Key Points
Tmprss6 siRNA induces hepcidin and diminishes iron in hemochromatosis or thalassemia mice, improving the anemia seen in the latter model.
Manipulation of TMPRSS6 with RNAi therapeutics may be an approach to treating iron overload diseases associated with low hepcidin levels.
Abstract
Mutations in HFE lead to hereditary hemochromatosis (HH) because of inappropriately high iron uptake from the diet resulting from decreased hepatic expression of the iron-regulatory hormone hepcidin. β-thalassemia is a congenital anemia caused by partial or complete loss of β-globin synthesis causing ineffective erythropoiesis, anemia, decreased hepcidin production, and secondary iron overload. Tmprss6 is postulated to regulate hepcidin production by cleaving Hemojuvelin (Hjv), a key modulator of hepcidin expression, from the hepatocyte surface. On this basis, we hypothesized that treatment of mouse models of HH (Hfe−/−) and β-thalassemia intermedia (Hbbth3/+) with Tmprss6 siRNA formulated in lipid nanoparticles (LNPs) that are preferentially taken up by the liver would increase hepcidin expression and lessen the iron loading in both models. In the present study, we demonstrate that LNP-Tmprss6 siRNA treatment of Hfe−/− and Hbbth3/+ mice induces hepcidin and diminishes tissue and serum iron levels. Furthermore, LNP-Tmprss6 siRNA treatment of Hbbth3/+ mice substantially improved the anemia by altering RBC survival and ineffective erythropoiesis. Our results indicate that pharmacologic manipulation of Tmprss6 with RNAi therapeutics isa practical approach to treating iron overload diseases associated with diminished hepcidin expression and may have efficacy in modifying disease-associated morbidities of β-thalassemia intermedia.
Introduction
Iron is essential for erythropoiesis and numerous other cellular processes. However, excess iron is toxic because of its ability to generate reactive oxygen species through Fenton-mediated chemistry, and therefore it must be tightly regulated. Mutations in HFE1 lead to the most common form of hereditary hemochromatosis (HH) in humans. Loss of HFE function results in chronically elevated dietary iron uptake because of dysregulation of hepcidin, a peptide hormone largely produced by hepatocytes in the liver2-6 that negatively regulates cellular iron export by promoting the degradation of ferroportin,7 an iron exporter present on the surface of macrophages of the reticuloendothelial system, duodenal enterocytes, and hepatocytes.8-10
β-Thalassemia is an inherited anemia caused by the partial or complete lack of β-globin synthesis. The most severe form, β-thalassemia major, requires chronic RBC transfusional support, leading to secondary iron overload.11 Less severe disruption of β-globin synthesis leads to the clinical phenotype of β-thalassemia intermedia, which by definition does not require chronic transfusions. Nonetheless, patients with β-thalassemia intermedia still develop iron overload as a consequence of chronic suppression of hepcidin synthesis by ineffective erythropoiesis.12,13 A mouse model of β-thalassemia intermedia (Hbbth3/+) mimics the human condition insofar as these mice have transfusion-independent anemia and ineffective erythropoiesis leading to splenomegaly and secondary iron overload14 due to inappropriately low hepcidin expression.15-18
TMPRSS6, also known as matriptase-2, is a membrane-bound serine protease19 expressed in hepatocytes that negatively modulates hepcidin production. Mutations of TMPRSS6 in both humans20-25 and mice26-29 result in excess hepcidin expression and iron-refractory iron deficiency anemia. In vitro, TMPRSS6 cleaves hemojuvelin (HJV) from the plasma membrane.30 In doing so, TMPRSS6 is thought to down-regulate the BMP/SMAD signaling pathway essential for iron-dependent regulation of hepcidin transcription.
Manipulation of hepcidin expression is a potential pharmacologic modality to treat iron overload disorders. For example, overexpression of hepcidin has been shown to ameliorate the iron overload phenotype in Hfe−/− mice,31 and deletion of Tmprss6 in Hfe−/− mice results in greatly increased hepcidin expression and iron deficiency anemia.29 Apart from the direct effect on iron storage, hepcidin pathway modulation by exogenous administration of transferrin in another murine model of thalassemia intermedia (Hbbth1/th1) was shown to ameliorate the anemia phenotype itself.32 Furthermore, dietary iron restriction or moderate overexpression of hepcidin in the Hbbth3/+ model reduces iron overload, improves anemia, and decreases splenomegaly.33 Most recently, it was shown that targeted deletion of Tmprss6 in Hbbth3/+ mice not only decreases iron loading, but also reduces ineffective erythropoiesis and splenomegaly, improving anemia.34 In toto, these studies indicate that there is a significant potential to exploit TMPRSS6-dependent hepcidin regulation as a therapeutic modality for iron overload disorders in which hepcidin expression is suppressed.
Previous studies have demonstrated that systemic administration of lipid nanoparticle (LNP)–formulated siRNAs can silence therapeutically relevant genes in rodents, nonhuman primates, and humans.35-39 To further interrogate the potential therapeutic utility of targeting Tmprss6 in β-thalassemia intermedia and primary genetic iron overload in vivo, we treated Hbbth3/+ and Hfe−/− mice with an RNAi therapeutic targeting Tmprss6. Our results indicate that LNP-formulated siRNAs can decrease Tmprss6 expression and greatly increase hepcidin mRNA, leading to diminished iron uptake in both models and improved erythropoiesis in β-thalassemic mice. These data are particularly noteworthy because TMPRSS6 siRNA therapy could lead to outcomes in β-thalassemia patients that are superior to those achieved with iron chelator treatment alone.
