Key Points
Rituximab use is associated with significant improvement in all outcomes for patients with HIV-associated CD20-positive lymphomas.
Infusional EPOCH chemotherapy is associated with better overall survival in patients with AIDS-related diffuse large B-cell lymphoma (DLBCL).
Abstract
Limited comparative data exist for the treatment of HIV-associated non-Hodgkin lymphoma. We analyzed pooled individual patient data for 1546 patients from 19 prospective clinical trials to assess treatment-specific factors (type of chemotherapy, rituximab, and concurrent combination antiretroviral [cART] use) and their influence on the outcomes complete response (CR), progression free survival (PFS), and overall survival (OS). In our analysis, rituximab was associated with a higher CR rate (odds ratio [OR] 2.89; P < .001), improved PFS (hazard ratio [HR] 0.50; P < .001), and OS (HR 0.51; P < .0001). Compared with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP), initial therapy with more dose-intense regimens resulted in better CR rates (ACVBP [doxorubicin, cyclophosphamide, vindesine, bleomycin and prednisolone]: OR 1.70; P < .04), PFS (ACVBP: HR 0.72; P = .049; “intensive regimens”: HR 0.35; P < .001) and OS (“intensive regimens”: HR 0.54; P < .001). Infusional etoposide, prednisone, infusional vincristine, infusional doxorubicin, and cyclophosphamide (EPOCH) was associated with significantly better OS in diffuse large B-cell lymphoma (HR 0.33; P = .03). Concurrent use of cART was associated with improved CR rates (OR 1.89; P = .005) and trended toward improved OS (HR 0.78; P = .07). These findings provide supporting evidence for current patterns of care where definitive evidence is unavailable.
Introduction
The incidence of non-Hodgkin lymphomas (NHLs) remains significantly increased in HIV-positive patients compared with the HIV-negative population, even in the era of combination antiretroviral therapy (cART).1-5 The prognosis of HIV-associated NHL is influenced by lymphoma-specific factors, HIV-specific factors, and treatment. HIV-associated lymphomas often present with a more aggressive histology and advanced stage. Impaired bone marrow reserve and underlying immunodeficiency contribute to higher rates of infectious complications compared with immunocompetent patients with NHLs.1,6,7
In the early days of the AIDS epidemic, treatment of HIV-positive patients diagnosed with NHL was mainly palliative, with median survival measured in months, and only ∼10% of patients alive at 2 years.8 The advent of cART in 1996 resulted in reduced morbidity and mortality from HIV infection, thus allowing more aggressive lymphoma-directed therapy.9-11 Several studies have shown that properly selected patients with HIV-associated NHL tolerate highly aggressive and potentially curative regimens typically used for immunocompetent patients without prohibitive toxicity.12-15
Despite these remarkable advances in outcomes, there are few randomized controlled clinical trials that define an optimal approach for the treatment of HIV-associated NHL. An important example is the role of rituximab, a monoclonal antibody directed against CD20: although overwhelming evidence supports its use in immunocompetent patients with B-cell NHL,16,17 the only randomized controlled clinical trial in the HIV-positive population showed no benefit.18 Another controversial topic is the concurrent use of cART, which some experts argue should be suspended during induction therapy.19 This dearth of comparative data motivated us to perform a systematic review to identify all prospectively performed clinical trials in HIV-associated NHL, extract patient-level information including lymphoma-specific, HIV-specific, and treatment factors, and perform a pooled analysis of this data. Our objective was to assess the influence of treatment on outcomes after adjustment for baseline covariates.
Materials and methods
Search strategy and selection criteria
We conducted a systematic review of the published literature by using the PubMed and Embase databases. We used an identical search strategy as used by the Cochrane Collaboration using the search terms lymphoma, non-Hodgkin, AIDS, HIV infection, and combinations of these terms as previously developed by The Cochrane Collaboration.20,21 Additionally, we searched all available online conference abstracts of the annual meetings of the American Society of Clinical Oncology, American Society of Hematology, AIDS, and International Conference on Malignancies in AIDS and Other Acquired Immunodeficiencies, by using combinations of the above search terms. To ensure that all relevant trials were included, we reviewed the bibliographic references of review articles and the retrieved publications and queried experts in the field for the existence of other published or unpublished trials. To be considered eligible, trials had to be prospective phase II or III clinical trials performed in North America or Europe, treat HIV-positive patients ≥18 years of age with newly diagnosed NHL, and measure either survival or response rate as their primary outcome. Studies that assessed patients with primary central nervous system lymphoma, or therapies such as radiation therapy, immunotoxins, radio-immunotherapy, stem cell transplantation, or salvage therapies, were excluded. The searches were restricted to studies performed in humans and limited to the time period between January 1, 1990 and October 31, 2010, without any restrictions on language.
