 50%). A logarithmic curve fit plot of the corrected E/T ratio versus % lysis was created using Microsoft Excel for each effector and leukemia cell target. We defined 1 Lytic Unit as 30% lysis of target cells at an E/T ratio of 10:1. This functional based “units” definition quantifies the amount of lytic activity in each transduced effector cell population being compared, whereas normalizing the differences seen in transduction efficiency of retroviral vector containing supernatants. For CAR blocking studies, CD22 CAR-transduced T cells were coincubated with indicated concentrations of CD22-Fc (R&D Systems) and effector T cells in a chromium release assay as above. CD19 CAR-transduced cells served as a positive control.
Cytokine levels were determined by analyzing supernatants from triplicate wells after 24-hours of coculture of 1 × 104 target cells with 1 × 105 effector T cells using a Meso Scale Discovery TH1/TH2 multi-array 96-well system. Averages and SEM are reported. All cellular assays were repeated 3 times with similar results.
In vivo studies
Animal studies were carried out under protocols approved by the NCI Bethesda Animal Care and Use Committee. The NALM6-GL cell line was previously described.25 Measurements of tumor burden were made using the Xenogen IVIS Lumina (Caliper Life Sciences). NSG Mice (NOD scid gamma, NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ JAX, Jackson ImmunoResearch Laboratories) were injected intraperitoneally with 3mg D-luciferin (Caliper Life Sciences) and 4 minutes after injection anesthetized mice were imaged with an exposure time of 30 seconds. Living Image Version 4.1 software (Caliper Life Sciences) was used to analyze the bioluminescent signals for each mouse as photons/s/cm2/sr. When tumor or other tissues were harvested from treated mice, T cells were detected using anti–human CD45-PerCP (eBioscience) and anti-CD3–APC (Invitrogen). CAR-expressing cells were detected with recombinant CD22-Fc (R&D Systems) and PE-anti–IgG-Fc (Jackson ImmunoResearch Laboratories), whereas tumor was detected with anti-CD22 APC (Invotrogen) and Pacific Blue anti-CD19 (BioLegend).
Results
CD22 expression by BCP-ALL
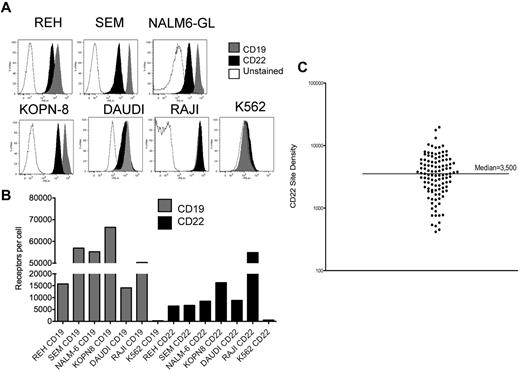
We measured CD22 and CD19 surface expression on the B-cell precursor ALL lines REH, SEM, NALM-6GL, KOPN-8, Daudi, and Raji (Figure 1). Figure 1B demonstrates a range of CD22 expression from 6485 to 54 878 molecules/cell versus a range of CD19 expression of 14 112 to 56 946 molecules/cell. Thus, all express both CD22 and CD19, with higher CD19 site density, except for Daudi and Raji, where CD22 and CD19 site density are equivalent. The negative control cell line, K562, does not express CD22 or CD19.
Expression of CD22 and CD19 B-cell precursor ALL cell lines. (A) Surface expression of CD19 and CD22 on the ALL lines REH, SEM, NALM6-GL, KOPN8, Daudi, Raji, and on K562, as determined by directly labeled flow cytometry antibodies. (B) Quantified surface antigen expression, y-axis, for each line as listed on the x-axis. (C) CD22 site density on 110 individual patient samples of BCP-ALL, with median indicated.
Expression of CD22 and CD19 B-cell precursor ALL cell lines. (A) Surface expression of CD19 and CD22 on the ALL lines REH, SEM, NALM6-GL, KOPN8, Daudi, Raji, and on K562, as determined by directly labeled flow cytometry antibodies. (B) Quantified surface antigen expression, y-axis, for each line as listed on the x-axis. (C) CD22 site density on 110 individual patient samples of BCP-ALL, with median indicated.