Methods
Animal care and analysis
Hbbth3/+ (B6;129P-Hbb-b1tm1UncHbb-b2tm1Unc/J) and Hfe−/− mice were generated previously14,40 and bred onto a C57BL/6J genetic background. All genetically modified mice were born and housed in the barrier facility at Children's Hospital Boston and handled according to approved protocols. Mice were maintained on the Prolab RMH 3000 diet (Lab Diet; 380 ppm iron). The facility employs a constant dark-night light cycle and all mice were provided both water and food ad libitum. Because of differences in iron metabolism between male and female mice, only females were analyzed. Mice were injected into the tail veins with 1.0 mg/kg of PBS, LNP-luciferase (LNP-Luc), or LNP-Tmprss6 at 6 weeks of age and then either killed at 8 weeks of age or reinjected at 8 and 10 weeks of age and killed 2 weeks later at 12 weeks of age.
Synthesis and LNP formulation of siRNAs
The chemically modified Tmprss6 and luciferase control siRNAs were synthesized by Alnylam and characterized by anion-exchange HPLC. The sequences of the siRNAs were as follows: Tmprss6 sense: 5′-UGGUAUUUCCUAGGGUACAdTdT-3′, antisense: 5′-UGUACCCUAGGAAAUACUAdTdT-3′; luciferase sense: 5′-CUUACGCUGAGUACUUCGAdTdT-3′, antisense 5′-UCGAAGUACUCAGCGUAAGdTdT-3′. The LNPs were prepared with an ionizable lipid, disteroylphosphatidyl choline, cholesterol, and PEG-DMG at a component molar ratio of approximately 50/10/38.5/1.5 using a previously described spontaneous vesicle formation procedure.41
Oligonucleotide primers
Oligonucleotide primers used in PCR genotyping and for quantitative PCR analysis are listed in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials list at the top of the online article).
PCR genotyping
PureLink Genomic DNA Mini Kits (Invitrogen) were used to prepare genomic DNA from tail snips. Transgenic Hbbth3/+ mice were genotyped by PCR with primers HPRT-2F and HPRT-9R to yield a 1000-bp band. Hfe−/− mice were genotyped by PCR using primers HFE-NeoF, HFE-KO, Map3, and Map4, yielding a 230-bp wild-type (WT) band and a 200-bp knockout band (supplemental Table 1).
Blood and tissue analysis
Whole blood for complete blood counts was collected retroorbitally into EDTA-coated Microtainer tubes (BD Biosciences) from mice anesthetized with 1.0% tribromoethanol in isoamyl alcohol (Avertin). Samples were analyzed on an Avida 120 analyzer (Bayer) in the Children's Hospital Boston Department of Laboratory Medicine Clinical Core Laboratories. Whole blood for other purposes was collected by retroorbital bleeding into serum separator tubes (BD Biosciences), and serum was prepared according to the manufacturer's instructions. Serum iron values were determined with the Serum Iron/UIBC kit (Thermo Fisher) according to the manufacturer's instructions. Liver and spleen tissues were collected and tissue nonheme iron concentrations were determined as described previously.42 Serum erythropoietin was measured using the Quantikine Mouse/Rat Epo Immunoassay kit (R&D Systems) according to the manufacturer's instructions.
Measurement of RBC lifespan
Female mice 6-8 weeks of age were injected by tail vein with LNP-Luc or LNP-Tmprss6, which was repeated every 2 weeks for the duration of the experiment. Three weeks after the initial treatment with LNP, mice were retroorbitally injected with 1 mg of NHS-PEG4-Biotin reagent (Pierce). After 24 hours, 10 μL of blood was collected retroorbitally and incubated with Alexa Fluor 488–conjugated streptavidin (Invitrogen). The fraction of residual labeled RBCs present was analyzed by flow cytometry at the Children's Hospital Boston Division of Hematology/Oncology and Harvard Stem Cell Institute Flow Cytometry Research Facility weekly for 5 weeks after the initial biotin injection. FlowJo Version 9.4.10 software (TreeStar) was used for data analysis.
Analysis of RBC membrane–associated globins
Blood samples collected retroorbitally into EDTA-coated Microtainer tubes (BD Biosciences) from WT and Hbbth3/+ mice after 6 weeks of siRNA treatment were lysed in 0.05× PBS. The insoluble membrane fraction was washed 4 times with 0.05× PBS by centrifugation and the membrane lipids were extracted with 0.1% Tween 20 in 56mM sodium borate, pH 8.0.43 The insoluble membrane fraction was electrophoresed on TAU gels,44 visualized by Coomassie staining, and α- and β-globin band intensities were quantified using ImageJ Version 1.46R software. Sample loading was normalized to packed RBC volume.
RNA extraction, RT-PCR, and semiquantitative and quantitative PCR
Total liver RNA was isolated from flash-frozen tissue in TRIzol reagent (Invitrogen). RNA was treated with DNase I (Roche) to remove contaminating genomic DNA. cDNA was synthesized using the High Capacity cDNA Reverse Transcription kit (ABI) or iScript cDNA Synthesis Kit (Bio-Rad) according to the manufacturer's protocol. Real-time PCR quantification of hepcidin (Hamp), Id1, β-actin, and Bmp6 mRNA transcript levels were performed as described previously.45 Tmprss6 mRNA expression was analyzed using a Tmprss6 TaqMan Gene Expression assay (Mm00551108; Applied Biosystems). All primers are listed in supplemental Table 1.
