Key Points
Human NK cells contain Golgi complex–associated intracellular stores of AICL, a ligand of the activating NK receptor NKp80.
Upon exposure to inflammatory cytokines, AICL surfaces on NK cells, rendering them susceptible to NKp80-mediated bystander NK cytolysis.
Abstract
NKp80 is a C-type lectin-like receptor broadly expressed on human natural killer (NK) cells, triggering cytotoxicity via an atypical cytoplasmic hemi-immunoreceptor tyrosine-based activation motif. As with other lectin-like NK receptors, NKp80 is encoded in the natural killer gene complex, but unlike most of these, adjacent to its ligand, ie, activation-induced C-type lectin (AICL). The reasons for the tight genetic linkage of this receptor–ligand pair remain elusive. Previous studies showed that NKp80 augments NK cell responses toward malignant and nonmalignant myeloid cells. Here, we report that resting human NK cells not only express NKp80 but also contain intracellular stores of AICL colocalizing with the Golgi complex. Domain-swapping experiments revealed that intracellular localization of AICL is determined by its C-type lectin-like ectodomain. Exposure of NK cells to monokines associated with conversion into memorylike cells induces substantial AICL cell surface expression, whereas NKp80 is downregulated, and NK cells become refractory to NKp80-mediated stimulation. AICL on monokine-exposed NK cells elicits NKp80-dependent effector responses by autologous NK cells and, hence, renders monokine-activated NK cells susceptible to NKp80-mediated cytolysis. Altogether, our data report a previously unrecognized regulatory circuit enabling autonomous control of human NK cell responses via the NKp80–AICL axis.
Introduction
Natural killer (NK) cells are innate lymphocytes equipped with cytotoxic capacity against infected and malignant cells.1-3 NK cells circulate in the peripheral blood but are also present in the lymphatics, at mucosal sites, and in the liver and are recruited to sites of inflammation.4 More recently, several studies revealed memorylike features of NK cells challenging the original view of NK cells as short-lived innate responder cells.5,6 Memorylike properties are induced by sustained pathogen-mediated ligation of activating NK cell receptors or exposure to cytokines interleukin (IL)–12 and IL-18,5,7 and long-lived “memory” NK cells arise following infection with cytomegalovirus or hantavirus.8-10
NK cells express a broad variety of germline-encoded activating and inhibiting cell surface receptors determining NK cell reactivity (ie, cytotoxicity and cytokine secretion) toward autologous cells according to the rules of “missing-self” and “induced-self” recognition, sparing “normal” major histocompatibility complex (MHC) class I–expressing cells and attacking MHC class I–deficient, stressed cells.11,12 The expression pattern of MHC class I–specific inhibitory receptors by NK cells is variegated, and responsiveness of individual NK cells depends on educational processes matching the inhibitory receptor profile with the polymorphic MHC class I equipment of the host.13,14 In contrast, many activating receptors are broadly expressed by NK cells and engage inducible ligands that are not or are barely expressed on normal cells.
NK cell receptors can be subdivided according to their structure into immunoglobulin-like and C-type lectin-like receptors (CTLR). The latter—including the activating receptors NKG2D, CD94/NKG2C, and NKp80—are encoded in the natural killer gene complex (NKC).15,16 NKp80 is a homodimeric CTLR expressed on virtually all human NK cells, but also on subsets of effector memory CD8 αβ T cells and γδ T cells.17-19 NKp80 contains an atypical hemi-immunoreceptor tyrosine-based activation motif at the aminoterminus of its cytoplasmic domain crucially involved in triggering NK cell cytotoxicity.20 The gene KLRF1, coding for NKp80, and the CLEC2B locus encoding the NKp80 ligand “activation-induced C-type lectin” (AICL) are adjacently located in the NKC in a tail-to-tail orientation.16,19 Similarly, genes of related receptor–ligand pairs of the NKRP1 and CLEC2 families are genetically linked both in mouse and man.15,16 Originally, PMA-stimulated peripheral blood mononuclear cells (PBMCs) were reported to transiently upregulate AICL transcripts in a manner analogous to the AICL-relative CD69.21 We previously reported pronounced surface expression of AICL glycoproteins by the human premonocytic cell line U937 stimulating NK cytotoxicity in an NKp80-dependent manner.19 In addition, NKp80–AICL interaction was shown to promote an activating cross-talk between NK cells and monocytes in the presence of inflammatory cytokines.19,22 In contrast to the MHC class I–related ligands of the activating NK receptor NKG2D that are broadly expressed on human cell lines of hematopoietic and nonhematopoietic origin, we identified only a few human myeloid cell lines expressing AICL at the cell surface.19 A more recent report describes AICL also on a few nonhematopoietic cell lines.23
The present study was initiated by the incidental observation of substantial AICL expression by human NK cell lines. Subsequently, we assessed AICL expression by human NK cells and found them to contain intracellular stores of AICL that surface upon activation. Functional AICL expression on activated NK cells allowing NKp80-dependent recognition and cytolysis by autologous NK cells provides new insights into the immunoregulatory function of this genetically coupled receptor–ligand pair and into the control of human NK cell responses.
Methods
Cells
U937, NK3.3, YT, Jurkat, K562, and Mono Mac6 cells were cultured in RPMI1640 medium, NK-92MI, NKL, and human primary NK cells in Iscove modified Dulbecco medium (IMDM) with cell lines NK3.3 and NKL being maintained in medium containing IL-2 (Promocell, Heidelberg, Germany) at 100 U/mL or 200 U/mL, respectively. HeLa, 293, and COS-7 cell lines were cultured in Dulbecco’s modified Eagle medium. We obtained 293 cells stably expressing an AICL–enhanced green fluorescent protein (eGFP) fusion protein by transfection with pEGFP-N3 containing AICL complementary DNA (cDNA) in front of the eGFP reading frame. PBMC of healthy human donors were isolated by gradient centrifugation with Ficoll-Paque Plus (GE Healthcare Europe, Munich, Germany). NK cells were isolated from PBMC using the human NK cell isolation kit (Miltenyi, Bergisch Gladbach, Germany) according to the manufacturer’s protocol. The purity of isolated CD3−CD56+ NK cells was always >95%. Human blood was used with the approval of the local ethics committee, and this study was conducted in accordance with the Declaration of Helsinki.