We next evaluated blasts from 110 patients with BCP-ALL by flow cytometry. CD22 expression was detected in all cases. Within individual patients, CD22 was detected on > 90% of blasts, with 2 exceptions. These 2 patients had apparent subpopulations of blasts without CD22 expression (22% and 75% of blasts CD22-positive). Notably, both of these cases had translocations involving the MLL gene (11q23). Determination of antibody binding capacity per cell revealed that the median antigen site density of surface CD22 within cases was 3500 sites/cell, and only 11 cases demonstrated < 1000 CD22 sites per cell (Figure 1C). We saw no significant difference based on patient age, such that the average CD22 site density in patients < 18 years was 3470 sites/cell (range 417-19 653, n = 82) versus 3792 sites/cell in patients > 18 years (range 539-9380, n = 23).
CD22-CARs mediate killing of BCP-ALL
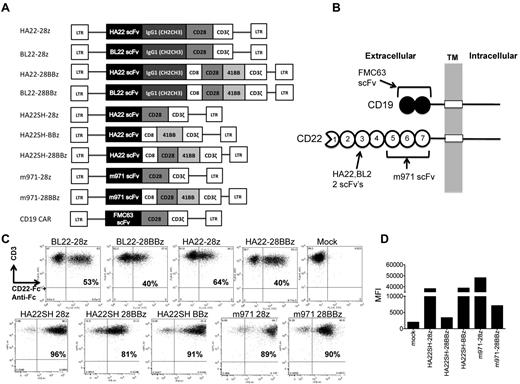
Ten separate retroviral gene vectors, depicted in Figure 2A, were generated to compare the effects of scFv affinity (BL22 versus HA22-derived) versus targeting of alternate CD22 epitopes (m971), as well as the presence of a CH2CH3 spacer domain and costimulatory motifs (CD28, 4-1BB, CD28 plus 4-1BB) on CD22-CAR bioactivity. A CD19-CAR known to confer lytic activity against human leukemia cell lines (MSGV-FMC63-28Z), was used as a comparator.19 The target antigen binding sites for the scFv domains used to generate our CAR constructs is shown in Figure 2B, illustrating the difference in the number of Ig extracellular domains between CD19 and CD22 and the difference in the HA22 and m971 binding sites on CD22. Second generation CD22 CAR T cells had higher transduction rates than third-generation CARs, and higher MFI on transduced cells (Figure 2C-D). Similar transduction rates were seen when anti-Ig antibody was used to detect CH2CH3 expressing CARs (not shown). CD19 CAR-transduced T cells showed consistent transduction efficiencies of approximately 60%, by protein-L staining (not shown). The increased expression levels, as determined by MFI, seen with second generation as opposed to third-generation vectors, indicates that the intracellular domains of the CAR affect surface expression levels (Figure 2D).
CD22 CARs expressing high affinity scFv (HA22), standard affinity (BL22), and membrane proximal binding (m971) anti-CD22 scFv-derived domains. (A) Second-generation CAR constructs (CD28 and CD3zeta or 4-1BB and CD3zeta signaling domains) and third-generation constructs (CD28, 4-1BB, and CD3zeta) expressed HA22, BL22, m971, or FMC63-derived scFv, ± an IgG1-derived CH2CH3 spacer domain. (B) HA22 and BL22 scFvs bind Ig domain 3, whereas m971 binds within Ig domains 5-7 of CD22. (C) Expression on transduced T cells of CD22 CARs containing CH2CH3 domains (top row) or in the shorter format, omitting this domain (bottom row). CD22 CARs were detected by CD3-APC (y-axis) and CD22-Fc followed by an anti–IgG-Fc-FITC stain. Percent transduction is noted in the top-right quadrant of each plot. (D) MFI, y-axis, of the indicated CD22 CAR constructs, x-axis. Results are representative of more than 3 retroviral supernatant preparations.
CD22 CARs expressing high affinity scFv (HA22), standard affinity (BL22), and membrane proximal binding (m971) anti-CD22 scFv-derived domains. (A) Second-generation CAR constructs (CD28 and CD3zeta or 4-1BB and CD3zeta signaling domains) and third-generation constructs (CD28, 4-1BB, and CD3zeta) expressed HA22, BL22, m971, or FMC63-derived scFv, ± an IgG1-derived CH2CH3 spacer domain. (B) HA22 and BL22 scFvs bind Ig domain 3, whereas m971 binds within Ig domains 5-7 of CD22. (C) Expression on transduced T cells of CD22 CARs containing CH2CH3 domains (top row) or in the shorter format, omitting this domain (bottom row). CD22 CARs were detected by CD3-APC (y-axis) and CD22-Fc followed by an anti–IgG-Fc-FITC stain. Percent transduction is noted in the top-right quadrant of each plot. (D) MFI, y-axis, of the indicated CD22 CAR constructs, x-axis. Results are representative of more than 3 retroviral supernatant preparations.