Procedure
We requested the complete data sets of all collected data in an electronic format stripped of any potential identifiers from the principal investigators (PIs), including gender, race, age, treatment center, enrollment date, date of last follow-up (or date of death), status at last follow-up, survival status, cause of death, relapse status, type and date of relapse if any, histological subtype, stage, LDH, number of involved extranodal sites, performance status at baseline, baseline CD4 count and viral load, date of HIV diagnosis, mode of HIV transmission, history of AIDS defining events prior to lymphoma, type of antiretroviral therapy, use of cART concurrently with chemotherapy, type of chemotherapy, and use of rituximab. If no response by the PI was received, 2 further attempts were made to contact the PI. We then pooled all patient-level data. Complete response (CR) was defined as per the individual study protocol. Overall survival (OS) was defined as the time from enrollment to death from any cause; progression-free survival (PFS) was the time to relapse. We analyzed all data centrally and checked for inconsistencies. The Albert-Einstein College of Medicine institutional review board approved the study. Informed consent was obtained in accordance with the Declaration of Helsinki.
Statistical analysis
We presented continuous data as mean ± standard deviation or median ± interquartile range (IQR). When comparing 2 groups, we used the Student t test for normally distributed data; otherwise, the nonparametric Mann-Whitney U test was used. For proportions, we used the Pearson χ2 test or the Fisher exact test for scarce data. The outcomes PFS and OS were plotted according to the Kaplan-Meier method and analyzed through the log-rank test as an initial examination. We then used a multivariate logistic regression model to estimate the odds ratio (OR) associated with exposure for the outcome CR and a Cox proportional hazards regression model to estimate hazard ratio (HR) for the time to event outcomes PFS and OS.
The variables of interest were the use of rituximab with chemotherapy, the type of chemotherapy defined as either (1) intensive regimes (alternating combinations of cytarabine, methotrexate, cyclophosphamide, ifosfamide, doxorubicin, teniposide, vincristine, dexamethasone [modified GMALL protocol] [GBALL]22 , LAL3/97 [modified GMALL protocol],12 and LMB6 regimens [detailed description as per Oriol et al23 ]), (2) cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP), (3) modified or low-dose CHOP, (4) infusional etoposide, prednisone, infusional vincristine, infusional doxorubicin, and cyclophosphamide (EPOCH), (5) vincristine and steroid (VS), (6) doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisolone (ABCVP) or LNHIV91 (for detailed description, see the original publication24 ), (7) infusional cyclophosphamide, doxorubicin, and etoposide (CDE), and (8) the oral regimen lomustine, etoposide, cyclophosphamide and procarbazine (Remick regimen),25 concurrent use of cART, and supportive use of granulocyte–colony-stimulating factor (G-CSF). Additionally, we grouped the chemotherapeutic regimens based on their relative intensity compared with CHOP for parts of the analysis as follows: less dose-intense regimens (VS, Remick regimen, modified, and/or low-dose CHOP) and dose-intense regimens (ACVBP and intensive regimens). The regimens EPOCH and CDE were also described as infusional regimens throughout the manuscript in contrast to all other regimens that are either given as bolus or orally. All estimates in the multivariate model were adjusted for covariates including age, gender, histological subtype (diffuse large B-cell lymphoma [DLBCL], Burkitt [BL], or Burkitt-like lymphoma [BLL], or other lymphomas), age-adjusted international prognostic index (aaIPI), CD4 count at baseline (< or ≥50 cells/μL), prior history of AIDS, enrollment period (years 1989-1995, 1996-1997, 1998-2000, 2001-2004, 2005-2010), rituximab use, and type of chemotherapy. OS was the primary outcome because it allows examination of net benefit of treatment combining efficacy and toxicity, whereas PFS and CR rate were considered secondary outcomes as they are mainly outcomes for examination of treatment efficacy. To examine possible differential associations between treatment factors and multiple causes of death, we used a polytomous regression model for nominal outcomes.26 We used the following categories to define cause of death: treatment-related mortality (TRM; any death from causes other than lymphoma that were judged by the PI to be possibly or probably associated with therapy), progressive disease (PD; death secondary to lymphoma progression or relapse); HIV (death due to nonmalignant complications of HIV disease), and other (all other causes of death, such as second malignancies, accidental death).
