Key Points
Clot retraction of sphingomyelin-rich raft-depleted platelets from sphingomyelin synthase knockout mouse is delayed.
Translocation of fibrin to sphingomyelin-rich rafts in platelet membrane is induced by thrombin in the presence of FXIII crosslinking activity.
Abstract
Membrane rafts are spatially and functionally heterogenous in the cell membrane. We observed that lysenin-positive sphingomyelin (SM)–rich rafts are identified histochemically in the central region of adhered platelets where fibrin and myosin are colocalized on activation by thrombin. The clot retraction of SM-depleted platelets from SM synthase knockout mouse was delayed significantly, suggesting that platelet SM-rich rafts are involved in clot retraction. We found that fibrin converted by thrombin translocated immediately in platelet detergent-resistant membrane (DRM) rafts but that from Glanzmann’s thrombasthenic platelets failed. The fibrinogen γ-chain C-terminal (residues 144-411) fusion protein translocated to platelet DRM rafts on thrombin activation, but its mutant that was replaced by A398A399 at factor XIII crosslinking sites (Q398Q399) was inhibited. Furthermore, fibrin translocation to DRM rafts was impaired in factor XIII A subunit-deficient mouse platelets, which show impaired clot retraction. In the cytoplasm, myosin translocated concomitantly with fibrin translocation into the DRM raft of thrombin-stimulated platelets. Furthermore, the disruption of SM-rich rafts by methyl-β-cyclodextrin impaired myosin activation and clot retraction. Thus, we propose that clot retraction takes place in SM-rich rafts where a fibrin-αIIbβ3-myosin complex is formed as a primary axis to promote platelet contraction.
Introduction
Membrane rafts are dynamic assemblies of sphingolipids, cholesterol, and proteins that can be stabilized into platforms involved in the regulation of a number of vital cellular processes.1 The important role of rafts at the cell surface may be their function in signal transduction. A number of studies provide considerable evidence that rafts are integral to the regulation of immune and neuronal signaling. Membrane rafts are also involved in hemostasis and thrombosis. Among blood cells, platelets are critical for maintaining the integrity of the blood coagulation system. Platelet rafts are critical membrane domains in physiological responses such as adhesion and aggregation.2 The localization of the adhesion receptor glycoprotein (GP)Ib-IX-V complex to membrane rafts is required for platelet adhesion to the vessel wall by binding the von Willebrand factor.3 Membrane rafts are also required for platelet aggregation via the collagen receptor GPVI,4 the adenosine 5′-diphosphate (ADP) receptor P2Y12,5 the Fcγ receptor FcγRIIa,6 and the C-type lectin-like receptor CLEC-2.7 Detergent-resistant membrane (DRM) rafts of platelets show round vesicles of heterogeneous sizes ranging from 20 to 500 nm, which are enriched in CD36 (GPIV).8,9 Recent reports have demonstrated that membrane rafts are spatially and compositionally heterogeneous in the cell membrane.10,11 However, little is known about raft heterogeneity in platelet membranes.
We have identified glycosphingolipid-binding proteins and investigated the signaling in membrane rafts.12-16 Previously, we reported on translocation of the heterotrimeric G protein Gαo to the DRM raft in the developing cerebellum.17 In this study, we demonstrated that sphingomyelin (SM)-rich rafts are localized in the central region of adhered platelet membranes where fibrin translocates on thrombin stimulation in combination with a coagulation factor XIII (FXIII), and this FXIII crosslinking occurs on SM-rich rafts of platelets and is involved in clot retraction.
Methods
The study was approved by the institutional ethics committee. The patient gave informed consent in accordance with the Declaration of Helsinki.
The experimental protocols were approved by the Animal Use and Care Committee.