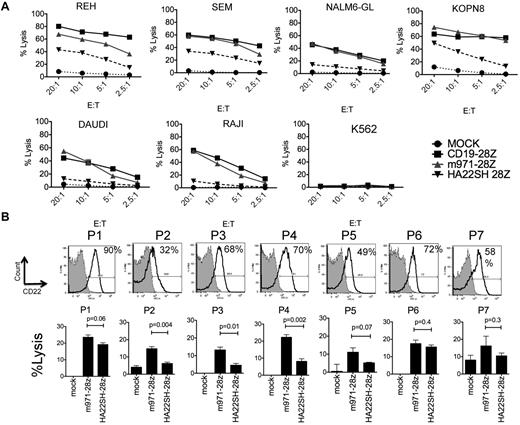
We next studied whether differences in surface expression levels of CD22 versus CD19 on the leukemia target cell influence efficiency of lysis by CD22 versus CD19-CARs (Figure 3A). The CD19-CAR is a second-generation construct and does not contain a CH2CH3 region, thus the most direct comparison is with our m971-CD28z and HA22SH-CD28z vector. In this experiment, the transduction efficiency with these constructs was similar (not shown). Despite reduced levels of CD22 target antigen compared with CD19 target antigen on ALL cell lines (REH, SEM, NLM6, KOPN8), the efficiency of m971 CD22-CAR killing on B-cell precursor ALL cell lines is comparable with CD19-28z–mediated lysis. The superior killing activity of the m971-scFv–derived vector over the HA22-scFv–derived vector highlights the importance of the CD22 epitope targeted by the CAR.
Comparison of CD22-CAR and CD19-CAR–mediated lysis. (A) 51Cr-release assay to evaluate lytic activity of CD22 HA22SH-28z second-generation CAR (inverted triangle), m971-28z second-generation CAR (gray triangle), CD19 CAR (squares), or mock transduced T cells (circles) against ALL lines, as listed. The E/T ratio is shown on the x-axis. (B) Top row, flow cytometric analysis of CD22 expression on 7 primary patient pre-B ALL samples (see “Methods”). Percentage expression over control is indicated. Bottom row, lysis of patient blasts with m971-28z or HASH-28z–expressing T cells in a 4 hours 51Cr-release assay, percent lysis indicated at E/T ratio of 30:1. Significant differences between the vectors is noted (average of triplicate wells). For m971 versus mock, P5 and P7 had P > .05, for HASH P2, P5, and P7 has P > .05, all other values versus mock were less than 0.05.
Comparison of CD22-CAR and CD19-CAR–mediated lysis. (A) 51Cr-release assay to evaluate lytic activity of CD22 HA22SH-28z second-generation CAR (inverted triangle), m971-28z second-generation CAR (gray triangle), CD19 CAR (squares), or mock transduced T cells (circles) against ALL lines, as listed. The E/T ratio is shown on the x-axis. (B) Top row, flow cytometric analysis of CD22 expression on 7 primary patient pre-B ALL samples (see “Methods”). Percentage expression over control is indicated. Bottom row, lysis of patient blasts with m971-28z or HASH-28z–expressing T cells in a 4 hours 51Cr-release assay, percent lysis indicated at E/T ratio of 30:1. Significant differences between the vectors is noted (average of triplicate wells). For m971 versus mock, P5 and P7 had P > .05, for HASH P2, P5, and P7 has P > .05, all other values versus mock were less than 0.05.
7 BCP-ALL patient samples (P1-7, Figure 3B) were analyzed for CD22 expression. A wide range of antigen expression was seen, as demonstrated by flow cytometric analysis. Some patients showed strong positivity, for example P1, whereas others had decreased CD22 staining, as seen for P2. In each case, CD22-specfic CARs conferred antileukemic lytic activity on transduced third-party lymphocytes.