Quantitative reverse transcription–polymerase chain reaction (PCR)
RNA was isolated using peqGOLD TriFast (Peqlab, Erlangen, Germany) and converted into cDNA using Moloney murine leukemia virus reverse transcriptase (Promega, Fitchburg, WI) after treatment with DNase I for 30 minutes at 37°C. cDNA was amplified with primer pairs specific for NKp80 (5′-cactcagtatgaggacactgg-3′; 5′-ggcatagtactgtctggtctg-3′), AICL (5′-taccaaatcgtttggcatga-3′; 5′-ctgcaaatccattttctttcg-3′), or 18S ribosomal RNA (5′-cggctaccacatccaaggaa-3′; 5′-gctggaattaccgcggct-3′) on the real-time PCR system StepOnePlus (Applied Biosystems, Foster City, CA) using SYBR Green Chemistry (Roche Diagnostics, Mannheim, Germany) for detection. Copy numbers of NKp80 and AICL transcripts were normalized with copy numbers of 18S rRNA (ΔCt method).
Transient expression of AICL/keratinocyte-associated C-type lectin (KACL) hybrids
Hybrid coding sequences of AICL and KACL were generated using standard PCR techniques and cloned with carboxyterminal FLAG-tag and hexahistidine-tag sequences into a pIRES2 vector (Clontech, Mountain View, CA). We transfected 293 cells using AppliFect (AppliChem, Darmstadt, Germany), and 3 days later we analyzed them by flow cytometry or immunoblotting using the FLAG-tag–specific mAb M2 (Sigma-Aldrich, St. Louis, MO) for detection. For microscopic studies, FLAG- and histidine-tagged hybrid sequences were cloned into the vector RSV.5neo.
Flow cytometry
Cells were washed with ice-cold fluorescence-activated cell sorter (FACS) buffer (phosphate-buffered saline [PBS], 2% fetal calf serum, 2 mM EDTA, 0.01% sodium azide), and Fc receptors were blocked with 30 μg/mL human immunoglobulin G (IgG) (Sigma-Aldrich) for 45 minutes at 4°C. Cells were then stained with relevant antibodies for 30 minutes at 4°C and washed again with FACS buffer. For intracellular staining, cells were fixed and permeabilized with Cytofix/Cytoperm (BD Biosciences, San Jose, CA) for 20 minutes on ice and Fc receptors blocked with human IgG (30 μg/mL) in saponin buffer (PBS, 0.5% bovine serum albumin, 0.1% saponin, 0.01% sodium azide) for 45 minutes at 4°C. Cells were incubated with antibodies for 30 minutes at 4°C and washed again with saponin buffer. Flow cytometry analysis was performed with a FACS Canto II (BD Biosciences) and data analyzed using FlowJo (Tree Star, Ashland, OR). Antibodies used were anti–CD3-PacificBlue, anti–CD56-PE, anti–CD107a-APC, anti-interferon (IFN)–γ-PE, anti–tumor necrosis factor (TNF)–PE (all from BD Biosciences), and anti-FLAG (M2; Sigma-Aldrich). Anti-NKp80 mAb 5D12 and anti-AICL mAb 7F12 were previously described.19 Unconjugated antibodies were stained with allophycocyanin-conjugated F(ab)2 fragments of goat anti-mouse IgG (Jackson ImmunoResearch, Newmarket, UK).
Confocal microscopy
Purified human NK cells or U937 cells were permeabilized with Cytofix/Cytoperm for 20 minutes on ice, washed with saponin buffer, and stained with the following antibodies after blocking Fc receptors: anti–LAMP1-Alexa488 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-Giantin (Abcam, Cambridge, UK), anti-Calreticulin (Cell Signaling Technology, Danvers, MA), anti-FLAG (M2), anti-AICL (7F12), or isotype control. Rabbit antibodies (anti-Giantin, anti-Calreticulin) were detected with AlexaFluor488-goat-anti-rabbit IgG, and mAb 7F12 with Alexa546-goat-anti-mouse IgG (both from Invitrogen, Eugene, OR). Subsequently, cells were resuspended in PreLong Gold with 4,6 diamidino-2-phenylindole (Invitrogen) and mounted on slides with cover slips. For localization of AICL hybrids, 293 cells were grown on poly-l-lysine-coated cover slips, transfected and cultured for 3 days. Cells were then fixed with 4% paraformaldehyde for 20 minutes at 37°C, permeabilized with 0.1% Triton X-100 for 5 minutes at room temperature, blocked with 10% fetal calf serum in PBS for 1 hour at room temperature, stained with relevant antibodies, and washed extensively with PBS before mounting on slides. Images were acquired using a LSM 510 META laser scanning microscope (Zeiss, Oberkochen, Germany) with a 63× oil immersion objective lens. Data were processed with ZEN software (Zeiss).
Immunoblotting
Cells were lysed with ice-cold lysis buffer (1% Triton X-100, 50 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 50 mM octylglucopyranosid, Complete Protease Inhibitor [Roche]) and centrifuged, and supernatants containing ∼100 µg cellular protein were separated by sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis (PAGE). For protein deglycosylation, cell lysates were treated with endoglycosidase H or PNGase F (both from New England Biolabs, Ipswich, MA) according to the manufacturer’s protocol prior to SDS-PAGE. After blotting onto a Hybond ECL Nitrocellulose Membrane (GE Healthcare Europe), the membrane was probed with anti-AICL mAb 7G419 or anti-FLAG M2, followed by detection with horseradish peroxidase-conjugated goat anti-mouse IgG (Jackson ImmunoResearch). Signals were generated with SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific, Rockford, IL) and detected by Hyperfilm enhanced chemiluminescence (ECL). Membranes were stripped with 1× Re-Blot Plus Mild Solution (Millipore, Temecula, CA) before reprobing with horseradish peroxidase–conjugated anti–β-actin (clone AC-15; Sigma-Aldrich).
Cell stimulation
PBMC were cultured in IMDM at a density of 5 × 106 cells/mL. For stimulation of cells, phorbol-12-myristate-13-acetate (PMA; 20 ng/mL) (Sigma-Aldrich) was added to the culture. NK cells isolated with the human NK cell isolation kit (Miltenyi) were cultured in IMDM at 1 × 106 cells/mL either without cytokines or with a combination of IL-12 and IL-18 (5 ng/mL each) (both R&D Systems, Minneapolis, MN) in the presence of IL-2 (100 U/mL).
NK cell cocultures
Purified NK cells were maintained in IMDM at 1 × 106 cells/mL for 2 days in parallel cultures either without cytokines (resting NK cells) or with a combination of IL-2 (100 U/mL), IL-12, and IL-18 (5 ng/mL each) (activated NK cells). There was no reduction in viability or cell numbers for short-term cultures (2 to 3 days) of resting NK cells in comparison with NK cells cultured with IL-2 (supplemental Figure 1, which can be found on the Blood Web site). Subsequently, cytokine-stimulated NK cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) (Invitrogen) and cocultured with the respective resting NK cells of the same donor at a ratio of 1:1 for 6 hours with GolgiStop (BD Biosciences) added 1 hour after start of coculture. Cells were stained with anti–CD107a-APC, fixed and permeabilized using Cytofix/Cytoperm, blocked with human IgG, and stained with anti–IFNγ-FITC or anti–TNF-PE, and subsequently, CFSE-negative cells were analyzed by flow cytometry.