We evaluated assumptions for all statistical models and found none were violated. We considered P < .05 as statistically significant; all statistical tests were 2-sided. We quoted 95% confidence intervals (95% CIs) whenever applicable. We used SAS software, version 9.2 (SAS Institute, Cary, NC) for statistical analysis. Missing data for continuous variables with heavy missing was imputed using multiple imputations, and P values were generated with the MIANALYXE procedure in SAS, but not for the subset analyses.
Results
Trials and patient characteristics
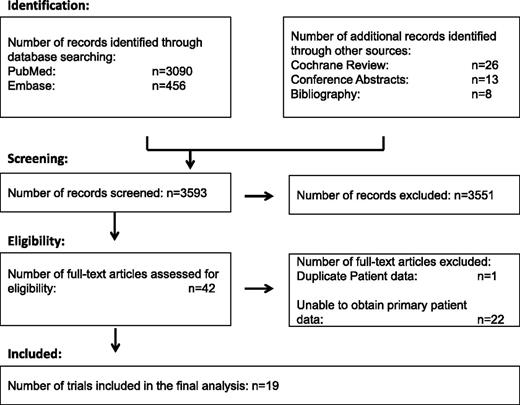
After reviewing the trials identified in the literature search, 42 trials were considered eligible. We were able to obtain patient-level data for 19 trials including 1546 patients (Figure 1). A median of 61 patients were enrolled per trial (range, 14-467), with 1 trial providing data on nearly one-third of the patients (Mounier et al27 ; n = 467). All trials were phase II clinical trials except for 2 phase III trials.18,27 Details of the characteristics of the included trials are listed in Table 1. Supplemental Table 1 on the Blood Web site details the excluded trials. The reasons for exclusion were as follows: duplicate data with another study already included (n = 1); unpublished data at the time of the analysis that was not released by the PI (n = 2); unsuccessful attempts at contacting the PI (n = 7); and the PI was unable to provide the patient data (n = 13). Demographic data and clinical characteristics of all 1546 patients are described in Table 2. The majority of patients (75%) were enrolled in the cART era. Most were men (84%), had DLBCL (69%), were intermediate-high or high risk (69%) by the age-adjusted IPI, and had a CD4 count ≥50 cells/µL (86%). Regarding treatment, rituximab was used in 35%, and the most common regimens included CHOP (41%), less dose-intense regimens (16%), infusional regimens (EPOCH in 11%, CDE in 12%), and the dose-intense regimens ACVBP/LNHIV91 and intensive regimens (10% each).
Diagram documenting the flow of information through the different phases of the systematic review as per the PRISMA statement.28
Diagram documenting the flow of information through the different phases of the systematic review as per the PRISMA statement.28
Rituximab and outcomes
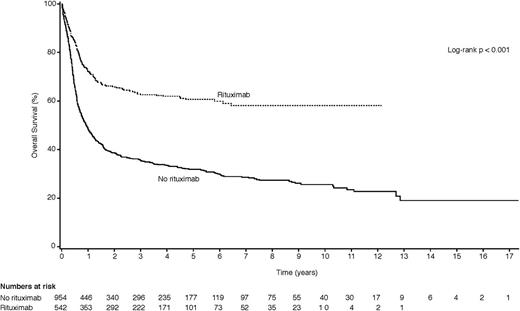
Table 3 shows the results of the univariate and multivariate analyses in which we correlated treatment factors with clinical outcomes (CR, PFS, and OS) for all patients. In univariate analysis, the use of rituximab was strongly associated with improved outcomes: CR rate (OR 2.49, 95% CI 1.98-3.15; P < .001), PFS (HR 0.53, 95% CI 0.44-0.63; P < .001), and OS (HR 0.43, 95% CI 0.37-0.51; P < .01), as also demonstrated in Figure 2. After adjustment for age, gender, baseline CD4 count, histological subtype, age-adjusted IPI, enrollment period, use of cART, prior history of AIDS, and chemotherapeutic regimen in the multivariate model, rituximab use remained significantly associated with improved outcomes, including CR rate (OR 2.89, 95% CI 1.64-5.08; P < .001), PFS (HR 0.50, 95% CI 0.34-0.73; P < .001), and OS (HR 0.51, 95% CI 0.38-0.71; P < .0001). We found a significant interaction between CD4 count ≥50 cells/μL and rituximab for all 3 outcomes. Rituximab use was only significantly associated with improved outcomes for patients with CD4 counts ≥50 cells/μL (OR for CR = 2.84, 95% CI 1.60-5.02; P < .001; HR for PFS = 0.48, 95% CI 0.32-0.72; P < .001; HR for OS = 0.55, 95% CI 0.39-0.77; P < .001), but not if the CD4 count was <50 cells/μL. To further examine if the lack of significant finding among patients with a CD4 count <50 cells/μL was a result of limited sample size, we calculated the statistical power to identify the same effects sizes on CR, PFS, and OS that we observed among patients with CD4 >50 cells/μL and obtained >90% power for each outcome with a 2-sided type I error rate ≤5%.