Analysis of affinity CAR affinity and domain structure in vitro
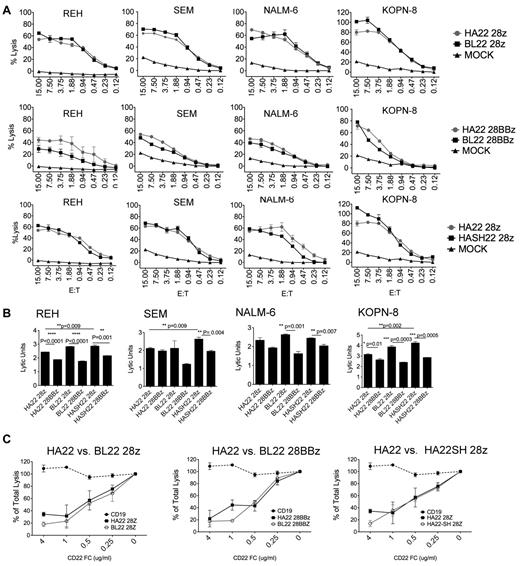
To directly assess the role of binding domain affinity on CAR efficiency, lysis of ALL lines was measured in standard chromium release assays (Figure 4A). We observed similar lytic activity between HA22 and BL22-derived vectors in the second-generation vector format (Figure 4A row 1). Note that many of the data points are from below an E/T ratio of 1:1, demonstrating the high activity of these constructs. We also found that BL22-scFv–derived and HA22-derived binding domains were essentially equivalent in third-generation vector formats, with the possible exception of the REH target cell line (Figure 4A row 2).
HA22 and BL22-derived CD22 CARs mediate similar lytic activity. (A) The lytic activity HA22 and BL22-derived CD22 CARs in the context of second and third-generation signaling constructs was compared against ALL cell lines at the indicated E:T ratios in a 4-hour 51Cr-release assay. SEM of triplicate wells is shown. The first row compares binding affinity of the scFv (BL22 versus HA22-derived) in second-generation vectors, the second row makes the comparison in the third-generation vectors, and the third row compares vectors with and without CH2CH3 domains (HA22-28z versus HASH22-28z). (B) Comparison of signaling domain structure on vector formats using REH, SEM, NALM-6, and KOPN-8 cell line targets. Lytic activity is expressed in lytic units, which corrects for transduction efficiency, at an E/T ratio of 10:1. Significant differences are noted, using an unpaired student t test. (C) Increasing concentrations of CD22-Fc, as designated on the x-axis, were added to triplicate wells in a 4-hour lytic assay using REH as the target and an E:T of 10:1. CD19-CAR, dashed line, served as a negative control. Assays were repeated 3 times with similar results.
HA22 and BL22-derived CD22 CARs mediate similar lytic activity. (A) The lytic activity HA22 and BL22-derived CD22 CARs in the context of second and third-generation signaling constructs was compared against ALL cell lines at the indicated E:T ratios in a 4-hour 51Cr-release assay. SEM of triplicate wells is shown. The first row compares binding affinity of the scFv (BL22 versus HA22-derived) in second-generation vectors, the second row makes the comparison in the third-generation vectors, and the third row compares vectors with and without CH2CH3 domains (HA22-28z versus HASH22-28z). (B) Comparison of signaling domain structure on vector formats using REH, SEM, NALM-6, and KOPN-8 cell line targets. Lytic activity is expressed in lytic units, which corrects for transduction efficiency, at an E/T ratio of 10:1. Significant differences are noted, using an unpaired student t test. (C) Increasing concentrations of CD22-Fc, as designated on the x-axis, were added to triplicate wells in a 4-hour lytic assay using REH as the target and an E:T of 10:1. CD19-CAR, dashed line, served as a negative control. Assays were repeated 3 times with similar results.
Previous studies evaluating the role of spacer domains on CARs activity have not conclusively demonstrated a benefit to the incorporation of this domain26 ; however, the distance between the T cell and target cell membrane was previously implicated as an important variable in the efficiency of CD22-CAR lysis.27 To determine whether lytic activity could be enhanced by altering the distance of the scFv domain from the T-cell membrane, we created CD22-CAR constructs with or without sequences encoding the CH2CH3 domains of IgG1, and compared them directly in a second generation vector format. As shown in Figure 4A row 3, the addition of the CH2CH3 domain did not have a significant impact on CAR potency.
