Key Points
Mouse umbilical arteries and VAs contain precursors that can mature into adult HSCs in the presence of interleukin 3.
Abstract
During mouse development, definitive hematopoietic stem cells (dHSCs) emerge by late E10.5 to E11 in several hematopoietic sites. Of them, the aorta-gonad-mesonephros (AGM) region drew particular attention owing to its capacity to autonomously initiate and expand dHSCs in culture, indicating its key role in HSC development. The dorsal aorta contains characteristic hematopoietic clusters and is the initial site of dHSC emergence, where they mature through vascular endothelial (VE)-cadherin+CD45–CD41low (type 1 pre-HSCs) and VE-cadherin+CD45+ (type 2 pre-HSCs) intermediates. Although dHSCs were also found in other embryonic niches (placenta, yolk sac, and extraembryonic vessels), attempts to detect their HSC initiating potential have been unsuccessful to date. Extraembryonic arterial vessels contain hematopoietic clusters, suggesting that they develop HSCs, but functional evidence for this has been lacking. Here we show that umbilical cord and vitelline arteries (VAs), but not veins, contain pre-HSCs capable of maturing into dHSCs in the presence of exogenous interleukin 3, although in fewer numbers than the AGM region, and that pre-HSC activity in VAs increases with proximity to the embryo proper. Our functional data strongly suggest that extraembryonic arteries can actively contribute to adult hematopoiesis.
Introduction
During mouse development, hematopoietic specification occurs from E7.5 in the yolk sac (YS), allantois, and chorion.1-3 Primitive hematopoiesis originating in the YS provides short-lived hematopoietic cells and progenitors for the developing embryo.3,4 Definitive hematopoietic stem cells (dHSCs), capable of long-term multilineage hematopoietic repopulation of adult recipients, emerge only by late E10.5 to E11. The capacity of the aorta-gonad-mesonephros (AGM) region to generate dHSCs in organotypic cultures indicated that this site plays an important role in HSC development.5,6 Around the time when dHSCs appear in the AGM region, HSCs can also be found in the placenta,7-9 YS,6,10 and umbilical cord (UC)/vitelline vessels (VVs).11 Hematopoietic potential has also been observed in cerebral vessels.12 By E12.5, the placenta and YS contain elevated numbers of dHSCs, which later migrate to the fetal liver, along with those from the AGM region, to undergo expansion before colonizing the bone marrow prior to birth.10 Although the extraembryonic sites harbor dHSCs and exhibit hematopoietic potential,9,13,14 previous attempts to demonstrate by ex vivo culture that these tissues can also generate dHSCs have been unsuccessful.8,11 The exact tissue origin of HSCs in mouse development remains open to debate.15
The dHSCs in the AGM region are localized to the ventral domain of the dorsal aorta,16 possibly within intra-aortic hematopoietic clusters that are closely associated with the endothelial lining.17-21 It has been reported that dHSCs emerge via an endothelial-to-hematopoietic transition in zebra fish,22,23 mouse,13,24,25 and embrionic stem cell models.26,27 Recently, we demonstrated that dHSCs in the AGM region mature in a stepwise manner from precursor cells: type 1 pre-HSCs that express vascular endothelial (VE)-cadherin+CD45–CD41low mature into type 2 pre-HSCs that are VE-cadherin+CD45+, before becoming fully functional adult-repopulating dHSCs.28,29
In the AGM region, intra-aortic hematopoietic clusters are thought to be associated with HSC formation.13,19,24,25,30 Indeed, at early stages, HSC activity is associated with the aorta but not with cardinal veins.11,16 Similar hematopoietic cell clusters observed within arterial components of UC and VVs (omphalomesenteric) led to the idea that HSCs develop in major arteries.11,19,20,31
Extraembyonic vessels develop through complex morphogenesis.32 The UC originates mainly from the allantois protruding from the posterior part of the embryo body shortly after gastrulation. The capacity of the allantois to generate hematopoietic cells was first shown in avian embryos.33,34 In the mouse, Runx1 expression occurs in the allantois prior to circulation, and its inherent hematopoietic capacity was confirmed.1,2 Intra-allantoic vessels form de novo by E7.5, and later umbilical vasculature fuses with the paired dorsal aorta.32,35 By E8.5, the tip of the allantois reaches the chorion and forms the chorio-allantoic fusion, which later becomes the embryonic part of the placenta.32 The VVs, which connect the YS and embryo proper, undergo extensive remodeling between E8.5 and E10.5,36 whereby the vitelline artery (VA) is fused to the dorsal aorta and the paired vitelline veins connect to the fetal liver.37 By late E10.5 to E11.5, the extraembryonic vessels contain some dHSCs,11 but it remains unclear which of these vessels harbored HSCs and whether these HSCs migrate from the AGM region or are independently generated in these vessels.
By using a quantitative limiting dilution approach, we demonstrate here that UC and VV contain low numbers of dHSCs, with not every vessel possessing HSC activity. After culture with exogenous growth factors (GFs), significant expansion of HSC numbers occurs in every explant, thus revealing the presence of pre-HSCs. Using a culture method that potently supports maturation of AGM-derived pre-HSCs, sorting experiments showed that, similar to the AGM region, UC and VV contain pre-HSCs.28 Subdissection of UC and VV followed by long-term transplantation experiments showed that pre-HSCs and dHSCs are localized exclusively to arterial but not venous vessels.