Chromium-release assay
The monokine-activated NK cells were labeled with 51Cr for 2 hours at 37°C, and the unstimulated NK cells were preincubated with F(ab)2 fragments of mAb 5D12 (anti-NKp80) or of an isotype control for 1 hour at 37°C. Subsequently, 51Cr-labeled cells (targets, T) and the unstimulated cells (effectors, E) were cocultured for 4 hours at 37°C at different E:T ratios. Supernatants were mixed with OptiPhase Supermix scintillation cocktail (PerkinElmer, Waltham, MA) and measured with a MicroBeta2 plate counter (PerkinElmer). To determine spontaneous and maximum lysis, we accordingly measured supernatants of target cells incubated without effectors or in presence of 2% Triton X-100, respectively. Specific lysis (percentage lysis) was calculated as follows: 100 × (experimental release – spontaneous release)/(maximum release – spontaneous release).
Results
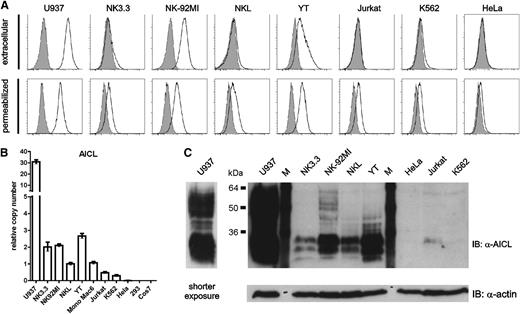
AICL expression by human NK cell lines
Previously, we reported that only a few myeloid cell lines, including U937 cells, exhibit substantial levels of surface AICL protein.19 When we reassessed expression of AICL by human cell lines including available NK cell lines, we unexpectedly observed pronounced surface AICL expression by the NK cell lines NK-92MI and YT (Figure 1A). Although for NK cell lines NK3.3 and NKL sparse or no AICL surface expression was detectable by flow cytometry, stainings of permeabilized cells indicated AICL proteins intracellularly (Figure 1A). Accordingly, AICL transcripts were detected for all these NK cell lines (Figure 1B); however, levels were much lower than in U937 cells, well in line with analyses of total cellular AICL protein assessed by immunoblotting using the AICL-specific mAb 7G4.19 The 2 characteristic AICL glycoisoforms at ∼30 kDa were detected for all abovementioned NK cell lines, but not for HeLa or K562 cells, which also contained no or low levels of AICL transcripts (Figure 1B-C). Additional AICL glycoisoforms of higher molecular mass (>40 kDa) detectable for NK-92MI cells and YT cells, and most prominently for U937 cells, are resistant to digestion with endoglycosidase H (not shown) and thus likely comprise AICL proteins at the cell surface, well in accord with flow cytometry data (Figure 1A). Notably, none of the 4 NK cell lines expressed the AICL receptor NKp80 (not shown).
AICL expression by human NK cell lines. Expression of AICL by human cell lines probed by flow cytometry, quantitative PCR (qPCR), and immunoblotting. (A) Cell surface expression of AICL (upper) by human NK cell lines (NK3.3, NK-92MI, NKL, YT) and other human cell lines (U937, Mono Mac6, Jurkat, K562, HeLa, 293) was assessed by flow cytometric analysis using anti-AICL mAb 7F12 (open histograms). For detection of total AICL protein, cells were permeabilized prior to incubation with 7F12 (lower). Isotype control stainings are shown in gray. (B) Levels of AICL transcripts in human cell lines. Relative levels of AICL transcripts in cell lines (including the primate cell line COS-7) were determined by qPCR and set relative to levels of NKL (arbitrarily set as 1). Means of triplicates are shown with standard deviations. (C) Total AICL protein in human cell lines as determined by immunoblotting. Cell lysates (same samples as in panel A) were separated by SDS-PAGE under reducing conditions and, after blotting, probed with anti-AICL mAb 7G4. For U937, signals after shorter exposure are also shown. Actin detection served as loading control. (A-C) One representative experiment out of 3 is shown.
AICL expression by human NK cell lines. Expression of AICL by human cell lines probed by flow cytometry, quantitative PCR (qPCR), and immunoblotting. (A) Cell surface expression of AICL (upper) by human NK cell lines (NK3.3, NK-92MI, NKL, YT) and other human cell lines (U937, Mono Mac6, Jurkat, K562, HeLa, 293) was assessed by flow cytometric analysis using anti-AICL mAb 7F12 (open histograms). For detection of total AICL protein, cells were permeabilized prior to incubation with 7F12 (lower). Isotype control stainings are shown in gray. (B) Levels of AICL transcripts in human cell lines. Relative levels of AICL transcripts in cell lines (including the primate cell line COS-7) were determined by qPCR and set relative to levels of NKL (arbitrarily set as 1). Means of triplicates are shown with standard deviations. (C) Total AICL protein in human cell lines as determined by immunoblotting. Cell lysates (same samples as in panel A) were separated by SDS-PAGE under reducing conditions and, after blotting, probed with anti-AICL mAb 7G4. For U937, signals after shorter exposure are also shown. Actin detection served as loading control. (A-C) One representative experiment out of 3 is shown.