We then compared second and third-generation CAR formats incorporating both HA22 and BL22 binding domains to assess the effects of intracellular T-cell signaling domains on lytic activity. The addition of the 4-1BB signaling domain to CAR constructs was previously shown to improve T-cell persistence and enhance both proliferation and functional activity.6 A 4-hour 51Cr release assay was carried out with 4 leukemia targets, comparing second and third-generation CARs using both HA22 and BL22-derived scFv. To normalize for differences in transduction efficiency between vectors, data are expressed in lytic units (Figure 4B). In 3 of 4 cell lines studied, the HA22SH-CAR was better than the HA22-CAR in the second-generation constructs, whereas in the third-generation CARs both HA22 and HA22SH had similar lytic activity. Thus, we consistently observed that second-generation CD22-CARs mediate more potent killing than third-generation CD22-CARs incorporating both CD28 and 4-1BB domains. This was also true for the m971 scFv-derived CARs, when tested on high CD22-expressing (Raji) and low CD22-expressing (NALM6) targets (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
We further evaluated differences in HA22 and BL22-derived CAR lytic activity by blocking interactions of the CAR with CD22 on the target cell using progressively increasing concentrations of CD22-Fc protein in the assay. As shown in Figure 4C, both higher-affinity (HA22-derived) and standard affinity (BL22-derived) CARs showed a dose-dependent reduction of lytic activity with essentially identical inhibition curves. We conclude that CD22-CAR lytic activity in these constructs did not vary based on scFv affinity, as encoded by HA22 and BL22-derived scFv. Thus, although enhancement of binding affinity in HA22-derived CAR appeared to improve the efficacy of a monomeric reagent, that is immunotoxins, our findings support recent studies with CARs, in which affinity enhancement of scFvs above a threshold level does not necessarily result in enhanced target-cell killing.28
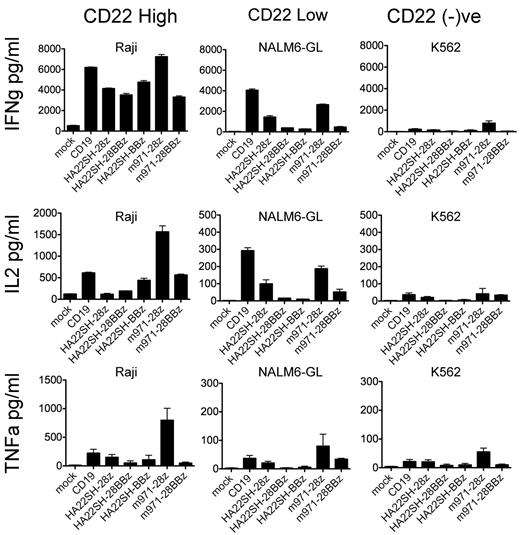
In addition to cytolysis of leukemia cell lines, we also tested cytokine production (Figure 5). Essentially the same pattern was seen as in lytic assays. The second-generation m971 and HA22 vectors were far more active in IFN-γ, TNF-α, and IL-2 production than the third-generation vectors. We included CD19-CAR in the cytokine-release assay to demonstrate the dependence of target antigen density for cytokine release. The Raji (high CD22 expressor) cell line induced more cytokine production from m971-28z vectors, whereas the CD22-low and CD19-high NALM6-GL line induced more cytokine production in PBMCs transduced to express CD19-CAR. We also tested the ability of CAR-transduced T cells to respond to restimulation with tumor-expressed antigen. After retroviral vector transduction, T cells were cultured in IL-2, and then on day 10 restimulated with irradiated NALM6-GL or Raji leukemia lines. When Raji cells were used to restimulate, second-generation vectors (m971-28z, HASH-28z, CD19) showed similar levels of expansion on day 15 and were superior to third-generation vectors (HASH-28BBz, m971-28BBz; supplemental Figure 2). Similar results were seen using NALM6-GL as a stimulator.
Cytokine release by CAR-transduced T cells. CAR-transduced T cells (vectors listed, x-axis) were incubated with irradiated CD22-high (Raji, column 1), CD22-low (NALM6-GL, column 2), or CD22-negative (K562, column 3) leukemia cell lines at a ratio of 10:1 for 24 hours and culture supernatants analyzed for IFN-γ (top row), IL-2 (second row), and TNF-α (third row). Averages and SD of triplicate wells are shown.
Cytokine release by CAR-transduced T cells. CAR-transduced T cells (vectors listed, x-axis) were incubated with irradiated CD22-high (Raji, column 1), CD22-low (NALM6-GL, column 2), or CD22-negative (K562, column 3) leukemia cell lines at a ratio of 10:1 for 24 hours and culture supernatants analyzed for IFN-γ (top row), IL-2 (second row), and TNF-α (third row). Averages and SD of triplicate wells are shown.