Interleukin 3 (IL-3) has been implicated in dHSC development in the AGM region.38 In contrast to the AGM region, UC and VV do not express detectable levels of IL-3, and dHSC production by vessel explants entirely depends on supplementation with exogenous IL-3, suggesting that UC- and VV-derived HSCs may have to relocate to the AGM region, placenta, or fetal liver to undergo further maturation and expansion.
Materials and methods
Mice and embryo manipulation
Embryos were obtained from C57BL/6 (CD45.2/2) mice. E11.0 to E11.5 embryos had 41 to 47 somite pairs, with morphologic characteristics described by Taylor et al.39 Independent experiments performed using different embryonic litters were repeated several times, as indicated. Mice were bred and used in experiments under UK Home Office regulations with approval of the University of Edinburgh Ethical Review Committee. Dissections were performed as described in the text and in Rybtsov et al28 and Taoudi et al.40 Embryonic tissues were dissected in 7% fetal calf serum in phosphate-buffered saline + MgCl2 + CaCl2 (Gibco) with penicillin/streptomycin. Peripheral blood (PBL) was collected either by draining the embryo after severing the head or by gently flushing the dorsal aorta using a glass pipette.
Explant and reaggregate cultures
Sorted embryo-derived cells (1 embryo equivalent [e.e.]) and 105 OP9 stromal cells were coaggregated by centrifugation in the yellow pipette tip as described previously.28 Explants or cell aggregates were placed onto 0.65- or 0.8-µm membranes (Millipore), floating on Iscove modified Dulbecco medium (Invitrogen) supplemented with L-glutamine (4 mM), penicillin/streptomycin (50 U/mL), batch-tested fetal calf serum (20%), and GFs (Peprotech: 100 ng/mL IL-3, 100 ng/mL stem cell factor [SCF], 100 ng/mL fms-like tyrosine kinase 3 [Flt3] ligand), unless otherwise indicated. In IL-3–only conditions, 200 ng/mL was used. Explants or coaggregates were cultured at the air-liquid interface for 4 to 5 days (37°C, 5% CO2).16,28 Cultures were dissociated using dispase/collagenase solution of 1 mg/mL (Roche) for 40 minutes (37°C), and single-cell suspensions prepared.
Repopulation assays
Donor cells from C57BL/6 (CD45.2/2) embryos were injected into C57BL/6 (CD45.1/1 or CD45.1/2) irradiated adult recipient mice (>6 weeks old), coinjected with 50 000 carrier bone marrow cells (CD45.1/2 or CD45.1/1, respectively) to provide short-term radioprotection. Recipient mice were γ-irradiated with a split dose of 1150 rad with an interval of 3 hours and transplanted on the same day. Donor cell doses are denoted as embryo equivalents, which refers to the proportion of cells relative to the number of those cells in the original tissue. Levels of donor cell contribution were assessed in PBL of recipients 12 to 14 weeks posttransplantation. PBL was collected into 5 mM EDTA/phosphate-buffered saline from the tail vein by venesection and depleted of erythrocytes (PharmLyse; BD Biosciences), before staining with monoclonal antibodies: anti-CD16/32 (Fc-block), anti-CD45.1-antigen-presenting cell (APC) (clone A20), anti-CD45.2-phycoerythrin (PE) (clone 104), or control isotype antibodies (eBioscience). ELDA software was used to calculate HSC numbers in limiting dilution analyses.41 Mice exhibiting >5% donor chimerism (>14 weeks) were considered to be repopulated with HSCs. Multilineage flow cytometric analysis was performed on PBL, thymus, bone marrow, and spleen using monoclonal antibodies against Ter119, Mac1, Gr1, CD3e, CD4, B220, or CD8 (BD Biosciences), conjugated with fluorescein isothiocyanate, PE, APC, or biotin, which was detected by secondary staining with streptavidin-PE or streptavidin-APC.
Flow cytometry
Flow cytometry was performed on Fortessa or FACSCaliburs. Sorting was performed on FACSAriaII or MoFlo (Cytomation), using FACSDiva software. Data were analyzed in FlowJo software (TreeStar). Single-cell suspensions were prepared by dispase/collagenase-mediated dissociation and stained with anti-CD45-PE or CD45-A488 (clone 30-F11) and VE-cadherin (clone 11.D4.1) (BD Biosciences), conjugated in-house to biotin and detected by secondary streptavidin-APC, or VE-cadherin clone BV13 conjugated to A647 (Biolegend). The 7-aminoactinomycin D viability staining solution was used to exclude dead cells, and gates were set using appropriate isotype antibodies and fluorescence minus 1 controls. Purity of sorted cells was checked before culture.
Hematopoietic colony assays
Hematopoietic progenitors were quantified in clonogenic colony forming unit (CFU) cell assays. Cells were seeded into methylcellulose supplemented with several cytokines (M3434; StemCell Technologies) that stimulate progenitors to differentiate into mature hematopoietic cell types. Colonies were counted after 8 to 10 days of incubation at 37°C and categorized based on hematopoietic cell content.
Microscopy
Photographs were taken on a Leica MZFLIII microscope using a Hamamatsu digital camera and processed in Volocity software or Adobe Photoshop.