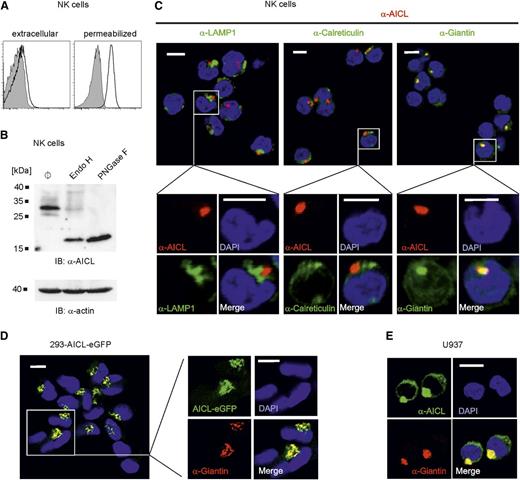
Resting human NK cells contain intracellular stores of AICL associated with the Golgi complex
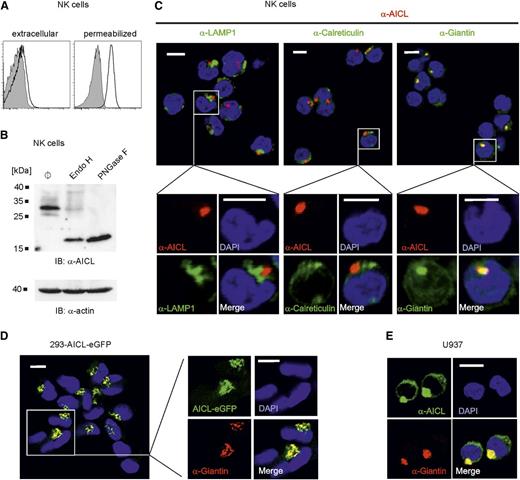
The observation of human NK cell lines commonly expressing AICL prompted us to reassess AICL expression by human primary NK cells. We previously detected substantial levels of AICL transcripts in human NK cells but had not observed corresponding AICL surface expression.19 Although no significant amounts of AICL are detectable at the surface of freshly isolated human NK cells, they were stained with anti-AICL mAb 7F12 subsequent to permeabilization, suggesting intracellular occurrence of AICL (Figure 2A). Intracellular expression of AICL was confirmed by immunoblotting, revealing AICL glycoproteins in lysates of resting NK cells that are largely susceptible to digestion with endoglycosidase H, compatible with a predominant intracellular localization (Figure 2B). Intracellular AICL expression was further corroborated by confocal microscopy visualizing intracellular clusters of AICL that primarily colocalized with the Golgi complex-resident protein Giantin, but neither with the lysosomal protein LAMP-1 nor with the endoplasmic reticulum marker protein calreticulin (Figure 2C). Because Golgi complex-associated localization of AICL protein was unexpected, we evaluated cellular AICL localization in other human cell types. Both in 293 cells ectopically expressing an AICL-eGFP fusion protein (Figure 2D) and in U937 cells (Figure 2E), the majority of cellular AICL protein colocalizes intracellularly with Giantin, showing that residence in the Golgi complex is an inherent and cell-type independent property of AICL.
Human NK cells contain intracellular stores of AICL associated with the Golgi complex. (A) Representative flow cytometric analyses of intact or permeabilized resting human NK cells (CD3−CD56+) for cell surface and total AICL expression, respectively, using mAb 7F12 (open histograms). Isotype control stainings are shown in gray. (B) Total AICL protein in resting human NK cells as determined by immunoblotting with mAb 7G4. Cell lysates were treated with either endoglycosidase H or PNGase F before SDS-PAGE, or left untreated (ø). Actin served as loading control. One representative experiment out of 3 is shown. (C) Confocal microscopy revealed predominant colocalization of AICL with the Golgi complex–resident protein Giantin in human resting NK cells. Freshly isolated NK cells were permeabilized and stained with anti-AICL mAb 7F12 (red) in combination with anti-Giantin, anti-LAMP1, or anti-Calreticulin (all green). Nuclei were counterstained with 4,6 diamidino-2-phenylindole (DAPI) (blue). Scale bars, 5 µm. Experiments with NK cells from 3 donors showed similar results. (D-E) Golgi complex–associated localization of AICL in human cell lines. (D) 293 cells stably ectopically expressing an AICL-eGFP fusion protein (green) were stained for Giantin (red) and analyzed by confocal microscopy. (E) U937 cells were costained with anti-AICL mAb 7F12 (green) and anti-Giantin (red). (D-E) Scale bars represent 10 µm.
Human NK cells contain intracellular stores of AICL associated with the Golgi complex. (A) Representative flow cytometric analyses of intact or permeabilized resting human NK cells (CD3−CD56+) for cell surface and total AICL expression, respectively, using mAb 7F12 (open histograms). Isotype control stainings are shown in gray. (B) Total AICL protein in resting human NK cells as determined by immunoblotting with mAb 7G4. Cell lysates were treated with either endoglycosidase H or PNGase F before SDS-PAGE, or left untreated (ø). Actin served as loading control. One representative experiment out of 3 is shown. (C) Confocal microscopy revealed predominant colocalization of AICL with the Golgi complex–resident protein Giantin in human resting NK cells. Freshly isolated NK cells were permeabilized and stained with anti-AICL mAb 7F12 (red) in combination with anti-Giantin, anti-LAMP1, or anti-Calreticulin (all green). Nuclei were counterstained with 4,6 diamidino-2-phenylindole (DAPI) (blue). Scale bars, 5 µm. Experiments with NK cells from 3 donors showed similar results. (D-E) Golgi complex–associated localization of AICL in human cell lines. (D) 293 cells stably ectopically expressing an AICL-eGFP fusion protein (green) were stained for Giantin (red) and analyzed by confocal microscopy. (E) U937 cells were costained with anti-AICL mAb 7F12 (green) and anti-Giantin (red). (D-E) Scale bars represent 10 µm.
Intracellular retention of AICL is determined by the C-type lectin-like ectodomain
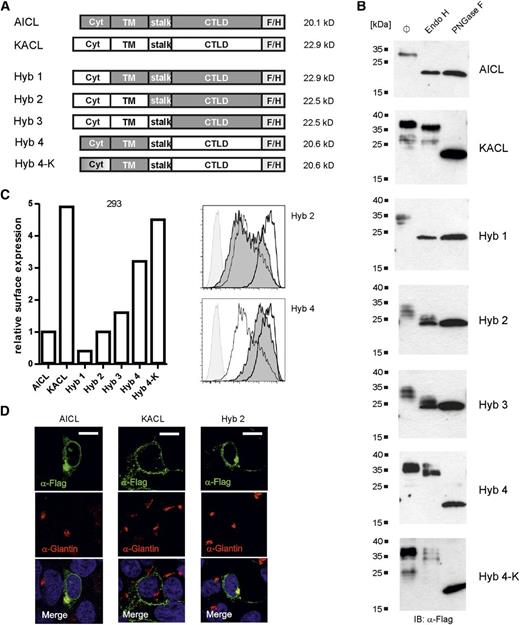
To delineate molecular mechanisms of the intracellular retention of AICL, we generated various hybrids of AICL and its close relative, keratinocyte-associated C-type lectin KACL.24 In contrast to AICL, KACL is predominantly expressed at the surface of transfected 293 cells (Figure 3 and our unpublished data). Hybrids built from KACL and AICL domains in various combinations (Figure 3A) were ectopically expressed in 293 cells and analyzed for endoglycosidase H resistance (Figure 3B), cell surface expression (Figure 3C), and Giantin colocalization (Figure 3D).
Intracellular retention of AICL is an inherent property of the lectin-like domain. (A) Schemes of the AICL/KACL hybrids with calculated molecular masses (deglycosylated proteins). AICL domains are shown in gray, KACL domains are open. Expression of hybrid proteins was analyzed 3 days after transfection of 293 cells. (B) Lysates of transfected 293 cells were left untreated (ø), or treated with endoglycosidase H or PNGase F, separated by SDS-PAGE, and KACL, AICL, and hybrid proteins detected using anti–FLAG-tag mAb M2 by immunoblotting. (C) Relative surface expression of AICL, KACL, and AICL/KACL hybrids by transfected 293 cells. Transfectants were stained with mAb M2 and mean fluorescence intensities (MFI) determined by flow cytometry with values set in relation to the MFI of AICL transfectants (arbitrarily set as 1) (left). Representative histograms of hybrids 2 and 4 (solid, dark gray) overlayed with those of AICL (thin line) and KACL (thick line) transfectants, and untransfected 293 cells (solid, light gray) (right). One representative experiment out of 3 is shown. (D) Confocal microscopy analysis of 293 cells ectopically expressing AICL, KACL, or hybrid 2, respectively. Transfectants were costained with mAb M2 (green) and anti-Giantin (red). Scale bars represent 10 µm.