Kaplan-Meier plots comparing the OS for patients treated with rituximab-containing regimens vs non–rituximab-containing regimens.
Kaplan-Meier plots comparing the OS for patients treated with rituximab-containing regimens vs non–rituximab-containing regimens.
Chemotherapy regimen and outcomes
Using CHOP as reference, we compared the effect of the choice of initial chemotherapeutic regimen while adjusting for rituximab use. Treatment with the less dose-intense regimens (dose-reduced or modified CHOP, vincristine/steroids, Remick regimen) was associated with significantly inferior clinical outcomes in both univariate and multivariate analysis except for the Remick regimen. The oral Remick regimen resulted in lower CR rates (OR 0.32, 95% CI 0.16-0.64; P = .001) and a worse OS on univariate analysis (HR 2.48, 95% CI 1.72-3.47; P < .001), but not in the multivariate model. In contrast, both the dose-intense ABCVP-based regimens and other intensive regimens generally resulted in improved outcomes, although this did not always reach statistical significance. The infusional regimen CDE was in univariate analysis associated with a reduced CR rate (OR 0.54, 95% CI 0.39-0.75; P < .001); however, this association was diminished in the multivariate model. Instead, CDE was found significantly associated with improved OS in the multivariate model (HR 0.49, 95% CI 0.23-0.95; P = .048). Infusional EPOCH had a higher CR rate (OR 1.73, 95% CI 1.17-2.57; P < .001) and improved PFS (HR 0.57, 95% CI 0.41-0.79; P < .001) and OS (HR 0.59, 95% CI 0.44-0.76; P < .001) in the univariate model, but not when adjusted for other covariates, including use of rituximab, in the multivariate model.
Concurrent use of antiretroviral therapy
When comparing the influence of supportive care measures, the concurrent use of cART with chemotherapy was associated with significantly higher CR rates and OS on univariate analysis, but this association only remained significant for a higher CR rate (OR 1.89, 95% CI 1.21-2.93; P = .005) and a trend toward better OS (HR 0.78, 95% CI 0.60-1.02; P = .07) after adjustment in the multivariate model. When the multivariate model was restricted to patients treated in the cART era (after 1995), we found similar results (OR for CR rate 1.84, 95% CI 1.17-2.89; P = .008; HR for OS 0.81, 95% CI 0.62-1.06; P = .12). We compared the effect of rituximab in concurrent cART users and in patients not using cART concurrently with chemotherapy, and neither clinically meaningful nor statistically significant differences between the groups were identified. As the use of G-CSF was nearly ubiquitous throughout the studies, no meaningful comparison could be performed.