Quantitative reverse-transcription polymerase chain reaction (qRT-PCR)
RNA was isolated from embryonic tissues (using 5-10 e.e. of tissue for each sample) with Qiagen RNeasy mini kit (+DNAse treatment) and single-strand complementary (cDNA) prepared using SuperScriptVILO cDNA Synthesis (Invitrogen) and Qiaquick purification kits (Qiagen). A universal probe library (UPL) strategy with Lightcycler 480 probes mastermix kit and machine (Roche) were used for qRT-PCR. Triplicate reactions were set up with 20 to 60 ng cDNA each. Tissue samples from 4 independent embryo litters were assessed. Gene expression levels were determined relative to the TBP housekeeping gene. Primers included the following: SCF (UPL probe 68) 5′ primer, agcgctgcctttccttatg, and 3′ primer, ccttggttttgacaagaggatt; Flt3L (UPL probe 25) 5′ primer, aggcctgccagaatttctct, and 3′ primer, tctagggctatgggactcctt; IL-3 (UPL probe 94) 5′ primer, tacatctgcgaatgactctgc, and 3′ primer, ggctgaggtggtctagaggtt; and TBP (UPL probe 97 or 11) 5′ primer, ggggagctgtgatgtgaagt, and 3′ primer, ccaggaaataattctggctca.
Results
UCs and VVs contain VE-cad+CD45+ dHSCs
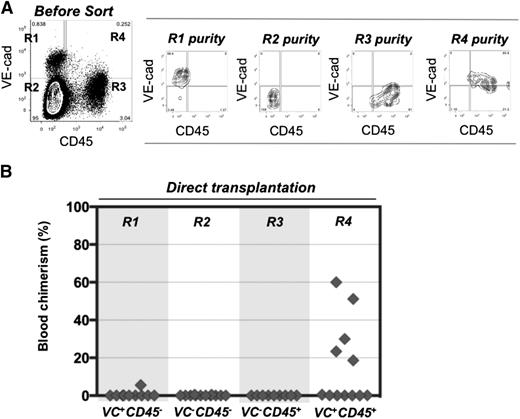
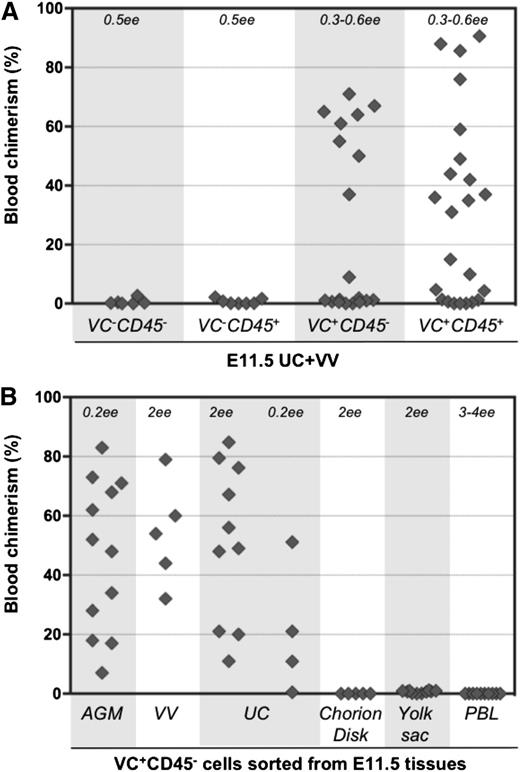
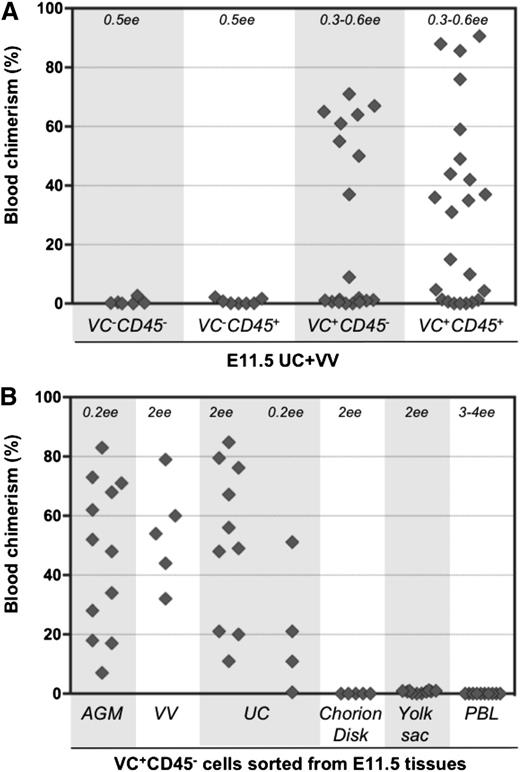
To determine the surface phenotype of dHSCs in extraembryonic vessels, cell suspensions were prepared from E11.5 UCs and VVs, and cells were sorted by fluorescence-activated cell sorter based on VE-cadherin and CD45 expression. Four cell populations were transplanted directly, without a culture step, into irradiated adult recipients (Figure 1A-B). Recipient PBL was assessed for donor cell contribution 13 to 14 weeks posttransplantation. In 6 independent sorting experiments, long-term definitive HSC activity resided only within the VE-cad+CD45+ fraction of extraembryonic vessels, similar to the AGM region.30,40 Because only 5 out of 12 recipients were repopulated, each of them likely received only 1 dHSC. Bone marrow from 2 primary recipients was harvested and injected separately into 3 secondary recipients, all of which showed high levels of donor-derived hematopoietic repopulation (supplemental Figure 1A, available on the Blood Web site).