Intracellular retention of AICL is an inherent property of the lectin-like domain. (A) Schemes of the AICL/KACL hybrids with calculated molecular masses (deglycosylated proteins). AICL domains are shown in gray, KACL domains are open. Expression of hybrid proteins was analyzed 3 days after transfection of 293 cells. (B) Lysates of transfected 293 cells were left untreated (ø), or treated with endoglycosidase H or PNGase F, separated by SDS-PAGE, and KACL, AICL, and hybrid proteins detected using anti–FLAG-tag mAb M2 by immunoblotting. (C) Relative surface expression of AICL, KACL, and AICL/KACL hybrids by transfected 293 cells. Transfectants were stained with mAb M2 and mean fluorescence intensities (MFI) determined by flow cytometry with values set in relation to the MFI of AICL transfectants (arbitrarily set as 1) (left). Representative histograms of hybrids 2 and 4 (solid, dark gray) overlayed with those of AICL (thin line) and KACL (thick line) transfectants, and untransfected 293 cells (solid, light gray) (right). One representative experiment out of 3 is shown. (D) Confocal microscopy analysis of 293 cells ectopically expressing AICL, KACL, or hybrid 2, respectively. Transfectants were costained with mAb M2 (green) and anti-Giantin (red). Scale bars represent 10 µm.
Although the vast majority of cellular AICL was sensitive to digestion with endoglycosidase H, cellular KACL proteins were almost fully resistant (Figure 3B). For AICL/KACL hybrids, sensitivity to digestion with endoglycosidase H was associated with the presence of the C-type lectin-like domain (CTLD) of AICL (hybrids 1 to 3; Figure 3) but not with the AICL transmembrane or cytoplasmic domain (hybrid 4; Figure 3). In flow cytometry, ectopically expressed AICL was detectable at the cell surface of transfected 293 cells, but at substantially lower levels than those of KACL (Figure 3C). In agreement with the biochemical data, cell surface expression of hybrids containing the AICL CTLD (hybrids 1-3) was impaired and comparable to the cell surface expression of the AICL protein (Figure 3C). The importance of the AICL ectodomain for the intracellular retention of AICL also became apparent by confocal microscopy, revealing colocalization of hybrid 2 (but not of KACL) with the Golgi complex marker Giantin (Figure 3D). In contrast, substitution of the AICL ectodomain by the ectodomain of KACL (hybrid 4) resulted in a substantial increase in surface expression, but not to levels of KACL surface expression (Figure 3C). Further mutational analysis revealed that lysine 7 in the short cytosolic domain of AICL also controls AICL surface expression, as the additional substitution of lysine 7 by alanine in hybrid 4 (hybrid 4-K) resulted in surface expression levels comparable to that of KACL (Figure 3C). However, similar resistance of hybrids 4 and 4-K to endoglycosidase H digestion indicates that lysine 7 regulates AICL surface expression by a mechanism different from intracellular retention. Altogether, these data suggested an essential role of the AICL ectodomain for the Golgi complex–associated retention.
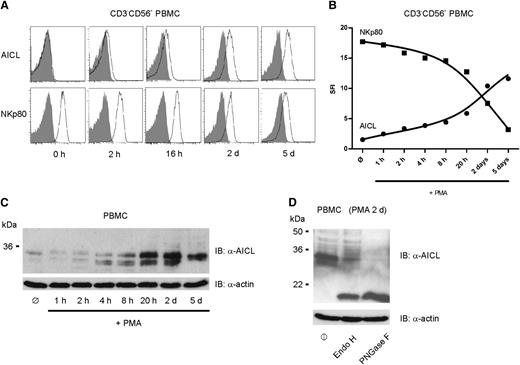
Activation-induced AICL surface expression by NK cells
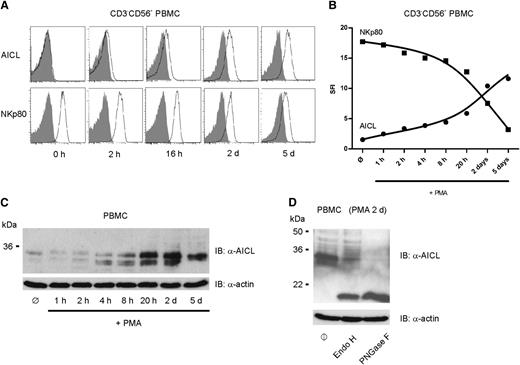
The original observation of a transient induction of AICL transcripts following treatment of PBMC with PMA gave rise to the designation activation-induced C-type lectin.21 We confirmed transient induction of AICL transcripts in PBMC upon PMA stimulation by quantitative PCR (qPCR) analysis (data not shown). Flow cytometric analyses of lymphocyte subpopulations among PMA-stimulated PBMC revealed a gradual and substantial increase of AICL surface expression on NK cells (Figure 4A). Of note, conversely to the induced AICL surface expression on PMA-stimulated NK cells, surface expression of NKp80 diminished over time (Figure 4A), accompanied by a decline in NKp80 transcript levels (data not shown). Overall, PMA-mediated activation led to a reversal in expression levels of NKp80 and AICL on resting versus activated NK cells (Figure 4B). In line with these results, PMA treatment led to a marked increase in cellular levels of AICL glycoproteins over time, as detected by immunoblotting (Figure 4C). However, AICL glycoproteins only partially gained resistance to digestion by endoglycosidase H (Figure 4D), indicating that a substantial portion of AICL remained intracellularly.
AICL upregulation and concomitant NKp80 downregulation by PMA-treated CD3−CD56+ PBMC. Freshly isolated human PBMC were stimulated with PMA and subsequently, at the indicated times, probed for expression of AICL or NKp80. (A-B) Kinetics of cell surface expression of AICL and NKp80, respectively, by NK cells among PMA-treated PBMC as determined by flow cytometry. PBMC were cultured with PMA for up to 5 days and stained for AICL (mAb 7F12), for NKp80 (mAb 5D12), or with isotype controls at various time points. One representative experiment out of 4 is shown. (A) Histograms show CD3−CD56+ cells stained with mAb 7F12 (upper) or mAb 5D12 (lower) (open histograms), and isotype controls (gray) at various time points. (B) Kinetics of AICL (●) and NKp80 (▪) surface expression on CD3−CD56+ cells among PMA-stimulated PBMC. MFI of AICL or NKp80 stainings was set in relation to those of isotype controls. (C) AICL glycoproteins in lysates of unstimulated PBMC (ø) or PBMC stimulated with PMA for various times were detected by immunoblotting with mAb 7G4. Actin detection served as loading control. Experiments with PBMC of 3 different donors gave comparable results. (D) Lysates of PBMC stimulated for 2 days with PMA were either left untreated (ø) or treated with endoglycosidase H or PNGase F, as indicated, before immunoblotting with mAb 7G4.