Treatment of DLBCL and BL/BLL
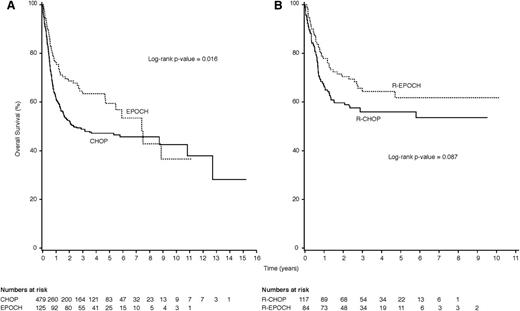
When we limited our analysis to only patients diagnosed with DLBCL, treatment with EPOCH resulted in improved OS compared with CHOP on univariate analysis (Figure 3A) and in the multivariate model (HR 0.33, 95% CI 0.11-0.85; P = .031). This association was similar when the analysis was limited to patients treated with rituximab (R-EPOCH vs R-CHOP; Kaplan-Meier analysis: log-rank P = .09; Figure 3B), but was of borderline statistical significance in the multivariate model (HR 0.34, 95% CI 0.11-0.97; P = .05). When we combined all infusional regimens (EPOCH and CDE), this resulted in a statistically significant OS benefit on multivariate analysis with adjustment for rituximab use (HR 0.72, 95% CI 0.52-0.99; P = .046).
Kaplan-Meier plots comparing OS for patients with DLBCL treated with EPOCH vs CHOP and R-EPOCH vs R-CHOP. (A) HIV-positive patients with newly diagnosed DLBCL treated with infusional EPOCH (dashed line) achieved significantly longer OS than patients treated with CHOP (solid line; P = .016). (B) This was also seen when the analysis was limited to patients with DLBCL treated in the rituximab era, where treatment with R-EPOCH (dashed line) compared favorably to treatment with R-CHOP (solid line; P = .087).
Kaplan-Meier plots comparing OS for patients with DLBCL treated with EPOCH vs CHOP and R-EPOCH vs R-CHOP. (A) HIV-positive patients with newly diagnosed DLBCL treated with infusional EPOCH (dashed line) achieved significantly longer OS than patients treated with CHOP (solid line; P = .016). (B) This was also seen when the analysis was limited to patients with DLBCL treated in the rituximab era, where treatment with R-EPOCH (dashed line) compared favorably to treatment with R-CHOP (solid line; P = .087).
In the subset of patients with either BL or BLL, treatment with either ABCVP/LNHIV91 or intensive regimens showed improved PFS in favor of the dose-intense regimens (HR 0.25, 95% CI 0.08–0.85; P = .03) when compared with the infusional regimens CDE or EPOCH. However, no difference in CR rate (P = .8) or OS (P = .28) between dose-intense and infusional regimens was observed in the multivariate model. The numbers became too small for meaningful analysis when the model was restricted to rituximab-treated patients only.
Effect of treatment on mortality from lymphoma, treatment-associated complications, and all-causes
In Table 4, we show the associations of treatment factors and causes of death. After adjusting for age, gender, history of AIDS, time of enrollment, type of lymphoma, age-adjusted IPI, rituximab use, and type of chemotherapy, we found that the less dose-intense (PD: OR 1.75, 95% CI 1.29-2.37; P < .001) and infusional (PD: OR 1.54, 95% CI 1.12-2.11; P = .008) chemotherapeutic regimens were associated with an increased risk of dying of progressive lymphoma compared with CHOP. The infusional regimens EPOCH and CDE were combined for this analysis because of the low number of events in the EPOCH-treated patients. Treatment with infusional regimens was associated with markedly less TRM compared with CHOP (TRM: OR 0.28, 95% CI 0.14-0.57; P < .001); conversely, a higher risk for death from disease progression or relapse was seen (OR 1.54, 95% CI 1.12-2.11; P = .008).
Treatment with rituximab-containing regimens was associated with a reduced risk of death secondary to TRM (OR 0.68; 95% CI 0.39-0.91; P = .09), progressive lymphoma (OR 0.30, 95% CI 0.21-0.41; P < .001), and other causes of death (OR 0.38; 95% CI 0.20-0.69; P = .002).
Death secondary to HIV-related causes was not significantly different between patients treated with or without rituximab-containing regimens (OR 0.58, 95% CI 0.30-1.12; P = .14). When the relationship between baseline CD4 count and causes of mortality was examined, there was no association between CD4 count and death due to treatment toxicities (TRM) or progressive lymphoma. We could demonstrate that the baseline CD4 count at the time of lymphoma diagnosis was significantly associated with subsequent death due to HIV-related causes. This association was strongest for patients with baseline CD4 counts <50 cells/µL, for whom the odds of dying of nonmalignant complications of HIV were reduced by 26% for every 10 cell/µL CD4 count increase (OR 0.74; 95% CI 0.57-0.97; P = .03) and by 4% per 10 cells/µL increase for patients with a CD4 count ≥200 (OR 0.96, 95% CI 0.92-0.99; P = .02). This association was not significant for patients with a CD4 count between 50 and 199 cells/µL.