Definitive HSCs in E11.5 UC and VV are enriched in the VE-cad+CD45+ fraction. (A) UC and VV were pooled and sorted on the basis of VE-cadherin and CD45 expression. (B) Four sorted cell populations were transplanted directly into irradiated adult recipients (8 e.e. per mouse), and the level of donor-derived engraftment in PBL was assessed 13 to 14 weeks posttransplantation (n = 6 independent experiments).
Definitive HSCs in E11.5 UC and VV are enriched in the VE-cad+CD45+ fraction. (A) UC and VV were pooled and sorted on the basis of VE-cadherin and CD45 expression. (B) Four sorted cell populations were transplanted directly into irradiated adult recipients (8 e.e. per mouse), and the level of donor-derived engraftment in PBL was assessed 13 to 14 weeks posttransplantation (n = 6 independent experiments).
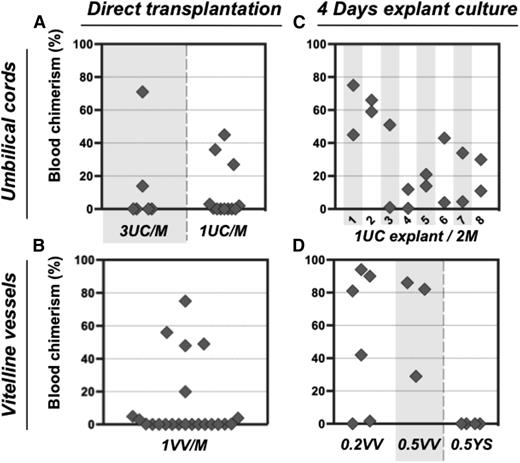
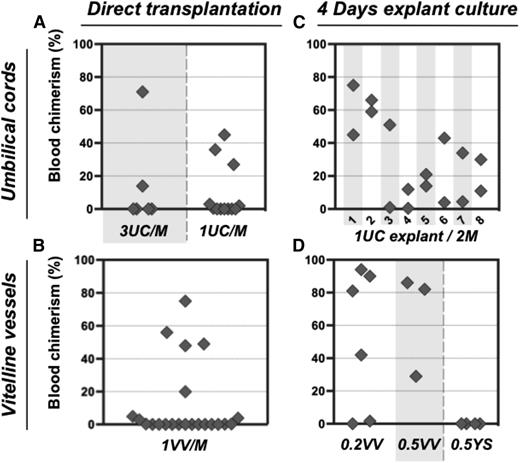
In order to assess the numbers of dHSCs present, E11.5 UC and VV were individually dissociated and injected without sorting into irradiated mice at doses of 1 or 3 e.e. per recipient. Only 5 out of 18 recipients that received in total 30 UCs showed donor-derived repopulation (Figure 2A), indicating a frequency of ∼0.17 dHSC per UC. Similarly, only 5 out of 22 mice were repopulated when a total of 22 VVs were injected (Figure 2B), indicating a frequency of ∼0.23 HSC per VV. Thus, both UC and VV appear to harbor fewer dHSCs than the AGM region, which reportedly contains 0.3 to 1.0 dHSC per AGM, depending on the exact stage.10,39
Pre-HSC potential is revealed in explant cultures of UC and VV. (A-B) Direct transplants. E11.5 UC or VV were separated on dissection and transplanted individually into irradiated recipients, and the level of donor-derived PBL chimerism (%) was determined 14 weeks posttransplantation. Only 5 out of 30 UCs transplanted into recipients and 5 out of 22 VV recipients were repopulated. (C-D) Transplantation of explant cultures. UC or VV explants were cultured for 4 days with GF (IL-3 + SCF + Flt3l) and transplanted separately into irradiated mice as follows: 1 UC explant per 2 recipients (8 explants in total) and 0.2 to 0.5 VV explants per 1 recipient. Note that after culture all UC and VV explants contained HSC activity. M, recipient mouse.
Pre-HSC potential is revealed in explant cultures of UC and VV. (A-B) Direct transplants. E11.5 UC or VV were separated on dissection and transplanted individually into irradiated recipients, and the level of donor-derived PBL chimerism (%) was determined 14 weeks posttransplantation. Only 5 out of 30 UCs transplanted into recipients and 5 out of 22 VV recipients were repopulated. (C-D) Transplantation of explant cultures. UC or VV explants were cultured for 4 days with GF (IL-3 + SCF + Flt3l) and transplanted separately into irradiated mice as follows: 1 UC explant per 2 recipients (8 explants in total) and 0.2 to 0.5 VV explants per 1 recipient. Note that after culture all UC and VV explants contained HSC activity. M, recipient mouse.
UCs and VVs can initiate dHSCs in explant culture supplemented with exogenous GFs
Unlike dHSCs, their precursors (pre-HSCs) are not yet able to repopulate the blood system of irradiated adult recipients. Therefore, to detect pre-HSCs in embryonic tissues, a culture step (organ or aggregate) was introduced in which they can mature into dHSCs.5,16,29 Using this approach, we determined whether extraembryonic vessels could initiate dHSCs in culture (Figures 2 and 3; supplemental Figure 2). Isolated UC explants were cultured in the presence of GFs (IL-3 + SCF + Flt3L) for 4 days. Transplantation of individual UC explants into 2 irradiated recipients each resulted in hematopoietic repopulation of at least 1 recipient (Figure 2C). Although there was <0.2 dHSC per fresh UC, after culture UCs contained at least 1.5 dHSCs per explant (Figure 2C). Notably, not every fresh UC contained dHSCs, but each developed HSC activity during culture, indicating that every vessel contained pre-HSCs.