Platelet preparation
Blood was collected into 3.8% sodium citrate at a ratio of 9:1. The blood was centrifuged (140× g) to prepare platelet-rich plasma (PRP). To prepare washed platelets, we incubated PRP with 4 mM citric acid and washed with Tyrode’s buffer (137 mM NaCl, 2.7 mM KCl, 3.75 mM NaH2PO4, 5 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), 0.35% bovine serum albumin, and 5 mM glucose; pH 6.8) containing 1 mM PGE1 and 1 U/mL heparin. Finally, the platelets were resuspended in Tyrode’s buffer (137 mM NaCl, 2.7 mM KCl, 3.75 mM NaH2PO4, 5 mM HEPES, 0.35% bovine serum albumin, and 5 mM glucose; pH 7.4) containing 1 mM CaCl2 and 1 mM MgCl2.
Cell staining
To stain SM-rich rafts, we incubated platelets with 10 μg/mL nontoxic mCherry-lysenin or nontoxic enhanced green fluorescent protein (EGFP)–lysenin in Tyrode’s buffer (pH 7.4) containing 1 mM CaCl2 and 1 mM MgCl2, and platelets were adhered onto a glass-bottomed culture dish coated with 100 μg/mL human fibronectin by treatment with 1 U/mL thrombin for 15 minutes and were fixed in 4% paraformaldehyde. To stain cholesterol-rich rafts, we incubated washed platelets with 2 μM fluorescent esters of (polyethylene glycol) cholesteryl ether (fPEG-Chol). To stain fibrin(ogen) or integrin β3 on the platelet surface, we adhered platelets onto a glass-bottomed culture dish coated with 100 μg/mL fibronectin by 1 U/mL thrombin, fixed in 4% paraformaldehyde, and incubated with the antifibrinogen rabbit polyclonal antibody or antiintegrin β3 monoclonal antibody TM8318 for 1 hour, and then with the Alexa Fluor 594 or 488-labeled secondary antibody. To stain myosin or activated myosin, we fixed adhered platelets in 4% paraformaldehyde with 0.05% Triton X-100, 1 mM Na3VO4, and 50 mM NaF, and incubated with the antimyosin rabbit polyclonal antibody or antiphosphomyosin light chain 2 (Ser19) monoclonal antibody for 1 hour, and then with the Alexa Fluor 488–labeled secondary antibody. The images were captured using a Carl Zeiss (Oberkochen, Germany) confocal imaging system (LSM510 META).
Sucrose density gradient analysis
We homogenized 600 000 000 platelets using a Teflon glass homogenizer in 2 mL of Tris/Triton buffer (0.05% Triton X-100, 25 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 1 mM EGTA). The sucrose content was then adjusted to 40% by adding 80% sucrose. A sucrose gradient (5% to 30%) in 6 mL of Tris buffer without Triton X-100 was layered over the lysate and was centrifuged for 17 hours at 39 000 × g at 4°C in a Hitachi (Tokyo, Japan) RPS40T rotor. The 10 fractions were collected from the top of the gradient. DRM raft fraction (fraction no. 5) was analyzed by 2-dimentional (2-D) polyacrylamide gel electrophoresis (PAGE) using a Multiphor II Electrophoresis Unit with Immobiline DryStrip pH 3-10 (GE Healthcare, Fairfield, CT).
Fibrin clot retraction
PRP was activated with 1 U/mL thrombin and 5 mM CaCl2, and the reaction mixtures were left unstirred at 37°C in siliconized tubes. The extent of clot retraction was monitored by taking photographic images, and measurement of clot areas was performed using ImageJ (National Institutes of Health, Washington, DC). Clot retraction was expressed as a percentage by ratio between a clot area and that of the entire reaction mixture (area ratio). Results are presented as mean ± standard deviation (SD) of 3 independent experiments, and analyzed using an unpaired Student t test. The area ratio method was comparable to the weight ratio method.19 Plasma-free clot retraction assay was performed with 300 000/μL washed platelets, 2 mg/mL fibrinogen, 0.5 U/mL thrombin, 1 mM CaCl2, and 5 mM glucose in HEPES-Tyrode’s buffer (pH 7.4). They were solubilized by adding an equal volume of a 2× solubilization buffer (252 mM Tris pH 6.8, 40% glycerol, 4% sodium dodecyl sulfate (SDS), 8 M urea, 32.5 mM dithiothreitol, 2 mM Na3VO4, and 0.25% Bromophenol Blue) at 95°C for 60 minutes. Protein phosphorylation was analyzed by immunoblotting with a phosphospecific antibody to myosin light chain (Ser19), phosphotyrosine (PY20).