AICL upregulation and concomitant NKp80 downregulation by PMA-treated CD3−CD56+ PBMC. Freshly isolated human PBMC were stimulated with PMA and subsequently, at the indicated times, probed for expression of AICL or NKp80. (A-B) Kinetics of cell surface expression of AICL and NKp80, respectively, by NK cells among PMA-treated PBMC as determined by flow cytometry. PBMC were cultured with PMA for up to 5 days and stained for AICL (mAb 7F12), for NKp80 (mAb 5D12), or with isotype controls at various time points. One representative experiment out of 4 is shown. (A) Histograms show CD3−CD56+ cells stained with mAb 7F12 (upper) or mAb 5D12 (lower) (open histograms), and isotype controls (gray) at various time points. (B) Kinetics of AICL (●) and NKp80 (▪) surface expression on CD3−CD56+ cells among PMA-stimulated PBMC. MFI of AICL or NKp80 stainings was set in relation to those of isotype controls. (C) AICL glycoproteins in lysates of unstimulated PBMC (ø) or PBMC stimulated with PMA for various times were detected by immunoblotting with mAb 7G4. Actin detection served as loading control. Experiments with PBMC of 3 different donors gave comparable results. (D) Lysates of PBMC stimulated for 2 days with PMA were either left untreated (ø) or treated with endoglycosidase H or PNGase F, as indicated, before immunoblotting with mAb 7G4.
Monokines IL-12 and IL-18 induce AICL surface expression on NK cells
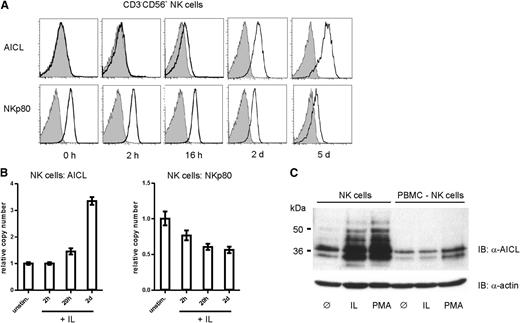
In order to address upregulation of AICL in a more physiological setting, freshly isolated NK cells were activated by triggering NK receptors or by exposure to cytokines. Although antibody-mediated crosslinking of activating NK cell receptors such as NKp46 was less efficient in AICL upregulation (not shown), combined treatment with cytokines IL-12 and IL-18 efficiently and substantially induced AICL expression on human NK cells to levels comparable to expression levels obtained upon PMA treatment (Figure 5A). Significant cell surface expression of AICL was observed >12 hours after beginning of cytokine treatment, and substantial levels were reached after 2 days of treatment. Conversely, NK cell exposure to these monokines led to a marked decrease of cell surface NKp80 (Figure 5A) that was accompanied by a loss of NKp80-mediated responsiveness (supplemental Figure 2). Changes in cell surface expression levels of NKp80 and AICL upon treatment with IL-12 and IL-18 were mirrored by corresponding changes of the respective transcript levels (Figure 5B). Similar to PMA-treated PBMC, AICL transcript levels of monokine-treated NK cells moderately but constantly increased, most noticeably 2 days after beginning of treatment, whereas NKp80 transcripts declined gradually. Induced expression of AICL glycoproteins by monokine-activated NK cells was further documented by immunoblotting (Figure 5C), whereas NK-depleted, monokine-treated PBMC showed no induction of AICL expression.
Exposure of NK cells to monokines IL-12 and IL-18 results in substantial AICL cell surface expression and NKp80 downregulation. NK cells purified from freshly isolated PBMC were cultivated in presence of IL-12 and IL-18 as well as IL-2. (A) Surface expression of AICL and NKp80 by NK cells cultivated for the indicated times. Flow cytometric analyses of cells stained with mAb 7F12 (anti-AICL, upper) or 5D12 (anti-NKp80, lower) (open histograms) or isotype controls (gray). One representative experiment out of 4 is shown. (B) Kinetics of AICL or NKp80 transcript levels of monokine-stimulated NK cells with levels of untreated NK cells arbitrarily set as 1. Means of triplicates are shown with standard deviations. Experiments with NK cells from 3 different donors gave similar results. (C) Total AICL glycoproteins in lysates of purified NK cells or NK cell–depleted PBMC cultivated for 2 days without additives (ø) or in the presence of IL-12 and IL-18 (IL) or PMA. AICL was detected with mAb 7G4 in immunoblotting, and actin used as loading control.
Exposure of NK cells to monokines IL-12 and IL-18 results in substantial AICL cell surface expression and NKp80 downregulation. NK cells purified from freshly isolated PBMC were cultivated in presence of IL-12 and IL-18 as well as IL-2. (A) Surface expression of AICL and NKp80 by NK cells cultivated for the indicated times. Flow cytometric analyses of cells stained with mAb 7F12 (anti-AICL, upper) or 5D12 (anti-NKp80, lower) (open histograms) or isotype controls (gray). One representative experiment out of 4 is shown. (B) Kinetics of AICL or NKp80 transcript levels of monokine-stimulated NK cells with levels of untreated NK cells arbitrarily set as 1. Means of triplicates are shown with standard deviations. Experiments with NK cells from 3 different donors gave similar results. (C) Total AICL glycoproteins in lysates of purified NK cells or NK cell–depleted PBMC cultivated for 2 days without additives (ø) or in the presence of IL-12 and IL-18 (IL) or PMA. AICL was detected with mAb 7G4 in immunoblotting, and actin used as loading control.