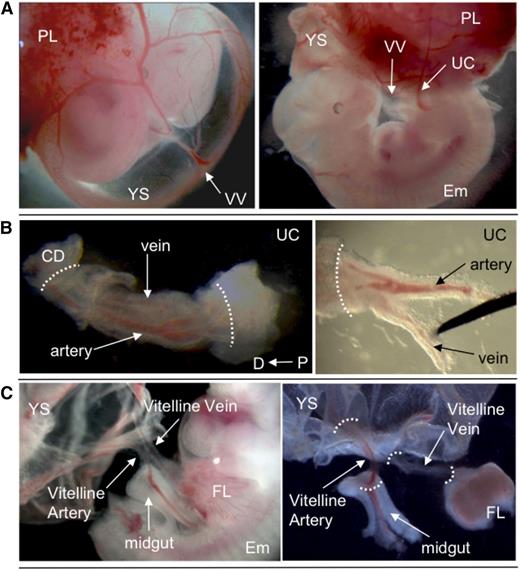
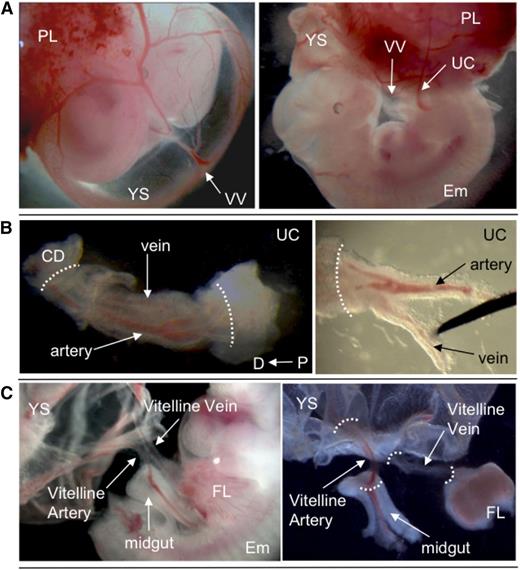
Dissection of extraembryonic vessels. (A) E11.5 wild-type embryo, showing position of VV and UC. (B) Dissection of UC. Separation of artery and vein is shown. (C) Dissection of VV. YS and midgut loop were removed. White dotted lines indicate points of dissection to isolate vessels. Original magnification ×8 (A), ×40 (B), and ×32 (C). CD, chorion disk; D, distal; Em, embryo proper; FL, fetal liver; P, proximal; PL, placenta.
Dissection of extraembryonic vessels. (A) E11.5 wild-type embryo, showing position of VV and UC. (B) Dissection of UC. Separation of artery and vein is shown. (C) Dissection of VV. YS and midgut loop were removed. White dotted lines indicate points of dissection to isolate vessels. Original magnification ×8 (A), ×40 (B), and ×32 (C). CD, chorion disk; D, distal; Em, embryo proper; FL, fetal liver; P, proximal; PL, placenta.
dHSC generation was also observed in VV explants accurately separated from YS at the point of entry. Although only ∼0.23 dHSC per VV was detected in freshly isolated VV, cultured VVs generated on average 2.6 dHSCs per VV (Figure 2D). By contrast, injection of YS explants carefully dissected from VVs did not lead to any repopulation (Figure 2D). dHSCs from both UC and VV explants successfully achieved multilineage repopulation of secondary recipients (supplemental Figures 1B-C, 3, and 4).
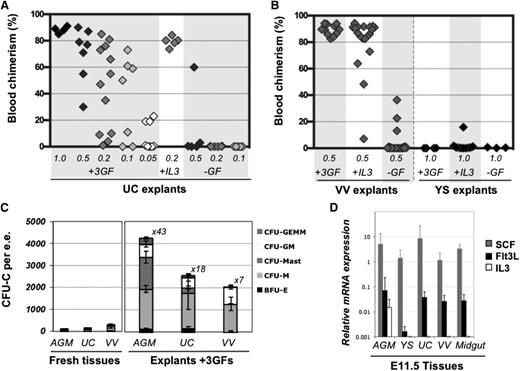
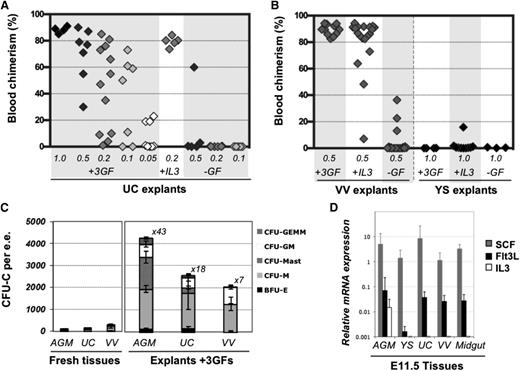
Limiting dilution analyses revealed that cultured explants exhibited extensive generation of dHSCs (Figure 4A). A frequency of 9.6 dHSCs per UC was observed (calculated by ELDA software, see Materials and methods), representing an expansion of 56-fold compared with fresh tissues. Cultured VV in the presence of GFs also showed significant expansion of dHSCs (Figure 4B). In the absence of GFs, dHSCs were only maintained in UC and VV explants (compare Figure 2A-B and Figure 4A-B), in contrast to the AGM region, which can initiate and expand HSCs self-autonomously by ∼10-fold.5 IL-3 or a combination of SCF, Flt3L, and IL-3 can substantially stimulate HSC maturation in the AGM region.29,38 Q-PCR analysis showed that the AGM region expresses all 3 of these GFs, although the IL-3 message was only detectable in 5 out of 8 preparations (each obtained from up to 10 AGM regions) (Figure 4D) and was on occasion detectable in placenta or fetal liver (supplemental Figure 5). By contrast, SCF and Flt3L, but not IL-3, were detected in freshly isolated UC and VV, which could explain why UC and VV explants failed to enhance dHSC activity in the absence of exogenous IL-3. Supplementation with IL-3 alone significantly stimulated dHSC generation in VV and UC explants by at least eightfold and 25-fold, respectively (Figure 4A-B).