Tissue staining and immunohistochemistry
Livers and spleens were fixed in 10% buffered formalin for 24 hours and embedded in paraffin. Deparaffinized sections of tissue were stained with H&E, Perls Prussian blue iron stain, or CD71 Ab (Tfr1) in the Children's Hospital Boston, Department of Pathology Histology Laboratory. Images were acquired using either a 10×/0.30 or 4×/0.13 objective lens on a BX51 microscope equipped with a DP71 Digital Camera using Olympus MicroSuite FIVE imaging software. May-Grunwald-Giemsa–stained (Sigma-Aldrich) peripheral blood smears were photographed using a 100×/1.3 objective lens.
Statistics
A 2-tailed Student t test (Microsoft Excel Version 12.3.5) with P < .05 was used to determine statistical significance.
Results
Silencing of Tmprss6 with LNP-formulated siRNA up-regulates hepcidin in a dose-dependent manner
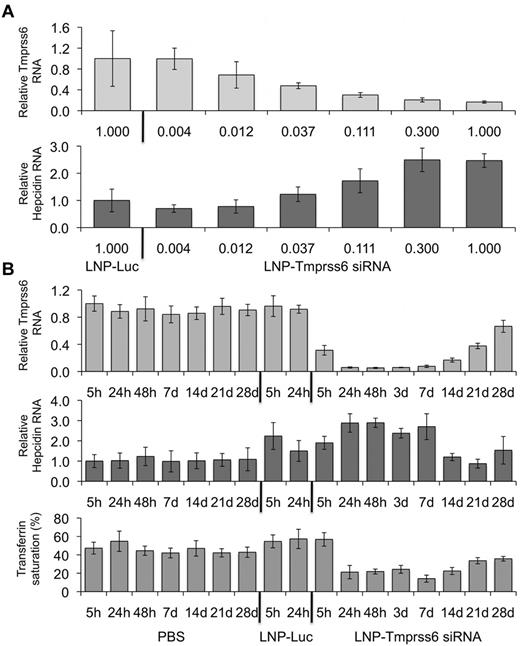
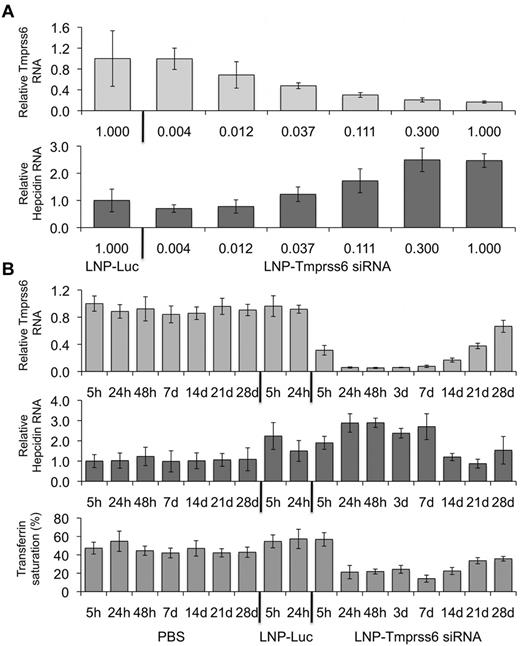
We sought to modulate hepcidin expression in vivo by targeting Tmprss6 with an LNP-formulated Tmprss6 siRNA (LNP-Tmprss6). We compared WT C57BL/6J mice injected with LNP-Tmprss6 or an LNP-Luc control siRNA. We found that treatment with increasing concentrations of LNP-Tmprss6 diminished Tmprss6 mRNA (Figure 1A top panel) and reciprocally increased hepcidin mRNA in a dose-dependent manner (Figure 1A bottom panel) with a median effective dose of 0.035 mg/kg. To ascertain the long-term effects of Tmprss6 siRNAs on hepcidin expression and iron metabolism, we injected mice with 0.3 mg/kg of PBS, LNP-Luc, or LNP-Tmprss6 and followed them over a 4-week period. We found that Tmprss6 silencing and hepcidin induction occurred as early as 24 hours after injection. Silencing of Tmprss6 continued for at least 14 days and hepcidin remained elevated for 7 days after administration (Figure 1B top and middle panels), resulting in decreased transferrin saturation (Figure 1B bottom panel). By 3 weeks after injection, Tmprss6 mRNA levels had recovered to nearly 50% of normal levels (Figure 1B top panel) and hepcidin had returned to WT expression levels (Figure 1B middle panel). However, transferrin saturation remained decreased even 28 days after the initial injection (Figure 1B bottom panel), likely related to the longer-term effects of treatment on intestinal iron uptake that result in diminished systemic iron stores. LNP-Luc treatment itself caused an acute, transient increase in hepcidin expression, but was not associated with a change in transferrin saturation (Figure 1B bottom panel). It is likely that LNP administration resulted in a transient inflammatory response in the liver that temporarily increased hepcidin expression through iron-independent pathways. These data demonstrate that siRNA carried in an LNP can effectively target Tmprss6, leading to a prolonged increase in hepcidin expression and a durable decrease in transferrin saturation.