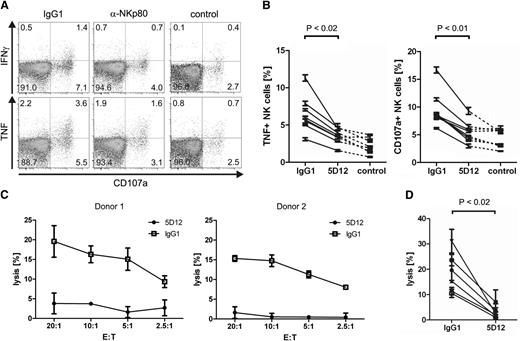
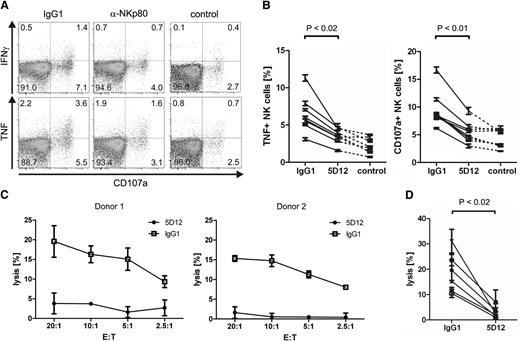
AICL on monokine-stimulated NK cells elicits cytolytic effector responses by autologous NK cells in an NKp80-dependent manner
To address the functional significance of induced AICL surface expression by monokine-activated NK cells, we cultivated freshly isolated NK cells in parallel for 2 days with or without IL-12 and IL-18, respectively. Subsequently, cultures were combined and assayed for effector functions. NK cells cultured without monokines (“resting” NK cells) strongly responded to exposure to monokine-activated AICL-expressing autologous NK cells by degranulation and TNF production (Figure 6A-B). Effector responses were at least partially NKp80-dependent, because both degranulation and TNF production were reduced to background levels in the presence of F(ab)2 fragments of the anti-NKp80 mAb 5D12, which has been shown to efficiently block NKp80-AICL interaction.19 In contrast, no substantial TNF or CD107a responses of monokine-activated NK cells were detectable (data not shown). Moreover, monokine-activated NK cells were lysed by autologous resting NK cells, and cytolysis was completely inhibited by the addition of 5D12 F(ab)2 fragments (Figure 6C-D). Hence, exposure of NK cells to monokines IL-12 and IL-18 results in cell surface appearance of AICL at substantial levels and as a consequence thereof, in the functional recognition by autologous NK cells stimulating cytokine secretion as well as cytolysis of monokine-stimulated NK cells in an NKp80-dependent manner.
NKp80-mediated recognition and lysis of monokine-activated NK cells by autologous NK cells. (A-B) NK cells were purified from freshly isolated PBMC and cultivated in parallel for 2 days in the presence of IL-2, IL-12 and IL-18 (activated NK cells) or without cytokines (resting NK cells), respectively. Subsequently, activated NK cells were labeled with CFSE and cocultured with resting NK cells of the same donor in the presence of blocking F(ab)2 fragments of mAb 5D12 (anti-NKp80) or of an isotype-matched IgG1 control. Subsequently, cultures were analyzed by flow cytometry for degranulation (CD107a+ cells) and production of IFN-γ or TNF. (A) Representative dot plots of resting (CFSE−) NK cells stained for CD107a, IFN-γ, or TNF, after coculture with autologous monokine-activated NK cells or, for control, without coculture. (B) Compiled data from independent experiments of TNF responses or degranulation of resting (CFSE−) NK cells after coculture with activated autologous NK cells or, for control, without coculture. Each value represents the mean of triplicates from a single experiment. P values were determined using the Wilcoxon signed-rank test. (C-D) Monokine-activated NK cells are lysed by autologous resting NK cells in an NKp80-dependent manner. Activated NK cells were labeled with 51Cr (target cells, T) and incubated with resting NK cells of the same donor (effector cells, E) that had been preincubated with blocking F(ab)2 fragments of mAb 5D12 (anti-NKp80) or of an isotype-matched IgG1 control. (C) Representative cytolysis data for 2 donors. Means of triplicates are shown with standard deviations. (D) Compiled cytolysis data for 6 different donors at an E:T ratio of 20:1.
NKp80-mediated recognition and lysis of monokine-activated NK cells by autologous NK cells. (A-B) NK cells were purified from freshly isolated PBMC and cultivated in parallel for 2 days in the presence of IL-2, IL-12 and IL-18 (activated NK cells) or without cytokines (resting NK cells), respectively. Subsequently, activated NK cells were labeled with CFSE and cocultured with resting NK cells of the same donor in the presence of blocking F(ab)2 fragments of mAb 5D12 (anti-NKp80) or of an isotype-matched IgG1 control. Subsequently, cultures were analyzed by flow cytometry for degranulation (CD107a+ cells) and production of IFN-γ or TNF. (A) Representative dot plots of resting (CFSE−) NK cells stained for CD107a, IFN-γ, or TNF, after coculture with autologous monokine-activated NK cells or, for control, without coculture. (B) Compiled data from independent experiments of TNF responses or degranulation of resting (CFSE−) NK cells after coculture with activated autologous NK cells or, for control, without coculture. Each value represents the mean of triplicates from a single experiment. P values were determined using the Wilcoxon signed-rank test. (C-D) Monokine-activated NK cells are lysed by autologous resting NK cells in an NKp80-dependent manner. Activated NK cells were labeled with 51Cr (target cells, T) and incubated with resting NK cells of the same donor (effector cells, E) that had been preincubated with blocking F(ab)2 fragments of mAb 5D12 (anti-NKp80) or of an isotype-matched IgG1 control. (C) Representative cytolysis data for 2 donors. Means of triplicates are shown with standard deviations. (D) Compiled cytolysis data for 6 different donors at an E:T ratio of 20:1.