Expansion of HSC and progenitor numbers in the presence of GFs. (A) In the absence of GFs, HSCs are only maintained in UC explants but show 56-fold increase with GFs (IL-3 + SCF + Flt3L). Addition of IL-3 alone was found to be sufficient to support HSC expansion; 0.05 to 1 e.e. was injected per recipient, as indicated, and engraftment was assessed 12 to 14 weeks posttransplantation in PBL. (B) VV or YSs were cultured for 4 days in the presence of the 3 GFs, with IL-3 only, or without GFs and injected into irradiated recipients (at 0.5 VV per mouse and 1 YS per mouse). Engraftment was assessed 12 to 14 weeks posttransplantation in PBL (n = 5 independent experiments). (C) CFU-C progenitor numbers increase after 4 days of explant culture in the presence of 3 GFs (n = 2-5 independent experiments). Values refer to fold increase in CFU-C in explants compared with their uncultured counterparts. (D) Levels of IL-3, SCF, and Flt3l expression were assessed by qRT-PCR relative to expression of the TBP housekeeping gene (n = 3-8 independent RNA preparations, representing up to 10 pooled tissues each).
Expansion of HSC and progenitor numbers in the presence of GFs. (A) In the absence of GFs, HSCs are only maintained in UC explants but show 56-fold increase with GFs (IL-3 + SCF + Flt3L). Addition of IL-3 alone was found to be sufficient to support HSC expansion; 0.05 to 1 e.e. was injected per recipient, as indicated, and engraftment was assessed 12 to 14 weeks posttransplantation in PBL. (B) VV or YSs were cultured for 4 days in the presence of the 3 GFs, with IL-3 only, or without GFs and injected into irradiated recipients (at 0.5 VV per mouse and 1 YS per mouse). Engraftment was assessed 12 to 14 weeks posttransplantation in PBL (n = 5 independent experiments). (C) CFU-C progenitor numbers increase after 4 days of explant culture in the presence of 3 GFs (n = 2-5 independent experiments). Values refer to fold increase in CFU-C in explants compared with their uncultured counterparts. (D) Levels of IL-3, SCF, and Flt3l expression were assessed by qRT-PCR relative to expression of the TBP housekeeping gene (n = 3-8 independent RNA preparations, representing up to 10 pooled tissues each).
Hematopoietic progenitors (CFU-C) as well as CD45+ cells in UC and VV also significantly increased in numbers after culture with exogenous GFs, but to a lesser extent than in the AGM region, and they were variable (Figure 4C; supplemental Figure 6), possibly due to variations in blood contents between individual embryos.39
Phenotypic characterization of pre-HSCs in extraembryonic vessels
To determine the phenotype of pre-HSCs in extraembryonic vessels, cell suspensions from UC and VV were sorted based on VE-cadherin and CD45 expression. Isolated cell fractions were coaggregated with OP9 stroma and cultured for 4 days in conditions known to promote maturation of AGM region pre-HSCs.28,29 Both VE-cad+CD45+ and VE-cad+CD45– cell fractions from UC and VV gave rise to dHSCs after culture (Figure 5A). Thus, similar to the AGM region, UC and VV contain immature VE-cad+CD45– type 1 pre-HSCs that are distinct from dHSCs (VE-cad+CD45+) (Figure 1). VE-cad+CD45+ type 2 pre-HSCs could not be distinguished from dHSCs in these experiments.
Type 1 pre-HSCsare present in UC and VV. (A) Four populations sorted based on VE-cadherin and CD45 expression were coaggregated with OP9 cells and transplanted after 4 days of culture (with IL-3 + SCF + Flt3l). The level (%) of donor engraftment in PBL was assessed 14 weeks after transplantation (n = 7 independent experiments). HSC activity correlated with VE-cad+CD45+ and VE-cad+CD45– fractions. (B) Type 1 pre-HSCs (VE-cad+CD45–) from different embryonic tissues were coaggregated with OP9 cells and transplanted after 4 days of culture. Note that VV and UC contain type 1 pre-HSCs.
Type 1 pre-HSCsare present in UC and VV. (A) Four populations sorted based on VE-cadherin and CD45 expression were coaggregated with OP9 cells and transplanted after 4 days of culture (with IL-3 + SCF + Flt3l). The level (%) of donor engraftment in PBL was assessed 14 weeks after transplantation (n = 7 independent experiments). HSC activity correlated with VE-cad+CD45+ and VE-cad+CD45– fractions. (B) Type 1 pre-HSCs (VE-cad+CD45–) from different embryonic tissues were coaggregated with OP9 cells and transplanted after 4 days of culture. Note that VV and UC contain type 1 pre-HSCs.