Analysis of hepcidin expression and serum transferrin saturation in WT mice treated with an RNAi therapeutic directed against Tmprss6. (A) Total mRNA harvested 24 hours after treatment from WT mice dosed with various concentrations of LNP-formulated Tmprss6 siRNA (LNP-Tmprss6) or a luciferase control (LNP-Luc) and Tmprss6 or hepcidin (Hamp) mRNA (A) was assessed by quantitative real-time PCR, normalized to β-actin (Actb), and then expressed relative to the LNP-Luc value the mean value of which was defined as 1.0. (B) Four-week time-course analysis of Tmprss6 or hepcidin mRNA expression and transferrin saturation after treatment with 0.3 mg/kg of PBS, LNP-Luc, or LNP-Tmprss6. Ratios are expressed ± SEM (n = 5 in each group).
Analysis of hepcidin expression and serum transferrin saturation in WT mice treated with an RNAi therapeutic directed against Tmprss6. (A) Total mRNA harvested 24 hours after treatment from WT mice dosed with various concentrations of LNP-formulated Tmprss6 siRNA (LNP-Tmprss6) or a luciferase control (LNP-Luc) and Tmprss6 or hepcidin (Hamp) mRNA (A) was assessed by quantitative real-time PCR, normalized to β-actin (Actb), and then expressed relative to the LNP-Luc value the mean value of which was defined as 1.0. (B) Four-week time-course analysis of Tmprss6 or hepcidin mRNA expression and transferrin saturation after treatment with 0.3 mg/kg of PBS, LNP-Luc, or LNP-Tmprss6. Ratios are expressed ± SEM (n = 5 in each group).
Tmprss6 siRNA treatment ameliorates the HH phenotype in Hfe−/− mice
To ascertain whether silencing of Tmprss6 expression could modulate systemic iron stores, we treated 6-week-old female Hfe−/− mice with IV PBS, LNP-Luc siRNA, or LNP-Tmprss6 siRNA at 1 mg/kg. Mice received either a single injection and were then killed 2 weeks later or received 3 sequential doses every 2 weeks beginning at 6 weeks of age and were killed at 12 weeks of age.
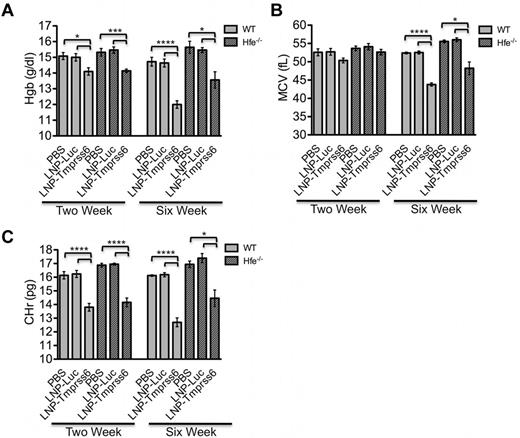
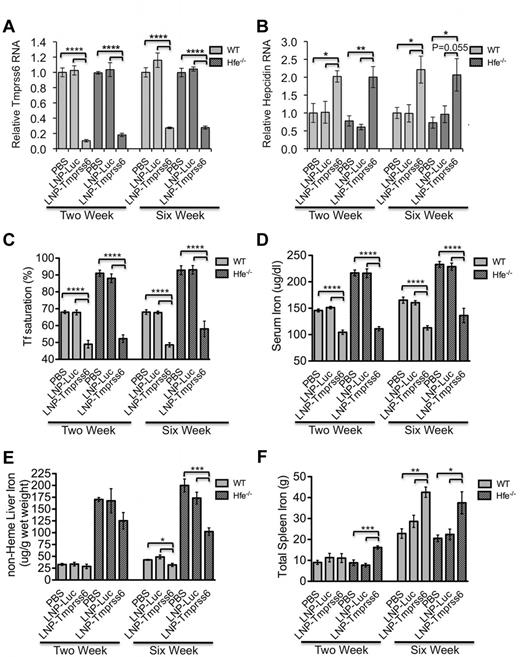
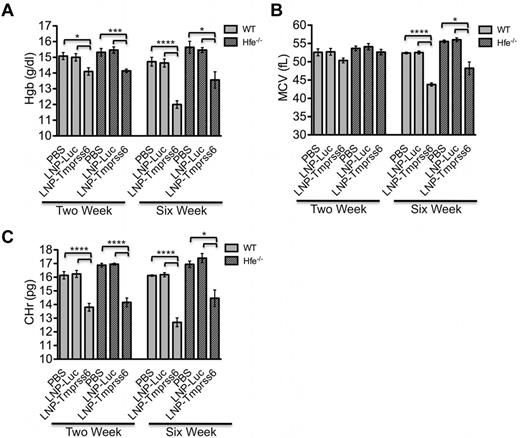
As expected, in both WT and Hfe−/− mice, treatment with LNP-Tmprss6 decreased Tmprss6 mRNA (Figure 2A) and increased hepcidin (Figure 2B) and Id1 expression (supplemental Figure 1A), the latter being another gene regulated by the BMP-SMAD signaling pathway in a manner similar to hepcidin. Concordantly, serum iron and transferrin saturation (Figure 2C-D) were decreased at 2 and 6 weeks; nonheme liver iron was decreased at 6 weeks (Figure 2E). In Hfe−/− mice, this effect on liver iron can be visualized in Perls-stained tissue sections as greatly decreased periportal hepatocellular iron (supplemental Figure 2A-B). There was an age-related increase in spleen iron in control mice, but by 6 weeks, LNP-Tmprss6 treatment also disproportionally increased total spleen iron in both WT and Hfe−/− mice (Figure 2F). The relative increase in spleen iron can be attributed to the effect of hepcidin expression on sequestering iron in iron-recycling macrophages in the spleen. In all cases, the effect on tissue iron levels was more pronounced in the chronically dosed cohort. Similarly, LNP-Tmprss6 treatment had a cumulative effect on erythropoiesis, leading to a hypochromic, microcytic, iron deficiency anemia in all mice by 6 weeks (Figure 3A-C). The data conclusively indicate that pharmacologic manipulation of Tmprss6 expression with LNP-siRNA can modulate iron stores and induce iron-deficient erythropoiesis, not only in WT mice, but also in mice with genetic hemochromatosis.