Assessment of CAR activity in vivo
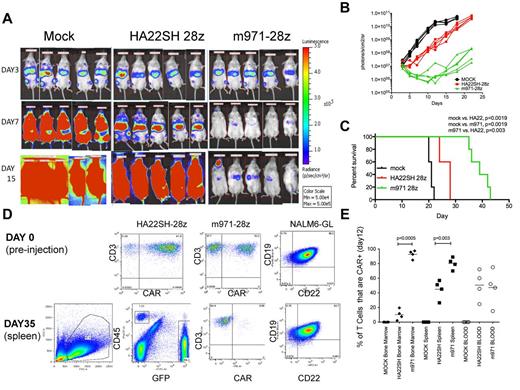
To assess antileukemic activity of CD22-CARs in a xenograft model, 0.5 × 106 NALM6-GL tumor cells were injected intravenously, followed 3 days later by 1 × 107 CAR-transduced T cells. Mice received T cells expressing the second best second-generation CD22-CAR vectors, m971-28z, or HA22SH-28z, or an equivalent number of mock transduced T cells. Mice were imaged after injection of luciferin on day 3 after injection of NALM6-GL leukemia, representing the disease burden before treatment, and on days 7 and 15 (Figure 6A). Signal intensity was plotted over time (Figure 6B) and survival of treated mice followed (Figure 6C). When mock versus either treatment was compared, CD22-CAR treatment induced significant survival advantage. However, survival of mice treated with m971-28z–expressing T cells was superior to those receiving HA22SH-28z–expressing T cells.
Evaluation of CD22 CARs in vivo. On day 0, NSG mice were injected intravenously with 5 × 105 NALM6-GL cells. On day 3 mice received 1 × 107 CAR+ T cells (HA22SH-28z (n = 5) or m971-28z (n = 5) or mock T cells (n = 5). (A) Bioluminescent imaging pre-treatment (day 3), day 7 and day 15 after intravenous injection of NALM6-GL. (B) Bioluminescent signal for each mouse over time, comparing mock, HASH-28z and m971-28z. (C) Kaplan-Meier survival curves for each group, listing significant differences between each group, survival statistics calculated using log-rank (Mantel-Cox) analysis. (D) Top row: flow cytometric analysis of transduced T cells used in the experiment, as well as CD22 and CD19 expression on NALM6-GL before injection. Bottom row: left panel, FSC versus SSC gating of splenocytes excised from a mouse on sacrifice; center-left panel, FSC versus SSC gated cells were analyzed for green fluorescence (NALM6-GL, x-axis) and CD45-PerCp (y-axis); center right, CD45-gated cells were stained for CD3-APC (y-axis) and for CAR expression; right panel, GFP+ gated cells were stained for CD22 and CD19. (E) In a separate experiment, carried out identically, mice were killed on day 12, and analyzed for CAR expression on gated human T cells in the bone marrow, spleen, and blood. Percentage of T cells expressing CARs is plotted (y-axis), and significant differences, using unpaired 2-tailed t test, are shown.
Evaluation of CD22 CARs in vivo. On day 0, NSG mice were injected intravenously with 5 × 105 NALM6-GL cells. On day 3 mice received 1 × 107 CAR+ T cells (HA22SH-28z (n = 5) or m971-28z (n = 5) or mock T cells (n = 5). (A) Bioluminescent imaging pre-treatment (day 3), day 7 and day 15 after intravenous injection of NALM6-GL. (B) Bioluminescent signal for each mouse over time, comparing mock, HASH-28z and m971-28z. (C) Kaplan-Meier survival curves for each group, listing significant differences between each group, survival statistics calculated using log-rank (Mantel-Cox) analysis. (D) Top row: flow cytometric analysis of transduced T cells used in the experiment, as well as CD22 and CD19 expression on NALM6-GL before injection. Bottom row: left panel, FSC versus SSC gating of splenocytes excised from a mouse on sacrifice; center-left panel, FSC versus SSC gated cells were analyzed for green fluorescence (NALM6-GL, x-axis) and CD45-PerCp (y-axis); center right, CD45-gated cells were stained for CD3-APC (y-axis) and for CAR expression; right panel, GFP+ gated cells were stained for CD22 and CD19. (E) In a separate experiment, carried out identically, mice were killed on day 12, and analyzed for CAR expression on gated human T cells in the bone marrow, spleen, and blood. Percentage of T cells expressing CARs is plotted (y-axis), and significant differences, using unpaired 2-tailed t test, are shown.
To determine whether treatment failure was because of antigenic loss, mice were euthanized because of disease burden according to institutional protocol, and harvested blood and tissue analyzed by flow cytometry. Before therapy (day 0, Figure 6D), high levels of CAR expression were seen on HA22SH-28z and m971-28z–transduced T cells, and the NALM6-GL cells expressed both CD19 and CD22. On sacrifice, splenocytes were analyzed first for human CD45 and GFP expression. Gated GFP-positive cells represent tumor, and on further analysis this gated population continued to express GFP. However, any CD45+/CD3+ and GFP-negative cells remaining no longer expressed the anti-CD22 CAR. This indicates that treatment failure in this model was not associated with antigen loss but rather with a failure of the CAR-transduced T cells to persist long term. When the experiment was repeated and mice killed on day 12 (when therapeutic T cells were still having an effect), CAR(+) T cells could be detected in the blood, spleen, and bone marrow (Figure 6E). Interestingly, the more effective CAR, m971-28z, showed a much great ability to infiltrate the bone marrow.