Notably, cells with pre-HSC type 1 phenotype sorted from the chorion disk, YS proper, and PBL coaggregated with OP9 cells did not give rise to dHSCs (Figure 5B and supplemental Figure 7). Thus, type 1 pre-HSCs are not detectable in PBL at this stage but appear to be localized within the AGM region and extraembryonic vessels.
Pre-HSC activity is associated with extraembryonic arteries but not veins
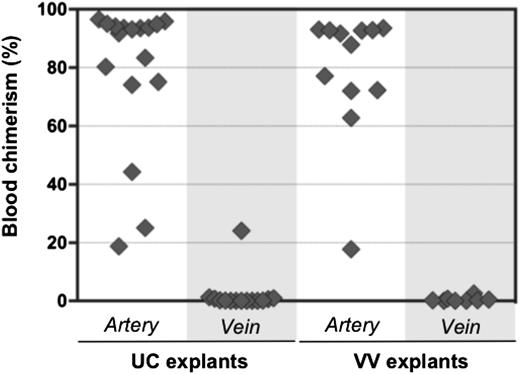
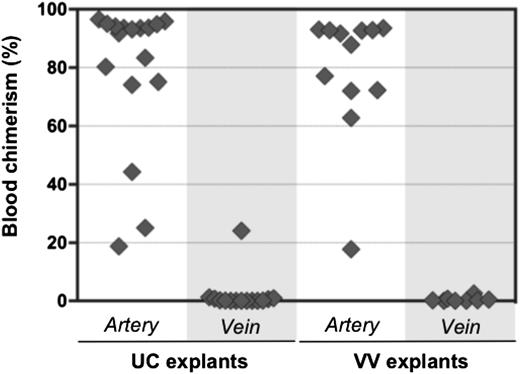
Initially, HSC activity in the AGM region correlates with the aorta, but not the cardinal veins.11,16 It was therefore of interest to test whether pre-HSCs are associated exclusively with extraembryonic arteries. UC and VV arteries and veins were subdissected and cultured separately for 4 days before transplantation into irradiated recipients (Figure 6). Umbilical arteries were distinguished from veins by the characteristic arterial bifurcation proximal to the embryo (Figure 3B). VAs and vitelline veins of the YS were distinguished by their connection to the dorsal aorta and fetal liver, respectively (Figure 3C).
Pre-HSC activity is associated with extraembryonic arteries, but not veins. Arteries and veins subdissected from UC and VV were cultured separately for 4 days with GFs (IL-3 + SCF + Flt3l) and transplanted into irradiated recipients (1 e.e. per mouse). Donor engraftment was assessed 12 to 14 weeks posttransplantation (n = 5 independent experiments).
Pre-HSC activity is associated with extraembryonic arteries, but not veins. Arteries and veins subdissected from UC and VV were cultured separately for 4 days with GFs (IL-3 + SCF + Flt3l) and transplanted into irradiated recipients (1 e.e. per mouse). Donor engraftment was assessed 12 to 14 weeks posttransplantation (n = 5 independent experiments).
In all 5 independent experiments, dHSC activity was mostly associated with extraembryonic arteries, but not veins. In 1 recipient, repopulation occurred from a cultured umbilical vein, which might arise from incomplete separation from a closely adjacent artery. Although venous explants contained CD45+ cells, CFU-C potential in veins was rare and variable (supplemental Figure 6).
Pre-HSC activity increases with proximity to the embryo proper
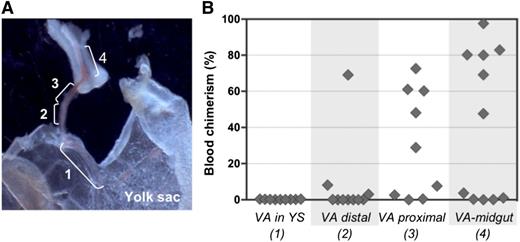
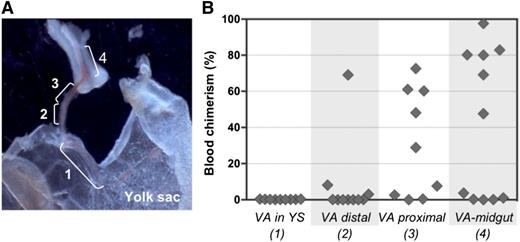
To examine the distribution of pre-HSC activity within the VA, vessels were subdissected into 4 portions (Figure 7A) and cultured separately before transplantation into irradiated adult recipients. Pre-HSC activity appeared to gradually increase in a distal-to-proximal direction, with more HSCs being generated closer to the embryo proper (Figure 7B). Interestingly, when entire VV explants included the midgut region that is associated with portion 4, as shown in Figure 7A, dHSC activity emerged even in the absence of GFs (supplemental Figure 8). No dHSC activity was detected in YS explants, in accordance with our previous explant and reaggregation experiments (Figures 4B and 5B).28 Distribution of pre-HSC activity in UC arteries was not assessed.
Proximal-distal gradient of pre-HSC activity in the VA. (A) Subdissection scheme of the VA: 1, portion of VA associated with YS; 2, distal VA portion; 3, proximal VA portion; 4, portion of VA associated with midgut. Original magnification ×32. (B) VA subsections were transplanted into irradiated recipients after 4 days of culture (1 e.e. per mouse). Donor engraftment was assessed 14 weeks posttransplantation (n = 2 independent experiments).