Phenotypic analysis of a HH mouse (Hfe−/−) model after treatment after treatment with LNP-Tmprss6 siRNA. Six-week-old female mice were injected once and then harvested 14 days later (left side of graphs, 2-week experiment) or injected a total of 3 times over 6 weeks and then harvested 14 days after the final injection (right side of graphs, 6-week experiment) with 1 mg/kg of PBS, LNP-Luc, or LNP-Tmprss6. Total mRNA was harvested from WT or Hfe−/− (n = 4 or 5 for each genotype) mice and Tmprss6 (A) or hepcidin (Hamp; B) mRNA was assessed by quantitative real-time PCR as in Figure 1. Ratios are expressed ± SEM. (C-F) Analysis of serum transferrin saturation (C; %), serum iron (D; μg/dL), nonheme liver iron (E) and total spleen iron (F) are depicted. (n = 5 for each genotype.) P values were calculated using the Student t test. ****P < .001; ***P < .005; **P < .01; *P < .05.
Phenotypic analysis of a HH mouse (Hfe−/−) model after treatment after treatment with LNP-Tmprss6 siRNA. Six-week-old female mice were injected once and then harvested 14 days later (left side of graphs, 2-week experiment) or injected a total of 3 times over 6 weeks and then harvested 14 days after the final injection (right side of graphs, 6-week experiment) with 1 mg/kg of PBS, LNP-Luc, or LNP-Tmprss6. Total mRNA was harvested from WT or Hfe−/− (n = 4 or 5 for each genotype) mice and Tmprss6 (A) or hepcidin (Hamp; B) mRNA was assessed by quantitative real-time PCR as in Figure 1. Ratios are expressed ± SEM. (C-F) Analysis of serum transferrin saturation (C; %), serum iron (D; μg/dL), nonheme liver iron (E) and total spleen iron (F) are depicted. (n = 5 for each genotype.) P values were calculated using the Student t test. ****P < .001; ***P < .005; **P < .01; *P < .05.
Analysis of erythropoietic parameters in treated Hfe−/− mice. The RBC parameters hemoglobin (Hgb; A), mean cell volume (MCV; B), and reticulocyte mean cell hemoglobin (CHr; C) were measured in female WT and Hfe−/− using the same treatment schedule as described in Figure 2. Data are presented as mean ± SEM (n = 5 for each genotype.) P values were calculated using the Student t test. ****P < .001; ***P < .005; **P < .01; *P < .05.
Analysis of erythropoietic parameters in treated Hfe−/− mice. The RBC parameters hemoglobin (Hgb; A), mean cell volume (MCV; B), and reticulocyte mean cell hemoglobin (CHr; C) were measured in female WT and Hfe−/− using the same treatment schedule as described in Figure 2. Data are presented as mean ± SEM (n = 5 for each genotype.) P values were calculated using the Student t test. ****P < .001; ***P < .005; **P < .01; *P < .05.
Tmprss6 siRNA treatment diminishes secondary iron overload and improves erythropoiesis, splenomegaly, and RBC survival in a mouse model of β-thalassemia intermedia
Hbbth3/+ mice have a heterozygous deletion of the β-minor and β-major hemoglobin genes, which results in transfusion-independent anemia similar to clinically mild human β-thalassemia intermedia. The anemia is multifactorial, resulting both from ineffective erythropoiesis and decreased RBC survival and manifesting as splenomegaly and reticulocytosis. Similar to their human β-thalassemia intermedia disease counterparts, Hbbth3/+ mice also have systemic iron overload secondary to suppression of hepcidin expression by ineffective erythropoiesis.46 Previously, Hbbth3/+ mice placed on an iron-deficient diet or expressing transgenic hepcidin were shown to have improved iron parameters and decreased ineffective erythropoiesis.33 Likewise, homozygous loss of Tmprss6 in Hbbth3/+ mice increases hepcidin expression and ameliorates the phenotype.34 On this basis, in the present study, we treated Hbbth3/+ mice with Tmprss6 siRNA to determine whether pharmacologic modulation of hepcidin production could have similar effects.