Discussion
We performed a pooled analysis of patient level data from 1546 patients with HIV-associated lymphoma enrolled in 19 clinical trials identified by systematic literature review. Our objective was to determine whether specific treatments were associated with better outcomes after adjustment for baseline covariates. Although this approach does not provide level 1 evidence supporting a specific treatment approach, it does provide level 2b evidence that may be useful in guiding treatment decisions given the dearth of level 1 evidence regarding the treatment of HIV-associated NHL.43
We made several important observations from this analysis. First, we found that the addition of rituximab to any chemotherapy backbone was associated with a nearly threefold increase in the CR rate and 50% reduction in the risk of progressive lymphoma or death from any cause. This is consistent with the overwhelming evidence indicating a beneficial effect of rituximab in immunocompetent patients with NHL. It is also reassuring given that the only phase III trial evaluating rituximab in HIV-associated NHL failed to show improved outcomes with the addition of rituximab; although the risk of death from lymphoma was reduced by rituximab in that trial, this was offset by an increased risk of infectious deaths in the rituximab arm that occurred almost exclusively in patients with a low baseline CD4 count of <100/μL.18 This is consistent with our observation that the rituximab benefit appears limited to patients with a CD4 count ≥50/μL. Second, the use of rituximab in this analysis was not associated with an increased risk of death due to treatment toxicities or HIV-related complications. Third, more dose-intense chemotherapy regimens (the multiagent dose-intense intensive regimens and ACVBP-based regimens) resulted in better clinical outcomes compared with treatment with CHOP in patients with the more aggressive BL or BLL. Moreover, for the most common lymphoma (DLBCL), OS was improved with infusional EPOCH therapy compared with CHOP. However, this analysis was limited by low patient numbers. Fourth, although immunocompetent patients with BL generally benefit from more intense chemotherapy regimens, in our analysis, the dose-intensive regimens did not result in an OS advantage compared with the less toxic infusional regimens EPOCH and CDE. Finally, patients who were using cART concurrently with induction chemotherapy experienced higher CR rates and a trend toward improved OS compared with patients who did not take cART during the initial treatment phase.
Whether infusional chemotherapy is superior to bolus regimens is a question that is currently under investigation in an ongoing phase III trial that compares R-CHOP with R-EPOCH in immunocompetent patients with DLBCL (CALGB50303; clinicaltrials.gov NCT00118209). Infusional regimens, such as EPOCH or CDE, were initially developed based on the hypothetical principle that highly proliferative tumors are more susceptible to prolonged continuous exposure to chemotherapeutic agents by overcoming kinetic resistance, and that prolonged infusion of cytotoxic therapy provides greater opportunity for therapeutic synergy with rituximab. At the same time infusional regimens are less toxic than commonly used high-dose multiagent regimens.44 Although death secondary to lymphoma was more common in patients treated with infusional regimens, toxicity-related death was less common. Although unexpected, given the high reported CR rates with EPOCH in 2 National Cancer Institute (NCI)-led studies,30,39 this result could have been influenced by our need to combine EPOCH and CDE in the analysis of infusional regimens secondary to small numbers. Another possibility is that the advent of rituximab might have offset some of the benefit of the infusional regimens over CHOP. Nevertheless, our findings support continued evaluation of continuous infusion approaches in combination with rituximab.
Our finding that concurrent cART is associated with improved clinical outcomes is consistent with other studies evaluating the role of continuous vs intermittent cART.45 Although concurrent use of chemotherapy and cART poses the risk of potential drug-drug interactions or overlapping toxicities, this appears to be offset by better HIV control and fewer infectious complications and AIDS-defining events. Experience derived from the treatment of other AIDS-defining illnesses, such as Pneumocystis jirovecii pneumonia and tuberculosis, show that prompt initiation of cART in antiretroviral-naïve patients leads to better clinical outcomes compared with deferred initiation.46-48 Our findings also demonstrate evidence for better outcomes with concurrent cART and support its use even during induction chemotherapy for AIDS-related lymphomas (ARLs).