Proximal-distal gradient of pre-HSC activity in the VA. (A) Subdissection scheme of the VA: 1, portion of VA associated with YS; 2, distal VA portion; 3, proximal VA portion; 4, portion of VA associated with midgut. Original magnification ×32. (B) VA subsections were transplanted into irradiated recipients after 4 days of culture (1 e.e. per mouse). Donor engraftment was assessed 14 weeks posttransplantation (n = 2 independent experiments).
Discussion
In the developing mouse embryo, the first dHSCs are detected in the AGM region by late E10 to E11. Organ culture and sorting experiments demonstrated that this tissue is able to initiate and support stepwise maturation of dHSCs from resident precursors.5,28,29 The placenta, YS, and UC/ VV also contain dHSCs,7,8,10,11 but it remains unclear whether they arise here de novo. Using a quantitative approach, we show that dHSCs are present at low frequencies in fresh E11.5 UC and VV and that, similar to the AGM region, they are enriched in the VE-cad+CD45+ fraction.40
The observation that umbilical arteries and VAs contain hematopoietic clusters11,19-21,42 has been broadly considered an indication of HSC development. Our data show that pre-HSCs exist in extraembryonic vessels and that explants in the presence of exogenous GFs are able to induce expansion of dHSCs, although to a lesser extent than the AGM region (∼50-fold compared with 150-fold, respectively).29 Sorting experiments revealed that UC and VV contain type 1 pre-HSCs (VE-cad+CD45–). Although these experiments did not allow direct detection of type 2 pre-HSCs, because of their phenotypic similarity to VE-cad+CD45+ dHSCs, it is reasonable to assume that dHSC maturation occurs through this intermediate, as in the AGM region.28 HSC activity was not detected after culture of sorted VE-cad+CD45– YS cells or in YS explants except for 1 low repopulated mouse, in contrast to previous report.38 This might be due to differences in culture methods or inadvertent inclusion of small fragments of extraembryonic vessels in the YS explants. Interestingly, we were not able to detect type 1 pre-HSCs (VE-cad+CD45–) in the E11.5 chorion disk or PBL, suggesting that these precursors localize to the AGM region, UC, and VV. Because PBL did not contain pre-HSC activity, it seems that pre-HSCs do not seed the extraembryonic vessels through the circulation, although this possibility cannot be fully excluded. Our previous detection of rare HSCs in circulation (∼0.1 HSC per e.e.)10 could be due to inadvertent inclusion of fragments of extraembryonic vessels in blood preparations.
Although HSC activity is exhibited at lower levels than in the AGM region, the extraembryonic arteries harbor large intraluminal hematopoietic clusters. The absence of such clusters in extraembryonic veins led to speculation about an exclusive role of arterial vessels in HSC formation.11 We tested this functionally in isolated extraembryonic arteries and veins and confirmed that pre-HSC/dHSC activity is associated only with the arteries. It is not yet clear why HSC development is associated with arteries, but it appears that to a certain extent, arterial and HSC specification are defined by common signaling pathways, such as Notch signaling.43-45
Development of HSCs is enhanced in culture by several key GFs: SCF, Flt3L, and IL-3.29,38 The AGM region expresses all 3 of these factors, although IL-3 was not detected in all AGM preparations, likely reflecting the rarity of IL-3–expressing cells.38 IL-3 could also be detected in placental and fetal liver preparations. However, in UC or VV samples, IL-3 was completely undetectable, and its addition alone was sufficient to stimulate dHSC generation. Because extraembryonic vessels do not exhibit autonomous HSC stimulatory capacity (in the absence of exogenous GFs), it is conceivable that resident pre-HSCs migrate either interstitially to the AGM region (against blood flow) or through circulation toward fetal liver or placenta to undergo maturation/expansion through the action of IL-3 or other factors. Exact migratory patterns of maturing HSCs remain to be elucidated. Interestingly, pre-HSC activity in the VA increases with proximity to the embryo proper, which could be defined by some stimulatory factors secreted by the embryo proper.46-48 In line with this, explants of VV with associated midgut were able to enhance dHSC activity in the absence of exogenous GFs.
We recently reported that in the human embryo the AGM region is the first site of HSC appearance.49 In contrast to the mouse, the human UC at this stage contains no HSCs. It is not clear at present which mechanisms underlie this difference between the 2 species.
In conclusion, we have shown that the extraembryonic arterial vessels contain pre-HSCs, which are capable of maturing into definitive HSCs. Whether these extraembryonic pre-HSCs emerge in situ or emigrate from another source remains to be elucidated. However, it is reasonable to speculate that in addition to the AGM region, these vessels are an extra source of maturing adult HSCs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Suling Zhao, J. Verth, C. Manson, R. McInnis, and Breeding Research Facility staff for assistance with mouse maintenance and breeding; I. Rybstov for plug settings; C. Watt, C. Flockhart, C. Forrest, and K. Anderson for irradiations; S. Monard and O. Rodriguez for cell sorting; and D. Hills and C. Souilhol for helpful comments.
This work was supported by Leukaemia and Lymphoma Research, Biotechnology and Biological Sciences Research Council, Medical Research Council, and Wellcome Trust.
Authorship
Contribution: S.G.-K., S.R., M.S., and K.M. performed experiments; A.M. designed the research; S.G.-K. and S.R. analyzed data and made figures; and A.M. and S.G.-K. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alexander Mevinsky, Institute for Stem Cell Research, Medical Research Council Centre for Regenerative Medicine, University of Edinburgh, SCRM Bioquarter, 5 Little France Dr, Edinburgh E16 4UU, Scotland, United Kingdom; e-mail: A.Medvinsky@ed.ac.uk.