As expected, LNP-Tmprss6 treatment of Hbbth3/+ mice severely abrogated Tmprss6 expression and increased hepcidin (Figure 4A-B) and Id1 mRNA expression (supplemental Figure 1B). Treated mice also had decreased transferrin saturation and serum iron (Figure 4C-D). Correspondingly, nonheme liver iron and total spleen iron (Figure 4E-F) were decreased, with the latter largely attributable to the decrease in spleen size. Treatment induced a mild microcytic, hypochromic anemia and induced erythropoietin production in WT mice (Figure 5A-B). However, treatment of Hbbth3/+ mice actually improved their anemia as determined by the hemoglobin (Figure 5A) and serum erythropoietin (Figure 5B), the latter occurring only by 6 weeks. This occurs even though treatment further diminished the mean cell volume (MCV) and reticulocyte mean cell hemoglobin (Figure 5C-D).
Phenotypic analysis of a thalassemia-intermedia mouse (Hbbth3/+) model after treatment with LNP-Tmprss6 siRNA. Six-week-old female mice were injected once (1 mg/kg) and then harvested 14 days later (left side of graphs, 2-week experiment) or injected a total of 3 times (1 mg/kg) over 6 weeks and then harvested 14 days after the final injection (right side of graphs, 6-week experiment). Total mRNA was harvested from WT or Hbbth3/+ (n = 4 or 5 for each genotype) and Tmprss6 (A) or hepcidin (Hamp; B) mRNA was assessed by quantitative real-time PCR as in Figure 1. Ratios are expressed ± SEM. (C-F) Analysis of serum transferrin saturation (C; %), serum iron (D; μg/dL), nonheme liver iron (E), and total spleen iron (F; g) are depicted (n = 5 or 6 for each genotype.) P values were calculated using the Student t test. ****P < .001; ***P < .005; **P < .01; *P < .05.
Phenotypic analysis of a thalassemia-intermedia mouse (Hbbth3/+) model after treatment with LNP-Tmprss6 siRNA. Six-week-old female mice were injected once (1 mg/kg) and then harvested 14 days later (left side of graphs, 2-week experiment) or injected a total of 3 times (1 mg/kg) over 6 weeks and then harvested 14 days after the final injection (right side of graphs, 6-week experiment). Total mRNA was harvested from WT or Hbbth3/+ (n = 4 or 5 for each genotype) and Tmprss6 (A) or hepcidin (Hamp; B) mRNA was assessed by quantitative real-time PCR as in Figure 1. Ratios are expressed ± SEM. (C-F) Analysis of serum transferrin saturation (C; %), serum iron (D; μg/dL), nonheme liver iron (E), and total spleen iron (F; g) are depicted (n = 5 or 6 for each genotype.) P values were calculated using the Student t test. ****P < .001; ***P < .005; **P < .01; *P < .05.
Analysis of erythropoietic parameters in treated Hbbth3/+ mice. (A,C,D) The RBC parameters Hgb (A), MCV (C), and reticulocyte mean cell hemoglobin (CHr; D) were measured in female WT and Hbbth3/+ mice. (B) ELISA erythropoietin analysis of serum collected from WT and Hbbth3/+ mice on same treatment schedule as described in Figure 4 (n = 5 or 6 for each genotype). Data are presented as mean ± SEM. P values were calculated using the Student t test. ****P < .001; ***P < .005; **P < .01; *P < .05.
Analysis of erythropoietic parameters in treated Hbbth3/+ mice. (A,C,D) The RBC parameters Hgb (A), MCV (C), and reticulocyte mean cell hemoglobin (CHr; D) were measured in female WT and Hbbth3/+ mice. (B) ELISA erythropoietin analysis of serum collected from WT and Hbbth3/+ mice on same treatment schedule as described in Figure 4 (n = 5 or 6 for each genotype). Data are presented as mean ± SEM. P values were calculated using the Student t test. ****P < .001; ***P < .005; **P < .01; *P < .05.
At baseline, Hbbth3/+ mice demonstrated many of the hallmarks of ineffective erythropoiesis, particularly an expansion of erythropoiesis manifesting as splenomegaly (Figure 6A). Decreased RBC survival, necessitating increased RBC production and indicated by a reticulocytosis (Figure 6B-C), likely also contributes substantially to the splenomegaly. In thalassemic mice, treatment with LNP-Tmprss6 siRNA led to a significant diminution in both spleen size and reticulocytosis accompanied by an increase in the number of mature RBCs in circulation (Figure 6A-C). This shift from reticulocytes to mature RBCs in part accounts for the decreased MCV because reticulocytes are larger than their mature RBC counterparts, but the diminution of the cellular hemoglobin of the reticulocyte (and MCH and MCVr, not shown) indicates that the reticulocytes themselves are smaller and contain less hemoglobin. The extent to which treatment improves ineffective erythropoiesis is difficult to assess; however, it is clear that treatment essentially normalized the RBC survival in Hbbth3/+ mice (Figure 6D). We assessed whether these changes in RBC parameters and survival also decreased membrane-associated α-globin, which is considered the primary cause of membrane damage and decreased RBC survival in β-thalassemia.47 We found that the absolute amount of membrane-associated α-globin was markedly decreased and the ratio of membrane-bound α- and β-globin was essentially normalized (Figure 6E). The effects of these changes were remarkably well demonstrated by a profound alteration in the RBC morphology that occurred with treatment of the Hbbth3/+ mice (supplemental Figure 3A-B) in which anisocytosis, polychromasia (indicative of reticulocytosis), and the abundance of target cells were substantially diminished. Indeed, after 6 weeks of treatment, there was a near normalization of the smear with an increased central pallor remaining as the hallmark of the treated erythrocytes.