To determine the impact of various signaling domain formats in vivo, the HA22-28BBz was compared with HA22-28z and HA22-BBz second-generation constructs. In these experiments, both second-generation vectors were expressed at high levels on transduced T cells, and lysed CD22 expressing targets (supplemental Figure 3A, and not shown); however, as in other experiments, surface expression of the third-generation CAR was less than the second-generation CAR. Each of the CAR-treated animals had a significant survival advantage compared with animals that received an equivalent number of mock transduced T cells (supplemental Figure 3B-C) but no statistical difference was observed between HA22-28z and HA22-BBz. Thus, we conclude that CD22-CAR mediates significant antitumor effects against CD22-expressing B-cell precursor acute lymphoblastic leukemia in vivo and that 4-1BB and CD28 second-generation CD22-CARs are of similar efficacy.
Discussion
CD22 is one of several developmentally regulated antigens expressed on developing and mature human B cells and human B-cell malignancies29 that have been targeted via immunotherapeutics.30 Given the clinical successes with CD22-linked immunotoxins, we sought to re-evaluate the HA22 CD22 binding moiety in the context of a CAR. Earlier studies using the standard affinity anti-CD22 scFv derived from RFB4 (BL22) showed that this moiety, when incorporated into a CAR, could mediate significant antitumor effects against B-cell malignancies.27 This report emphasized the importance of cell membrane proximity, by altering the RFB4-specific epitope using engineered CD22 molecules. Given the availability of a new higher affinity binding domain, HA22-scFv, the availability of an anti-CD22 scFv targeting a more proximal epitope on CD22, m971, and the recent clinical advances with CD19-CAR therapy, we sought to further explore the optimal configuration for CD22-CAR therapy and to compare the relative efficacy of CD19-CAR versus CD22-CAR against B-cell precursor acute lymphoblastic leukemia. Our results clearly demonstrate antileukemic activity of CD22-CAR modified T cells both in vitro and in vivo and demonstrate that the efficacy of m971-based CARs is similar to that of CD19-CAR, which has already demonstrated clinical responses in the clinic. We further demonstrate that neither increasing the distance between the T cell surface and the CD22 epitope with a CH2CH3 domain nor incorporating 2 costimulatory domains significantly enhances activity.
Our CAR constructs also allowed us to test the effect of binding affinity on CAR function. BL22 was derived from the original RFB4 anti-CD22 antibody and developed as the binding moiety for an immunotoxin conjugate, then subsequently matured for affinity and incorporated into a second-generation immunotoxin designated HA22.31 HA22-linked immunotoxins were more potent in animal tumor models than BL22, and we sought to determine whether this would also be the case for CAR-modified T-cell activity. In direct comparison of lytic activity mediated by CAR-transduced T cells, or by inhibition assays using CD22-Fc to block CAR binding to its target, there were no appreciable differences in the activity of HA22-scFv and BL22-scFv CAR-transduced T cells (Figure 4). In 2004 Chmielewski et al, conducting similar studies on the impact of scFv affinity, demonstrated that affinity is not a driving characteristic for CAR activity, until low Kd values for the scFv are reached.28 Because BL22 (Kd = 5.8 nM), is already selected for high binding affinity, our studies support this report, in that further increasing the affinity of the scFv-binding domain, as seen in HA22 (Kd = 2.3nM), did not have a significant impact on the activity of transduced T cells bearing this receptor.20 Importantly however, we cannot rule out the possibility that affinity might affect function against antigen-limiting targets, as all of the lines tested had similar expression levels of CD22, with none less than 2000 receptors per leukemic cell (Figure 1).