Discussion
Human resting NK cells are known to uniformly express the activating NK cell receptor NKp80. We here now report that human NK cells also contain intracellular stores of the NKp80 ligand AICL and that these are associated with the Golgi complex. Upon exposure of NK cells to monokines IL-12 and IL-18, AICL glycoproteins surface and accumulate at the cell surface at substantial levels, resulting in functional recognition and cytolysis by autologous bystander NK cells in an NKp80-dependent manner. AICL belongs to a subgroup of NKC-encoded CTLRs, termed CLEC2 family, that is characterized by peculiar sequence features in the CTLD.16 Human members of the CLEC2 family include KACL (encoded by the CLEC2A gene), AICL (CLEC2B), CD69 (CLEC2C), and LLT1 (CLEC2D). They all are homodimeric glycoproteins with a restricted expression pattern: KACL is restricted to keratinocytes, and the other CLEC2 family members appear almost exclusively expressed by various cells of the hematopoietic lineage.16,24 Except for CD69, they all are known to engage likewise homodimeric CTLR of the NKRP1 family adjacently encoded in the NKC. Whereas NKp80 and its inhibitory counterpart NKR-P1A, the receptor of LLT1, broadly are present on NK cells and subsets of T cells, the physiological expression of the KACL receptor NKp65 remains to be determined.24 Previously, AICL was shown to be expressed by myeloid cells and LLT1 by B cells and dendritic cells, respectively, with both modulated by TLR ligands.19,25,26 In vitro studies further showed that NKp80-AICL contributed to the activating cross-talk of monocytes and NK cells under inflammatory conditions, whereas NKR-P1A-LLT1 interaction dampened NK cell responses toward B cells.19,25,26
Whereas LLT1 is not detectable in resting lymphocytes,25 intracellular presence in resting lymphocytes has also been reported for the AICL-relative CD69,27 though the specific localization of intracellular CD69 is unclear. CD69 is rapidly recruited to the cell surface upon lymphocyte activation and serves as an early marker of lymphocyte activation. More recently, association of CD69 with the sphingosin-1-phosphate receptor 1 was shown to regulate cellular trafficking and to mediate depletion of CD69 from the surface of resting lymphocytes.28 A similar role of sphingosin-1-phosphate receptors in regulating AICL surface expression remains to be addressed. Our domain-swapping experiments reveal a crucial role of the AICL ectodomain for the intracellular retention of AICL, possibly by interacting with a Golgi complex–resident molecule. We also provide evidence that lysine 7 of the rather short cytoplasmic tail of AICL controls cell surface levels of AICL. It has been shown that the Kaposi’s sarcoma-associated herpes virus protein K5 downregulates surface expression of AICL due to ubiquitination of cytoplasmic lysines resulting in lysosomal degradation of AICL,29 suggesting that ubiquitination may be responsible for lysine 7-dependent regulation of AICL. Hence, our data suggest that AICL expression is regulated at various levels, eg, at the level of transcripts, by intracellular retention and via ubiquitination. Regulation of AICL expression may also occur at the posttranscriptional level because AICL transcripts comprise extensive noncoding sequences. This multilayered regulation of AICL expression is reminiscent of the regulation of expression of ligands of the NKG2D receptor.30-32 Regulation of NKG2D ligand expression at various levels is thought to fine-tune responses of this potent activating receptor.30-33 The observation of AICL being coexpressed together with its receptor NKp80 by human NK cells raises questions on functional consequences thereof and sheds new light on the tight genetic linkage of such a nonpolymorphic receptor–ligand pair that may allow for an interdependent regulation of coexpression in a given cell type, eg, at the transcriptional level.
Recently, induced LLT1 surface expression upon activation of lymphocytes was shown.25 Hence, activation-induced surface expression by leukocytes represents a common theme of the 3 human CLEC2 family members CD69, LLT1, and AICL. It is tempting to speculate that these molecules enable the immune system to sense activated leukocytes in order to regulate and limit cellular immune responses. LLT1 is expressed by the NK cell line YT and several B cell lines, but no expression by nonhematopoietic cell lines has been reported.25,26,34 Similarly, we found human NK cell lines and some other hematopoietic cell lines to express AICL, but in contrast to a previous report,23 no AICL expression by the epithelial cell line HeLa or other nonhematopoietic cell lines. However, reports on expression of LLT1 and AICL by glioblastoma and liver cancer, respectively, indicate that tumor cells may aberrantly express these CLEC2 proteins.23,35
We find that NK cell activation by monokines results in an upregulation of AICL and downregulation of NKp80 at the cell surface that is paralleled by changes in levels of the corresponding transcripts. Because changes in NKp80 transcript levels were moderate, additional regulatory mechanisms may contribute to the observed changes in cell surface expression. For example, cellular upregulation of AICL may lead to complex formation with NKp80 and enhanced NKp80 downregulation from the cell surface. However, flow cytometric analyses of NK-92MI cells ectopically expressing NKp80 did not reveal any evidence for a mutual interference of cell surface expression of coexpressed NKp80 and AICL glycoproteins (data not shown). We observed a biphasic increase of the AICL glycoproteins on the surface of monokine-activated NK cells with an immediate moderate increase (first 24 hours), followed by a more substantial upregulation in the course of the following days. Analogous to CD69, the initial appearance of extracellular AICL protein on NK cells at the onset of activation may result from the mobilization from intracellular stores,27 and the second more substantial increase most likely is due to neosynthesis of AICL proteins well in agreement with the delayed upregulation of AICL transcripts. The latter may be caused by a “reprogramming” of NK cells by the monokines IL-12 and IL-18. Of note, mouse NK cells stimulated with proinflammatory cytokines IL-12 and IL-18 were shown to develop memory-like properties.7 In comparison with resting NK cells, these cells displayed enhanced responsiveness upon restimulation with cytokines as measured by IFN-γ secretion and exhibited a longer life span than did untreated NK cells. In humans, NK cells are exposed to monokines IL-12 and IL-18 in secondary lymphoid organs and at sites of inflammation. Whereas we observed that NKp80 is downregulated by treatment with IL-12 and IL-18, IL-12 has previously been shown to upregulate expression of NKR-P1A in a biphasic manner.36 Converse regulation of the activating receptor NKp80 and the inhibitory receptor NKR-P1A by monokines including IL-12 may reflect their opposing function during immune responses. Upregulation of AICL on NK cells exposed to an inflammatory milieu and prone to differentiate into memory-like NK cells may allow NKp80-mediated regulation of cytokine-activated NK cells. Taking advantage of the cytotoxic capacity of NK cells to limit cellular immune responses has previously been reported for NKG2D-mediated recognition and cytolysis of activated T cells upregulating NKG2D ligands.37 Although resting NK cells are commonly viewed as poorly cytolytic,38 we and others observed that cytotoxicity of resting NK cells can be triggered via NKp80.18,19 Although cytolysis of monokine-activated NK cells by resting autologous NK cells appeared NKp80-dependent, our data do not exclude involvement of additional activating NK receptors.
In summary, we demonstrate that AICL glycoproteins accumulate in intracellular stores of resting human NK cells and are released to the cell surface upon activation by the monokines IL-12 and IL-18. Hence, AICL surface expression tags monokine-activated NK cells for functional recognition and cytolysis by autologous NK cells. On the basis of our findings, we hypothesize that AICL and NKp80 generate a negative regulatory circuit loop that allows autonomous control of NK cell responses during inflammation and infection and may limit generation of “memory” NK cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Christina Rohe and Wiebke Ruschmeier for excellent technical assistance and Evelyn Ullrich for sharing unpublished data.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (STE 828/6-1) and the Deutsche Krebshilfe (108574).
Authorship
Contribution: S.N.K. and Y.B. designed and performed experiments; S.W. performed initial experiments; and A.S. conceptualized research and wrote the manuscript.
Conflict-of-interest disclosure: A.S. and S.W. have filed a patent application on the blockade of NKp80–AICL interaction. The remaining authors declare no competing financial interests.
Correspondence: Alexander Steinle, Institute for Molecular Medicine, J.W. Goethe-University Frankfurt am Main, Theodor-Stern-Kai 7, 60590 Frankfurt am Main, Germany; e-mail: alexander.steinle@kgu.de.
References
Author notes
S.N.K. and Y.B. contributed equally to this study.