Analysis of RBC maturation in treated Hbbth3/+ mice. (A-C) Total spleen weight (g; A), RBC count (B), and reticulocyte count (Retic; C) are depicted. (D) Murine RBCs were biotinylated and flow cytometric analysis of streptavidin-labeled cells was completed every 7 days (WT LNP-Luc, n = 5; WT LNP-Tmprss6, n = 4; Hbbth3/+ LNP-Luc, n = 2 or Hbbth3/+ LNP-Tmprss6, n = 3) to determine RBC survival. (E) RBC membrane-bound globins were analyzed using TAU gel electrophoresis. The number below each lane is the intensity of α-globin compared with β-globin. Globins from the WT-soluble fraction were used as a standard. Data are presented as mean ± SEM. P values were calculated using the Student t test. ****P < .001; ***P < .005.
Analysis of RBC maturation in treated Hbbth3/+ mice. (A-C) Total spleen weight (g; A), RBC count (B), and reticulocyte count (Retic; C) are depicted. (D) Murine RBCs were biotinylated and flow cytometric analysis of streptavidin-labeled cells was completed every 7 days (WT LNP-Luc, n = 5; WT LNP-Tmprss6, n = 4; Hbbth3/+ LNP-Luc, n = 2 or Hbbth3/+ LNP-Tmprss6, n = 3) to determine RBC survival. (E) RBC membrane-bound globins were analyzed using TAU gel electrophoresis. The number below each lane is the intensity of α-globin compared with β-globin. Globins from the WT-soluble fraction were used as a standard. Data are presented as mean ± SEM. P values were calculated using the Student t test. ****P < .001; ***P < .005.
Histologic analysis of Hbbth3/+ control spleens demonstrated nearly complete obliteration of the white pulp with greatly increased red pulp (supplemental Figure 4A-B). Immunostaining for transferrin receptor 1 (Tfr1; supplemental Figure 4C-D) confirmed the histologic impression of markedly expanded splenic erythropoiesis. Tmprss6 siRNA treatment restored the splenic architecture and markedly decreased Tfr1 expression (supplemental Figure 4A-D). These data indicate that increasing hepcidin production through pharmacologic manipulation of Tmprss6 expression is able to diminish the ineffective erythropoiesis and secondary iron overload and improve RBC survival in a murine model of β-thalassemia intermedia.
Discussion
There is a rapidly expanding body of preclinical data supporting the manipulation of the hepcidin-ferroportin iron regulatory pathway as a therapeutic strategy for primary or secondary iron overload disorders. Because of its unusual secondary structure, high serum levels, and rapid turnover, the use of hepcidin itself as a drug poses particular challenges. The development of biomimetic “minihepcidins” that are technically easier to synthesize and have better pharmacodynamic properties than the native peptide has been one approach that has shown some promise.48 Another strategy is to manipulate the liver's own prodigious ability to synthesize endogenous hepcidin either by inducing or repressing positive and negative regulators, respectively. Genetic studies in which iron-overloaded Hfe−/− mice29 or iron-overloaded, thalassemic Hbbth3/+ mice34 were intercrossed with Tmprss6−/− mice demonstrated the potential for pharmacologic inhibition of TMPRSS6 in reducing iron overload; in the thalassemia intermedia model, loss of Tmprss6 also had the added benefit of improving the anemia. In the present study, we sought to demonstrate that RNAi-mediated pharmacologic modulation of Tmprss6 expression could be achieved and has the potential to be equally efficacious.
Data presented herein demonstrate that pharmacologic inhibition of Tmprss6 expression is not only achievable, but also results in the expected physiologic and therapeutic effects. We show that an LNP-formulated siRNA durably inhibits liver Tmprss6 expression with a concomitant elevation of hepcidin expression. In WT mice, silencing of Tmprss6 was maintained at greater than 80% inhibition for 14 days. Levels of liver hepcidin mRNA were increased 2- to 3-fold from 24 hours through 7 days after administration. This led to approximately 50% decreases in serum iron concentration and transferrin saturation through 14 days, as well as decreases in hemoglobin levels and changes in other hematologic parameters between 14 and 28 days.
In the Hfe−/− model of HH, serial treatments with Tmprss6 siRNA also greatly increased hepcidin expression and led to significantly decreased transferrin saturation, serum iron, and nonheme liver iron. Likewise, repeated dosing of thalassemic Hbbth3/+ mice reduced serum and tissue iron parameters. As was demonstrated in Tmprss6−/−Hbbth3/+ compound mutant mice, we also observed an improvement of the anemia and splenomegaly by inhibiting Tmprss6 expression. The physiologic significance of the increase in hemoglobin was further demonstrated by the suppression of serum erythropoietin in chronically siRNA-treated mice compared with untreated Hbbth3/+ mice. Others have reported that genetic ablation of Tmprss6 improves erythropoiesis and decreases splenomegaly without a concomitant decrease in erythropoietin production.34 Furthermore, although limited transgenic overexpression of hepcidin33 was able to ameliorate erythropoietic defects and secondary iron overload in Hbbth3/+ mice, erythropoietin levels also did not change. There was no readily apparent cause for this discrepancy, other than perhaps the incomplete Tmprss6 suppression and fluctuation in endogenously produced hepcidin due to the cyclic effects of recurrent drug administration or the genetic background of mice used in each case. Alternatively, the initiation of Tmprss6 inhibition therapy at 6 weeks of age could have different effects on erythropoietin synthesis than the complete absence of the protein since birth.