With patient-level data for >1500 patients from most major prospectively performed clinical trials for patients with ARLs in Europe and the United States, our study represents the largest and most comprehensive analysis of factors influencing outcomes for HIV-positive patients newly diagnosed with aggressive NHL to date. Although meta-analyses in the past have attempted to examine heterogeneity in treatment effects, such as the use of rituximab, using a fixed effects or a mixed effects model, the results were limited by the fact that many trials were small, nonrandomized, and heterogeneous in many aspects such as supportive care, patient-eligibility criteria, and interventions studied.20,49 It is important to recognize that during the 20-year span of the included studies, different antiretroviral therapeutic eras and lymphoma classification systems were used. Here we were able to diminish this bias first by using histological classifications uniformly for purposes of our review that have been consistently described in published studies based on the contemporary classification schema at the time (DLBCL, BL, and BLL) and second by adjusting all outcome measures equally for known important prognostic variables and other significant confounders, such as the time of enrollment. Each patient was treated with standard state-of-the art lymphoma and HIV care at the time of respective enrollment by physicians experienced in the care of this patient population, which accounts for a certain homogeneity.
Several limitations hamper our analysis. Although we adjusted for enrollment time, certain unmeasured factors could still confound our estimates, especially as the use of rituximab, more powerful antiretroviral therapy, and better supportive care measures, all increased over time in a collinear fashion. Missing data might have led to imprecise estimates (although multiple imputation was used to address imprecision due to random missing), as well as reclassification errors for histological subtypes necessitated by changes in the histological classifications for NHL over the last 2 decades. Additionally, histological diagnosis was not centrally reviewed or confirmed. Outcomes, such as CR, PFS, and treatment-related deaths, might have been defined slightly differently in each study, which could account for some inaccuracy. The outcome CR is especially problematic, as achievement of CR was neither uniformly defined or assessed in the different studies, nor centrally verified. This bias, however, should not affect our results for the main measure of clinical benefit, OS. Although we were not able to obtain data for 23 studies, these studies were generally small (18 of 23 trials had <50 patients enrolled; the largest trial [n = 159] contained duplicate data with a trial included in our analysis) or conducted in the pre-cART era often using outdated treatment that mostly did not contain rituximab (21 of 23). Last, because of small numbers, some chemotherapeutic regimens had to be combined in the subset analyses, which may have led to some difficulties in the interpretation of the results.
In conclusion, our results support the benefit of rituximab, infusional regimens, and cART in the treatment of AIDS-related lymphomas. Ongoing prospective clinical trials address the important questions of which regimen should become the standard of care for any patient with DLBCL (CALGB50303) and BL (AMC048 and NCI#9177). Until such definitive evidence is available, the presented results can serve as supporting evidence for current patterns of care. In the interim, treatment decisions for individuals with HIV-associated lymphomas should be guided by experienced physicians or specialized centers.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Xin Zheng and Rimma Belenkaya for help in creating the patient database.
This work was supported in parts by the AIDS Malignancy Consortium (grant UO1CA121947), the Paul Calabresi Career Development Award for Clinical Oncology (grant K12CA132783-03), the American Society of Clinical Oncology Cancer Foundation 2010 Young Investigator Award, Red Temática de Investigación Cooperativa en Cáncer grant RD12/0036/0029, Instituto de Salud Carlos III, Spain, Clinical and Translational Science Award grants UL1 RR025750 and KL2 RR025749, and TL1 RR025748 from the National Center for Research Resources, a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Authorship
Contribution: S.K.B. and J.A.S. designed the research; S.K.B., R.T., and J.A.S. performed the literature search and systematic review; J.Y.L., N.M., L.D.K., J.-M.R., M.S., U.T., R.W., L.G., F.B., W.H.W., C.W., A.O., J.-T.N., K.D., R.F.L., L.R., O.G., M.M., S.C.R., and J.A.S. performed the research; S.K.B., X.X., D.W., and J.A.S. analyzed the data; and S.K.B., X.X., A.N., and J.A.S. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stefan Klaus Barta, Albert-Einstein Cancer Center, Montefiore Medical Center, Department of Oncology, 111 East 210th St, Bronx, NY 10467; e-mail: sbarta@montefiore.org.