Surprisingly, we did see differences in cytolytic activity when we compared signaling domains in our CD22-CAR vectors. In most cases, the second generation CAR was superior to the third-generation CAR in vitro (Figures 4–5, supplemental Figure 2). This was an unexpected finding, as third-generation CAR vectors containing the CD137/4-1BB signaling motif were assumed to be superior because of the additive effect of these signaling domains. Stephan et al, demonstrated that providing CD80 and 4-1BBL–mediated signaling greatly enhanced the antitumor activity of a first generation CAR.32 This built on earlier work showing that inclusion of 3 signals, 4-1BB, anti-CD28, and anti-CD3, on artificial antigen presenting cells greatly augmented in vitro T-cell expansion.33 In 2009 Milone et al, reported that third-generation CAR vectors targeting CD19 were superior; however, more recent work from this same group has focused on CD137 and CD3-ζ chain only vectors, omitting CD28, reasoning that the IL-2 supplied in tissue culture rendered this signaling unit redundant.34,35 Finney et al,21 demonstrated that inclusion of CD134 (OX40) or CD137 (4-1BB) induces Bcl-2 expression in multi-domain CARs and Zhao et al,36 demonstrated that 4-1BB containing third-generation vectors had enhanced lytic activity, induced Bcl-xL and showed better in vivo tumor efficacy. Given this background, we anticipated that in vivo CAR-modified T cells expressing a CD137 domain would be superior to second-generation vectors that lack this domain, as T cells transduced with third-generation vectors would be more resistant to apoptosis by the induction of Bcl family members, and thereby should have prolonged the antitumor effect of CAR-modified T-cell infusion in vivo. In fact, our data shows the addition of CD137 signaling domains did not improve therapeutic effect in our model (supplemental Figure 3). Experiments in the murine anti-CD19 CAR system showed that a CD28-signaling domain that had 2 of 3 ITAM motifs mutated, was superior to the wild-type signaling sequence8 raising the possibility that high levels of activating signals may result in T-cell “tuning” and subsequent induction of compensatory negative regulators that offset positive signals.
The final parameter we evaluated was to change entirely the binding site of the CAR on the CD22 antigen by generating m971-scFv–based constructs. Creation of this CAR taught us that binding site choice superseded all other parameters that we examined, thus finally approaching CD19-CAR in efficacy. Further studies comparing these CARs, as well as using them in combination are planned.
Down-regulation or loss of target proteins is a potential mechanism for immune evasion by cancer. Recently, a few adults with ALL have been reported to relapse with CD19-negative blasts after treatment with the anti-CD3/CD19 bi-specific antibody blinatumomab.37,38 Therefore, we hypothesize that the efficacy of mAb-based therapy of ALL, including CARs, will be improved if multiple antigens are targeted. Our work demonstrates that CD22 is universally expressed on BCP-ALL, and that all blasts in an individual case express the target antigen, which appears to be the case in almost all patients studied (109/111 showing CD22 on > 90% of blasts). CD22 expression was also recapitulated in human ALL-murine xenografts established with limiting dilution studies, which raises the possibility that CD22 might be expressed on the leukemia-initiating cell.39 Furthermore, there was no loss of CD22 expression in serial studies of 45 patients, including 39 treated with anti-CD22 directed therapy (data not shown), suggesting that the development of CD22-negative BCP-ALL is probably not a common event.
In conclusion, CD22 represents a promising target for the treatment of ALL and anti-CD22 CAR-transduced T cells hold therapeutic promise for children and adults with this malignancy. The development of a CAR that binds a membrane-proximal domain of CD22 proved to be the key element in developing a highly active CAR. Target antigen epitope selection should be considered in other vectors, alongside evaluation of CAR binding affinity, and the inclusion of appropriate signaling and activation domains.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank James Kochenderfer, MD, of the NIH for supplying the CD19-CAR used in these studies. A patent has been filed for CARs expressing HA22 (R.J.O., I.H.P., C.L.M.) or m971 (R.J.O., D.SD, I.H.P., C.L.M.) by the Office of Technology Transfer, National Institutes of Health, US Department of Health and Human Services.
This research was supported by the Intramural Research Program of the NIH, CCR, NCI.
National Institutes of Health
Authorship
Contribution: W.H. carried out experiments, helped design and carried out animal studies, and wrote the paper; D.W.L. assisted in CD19-based studies and comparison studies to CD22; C.L.M. provided laboratory and animal support, helped design studies, and wrote the paper; N.N.S. and A.S.W. wrote clinical portions of the paper, secured samples, obtained institutional approvals, and worked with C.M.Y. and M.S.-S., who analyzed clinical samples and authored clinical methodology; R.J.O. created and evaluated CAR constructs in association with I.H.P. and D.J.F., who provided BL22 and HA22 binding motifs; D.S.D. provided m971 motifs; R.A.M. created gene vector backbones used in these studies; and R.J.O. served as primary author.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rimas J. Orentas, Pediatric Oncology Branch, CCR, NCI, NIH, 10 Center Dr, 1W3840, Bethesda, MD 20892; e-mail: rimas.orentas@nih.gov.