For the first time, we show herein that normalization of RBC survival is a significant component of the effects of Tmprss6 inhibition on hemoglobin and spleen size. Similar in vivo assays were used to demonstrate that transferrin therapy32 and transgenic expression of hepcidin33 are also able to prolong the duration of RBC lifespan. As in those cases, we do not know to what extent amelioration of ineffective erythropoiesis contributes to these observations, because this is impossible to ascertain definitively without the assessment of turnover of serum iron or newly synthesized heme. Nevertheless, it is difficult to account for the near normalization of spleen size and the doubling of the erythrocyte number solely on the basis of an increased RBC half-life.
It also remains to be seen if the effect of hepcidin on erythropoiesis occurs indirectly through its effect on serum transferrin-bound iron available for erythropoiesis, by a direct effect on erythroid progenitors through modulation of Fpn1 expression,49 or both. It has been suggested that transferrin administration favorably affects thalassemic hematopoiesis by inducing a state of “functional iron deficiency” by reducing the transferrin saturation.32 That dietary iron deficiency exerts similar physiologic effects on thalassemic erythropoiesis also suggests that it is iron availability itself that is the dominant factor.33 Although it would seem likely that the reduction of the physiologically normal slight excess of α- compared with β-globin chains by iron deficiency is the proximal cause of the improvement in RBC survival and anemia,50 no one has yet demonstrated that any intervention in the hepcidin-ferroportin axis in any thalassemic model can modulate the rate of α- or β-chain synthesis. In addition to inducing iron deficiency, stimulation of hepcidin production might also have beneficial effects by redistributing stored iron from hepatocytes to macrophages, where it is less toxic.51
Before the present study, LNP formulations of siRNAs have been successfully used to decrease expression of several hepatic proteins.35,36,39 The liver is an especially good target organ for the LNP-mediated delivery of siRNA because it is well supplied with blood, has a highly permeable endothelium, and abundantly expresses receptors specific for lipid particles.37 Compared with small molecules and Abs, an RNAi therapeutic strategy has the distinct advantage of enabling very rapid development of a highly specific drug that can be used both for target validation and, ultimately, as a therapy. The reasons for this rapid development include ease of design and synthesis of target-specific complementary nucleic acids, rapid in vitro and in vivo assessment of efficacy via target knock-down, and ease of formulation. Furthermore, the potential to target classes of proteins that are not generally viewed as particularly accessible to inhibition by small molecules or Abs, such as transcription factors and nonenzyme intracellular proteins, are distinct advantages. In the research environment, RNAi strategies such as those used here have the potential to supplant and/or complement gene-targeting approaches, particularly when hepatic-specific deletion is lethal or when it is desirable to rapidly evaluate the desirability of initiating a gene-targeting approach. This liver-specific inhibition is uniquely desirable for the development of therapeutics targeting iron metabolic pathways because this organ plays a central role in the regulation of iron uptake and distribution through the modulation of hepcidin expression. This method allows for reversible, titratable gene silencing, which could be crucial when modulating hepcidin expression and, by extension, iron metabolism. We have noted a small, transient induction of hepcidin by the control LNP-Luc. This suggests that the LNP itself can cause a minimal inflammatory response in mice. These observations are consistent with the dose-dependent findings recently published for other LNP-formulated siRNAs.52 However, in the present study, hepcidin induction was minor and transient (resolved by 24 hours after dosing), whereas the induction of hepcidin through silencing of Tmprss6 was maintained past 7 days.
In conclusion, the results of the present study demonstrate that pharmacologically targeting Tmprss6 expression by systemic administration of an RNAi therapeutic is able to modulate hepcidin expression in mouse models of HH and β-thalassemia intermedia. Up-regulation of hepcidin diminishes iron overload in both models. The limitation of iron available for erythropoiesis in β-thalassemic mice ameliorates ineffective erythropoiesis and splenomegaly. These data provide proof of principle that pharmacologic manipulation of hepcidin may be an effective treatment for human diseases of iron dysregulation. In particular, an RNAi therapeutic could serve as a substitute for, or adjunct therapy to, phlebotomy in HH patients or iron chelation treatment in patients affected by β-thalassemia. Further research will seek to demonstrate the effectiveness of siRNA modulation of hepcidin in human diseases themselves.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Tom Bartnikas for technical advice and helpful suggestions, Dean Campagna and Sherika Blevins for excellent technical assistance, and members of the Fleming laboratory for helpful discussions.
This work was supported by Alnylam Pharmaceuticals.
Authorship
Contribution: P.J.S. analyzed the mouse data and wrote the manuscript; P.J.S., I.T., D.B., and M.D.F. conceived and designed the murine experiments; A.K.S. analyzed the globin content of RBCs; T.R. performed the tail injections; S.M. and B.R.B. performed the design and in vitro screening to select the TMPRSS6 siRNAs; J.H. performed the mouse serum analyses; and I.T., D.B., and M.D.F. designed the research and wrote the manuscript.
Conflict-of-interest disclosure: I.T., T.R., S.M., B.R.B., J.H., and D.B. are employees and/or stockowners of Alnylam Pharmaceuticals. M.D.F. receives research funding from Alnylam Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Paul J. Schmidt, PhD, Department of Pathology, Children's Hospital Boston, Enders Research Bldg, Rm 1110, 320 Longwood Ave, Boston, MA 02115; e-mail: pschmidt@enders.tch.harvard.edu.