Results
SM-rich rafts colocalize with fibrin in the central region of adhered platelets induced by thrombin
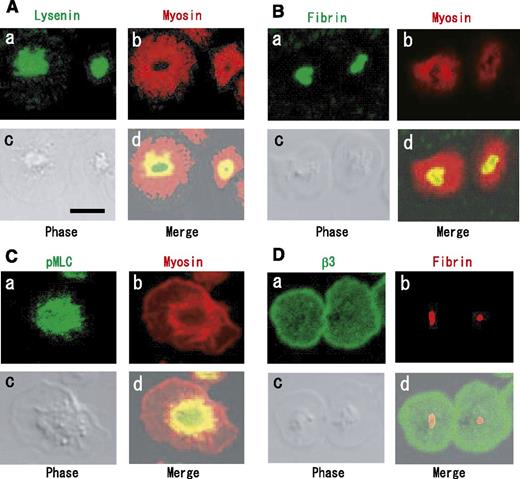
The perfringolysin O derivative BCθ binds selectively to a subpopulation of platelet DRM rafts (cholesterol-rich rafts), suggesting that a heterogeneous population of membrane rafts exists in platelets.20 SM is a major component of raft lipids in platelets.2,20 Lysenin is a specific probe of SM-rich rafts.21 A previous study showed that BCθ-positive cholesterol-rich rafts are uniformly distributed on the cell surface or at the leading edge of spreading platelets.22 Therefore, we investigated a subcellular distribution of SM-rich rafts in spreading platelets. Lysenin-positive SM-rich rafts were mainly localized in the central region of adhered platelets by thrombin (Figure 1Aa, red). In contrast, polyethylene glycol-derivatized cholesterol (PEG-Chol), a probe for cholesterol-rich rafts,21 was localized evenly on the membrane (Figure 1Ab, green). These observations suggest that SM-rich rafts are a subset of cholesterol-rich rafts at the plasma membrane of adhered platelets by thrombin (Figure 1Ad). To clarify the specific function of SM-rich rafts, we first focused on the distribution of fibrinogen and SM clusters. Previous immunocytochemical studies showed that fibrin(ogen) is present at the center of spreading platelets treated by thrombin.23 Lysenin-positive SM-rich rafts (Figure 1Ba, green) and fibrin(ogen) (Figure 1Bb, red) were mostly colocalized as a patch in double-staining the central region of adhered platelets by thrombin (Figure 1Bd). Superresolution photoactivated localization microscopy and direct stochastic optical reconstruction microscopy (PALM/dSTORM) also indicated the colocalization between fibrin(ogen) and SM-rich rafts (Figure 1C), suggesting that SM-rich rafts act as platforms of fibrin(ogen)-mediated outside-in signal, leading to clot retraction.
Immunocytochemical colocalization of fibrin(ogen) with sphingomyelin-rich rafts in central region of adhered platelets in the presence of thrombin. (A) (a) mCherry-lysenin-positive sphingomyelin (SM)-rich rafts (red), (b) fPEG-Chol-positive cholesterol-rich rafts (green), (c) phase-contrast cell morphology, (d) merged images of (a-c). (B) colocalization of fibrin (red) with green fluorescent protein–lysenin-positive SM-rich rafts (green). Scale bar, 5 μm. (C) Photoactivated localization microscopy and direct stochastic optical reconstruction microscopy (PALM/dSTORM) imaging of lysenin-positive SM-rich rafts (green) and fibrin(ogen) (red). The white line represents the shape of an adhered human platelet.
Immunocytochemical colocalization of fibrin(ogen) with sphingomyelin-rich rafts in central region of adhered platelets in the presence of thrombin. (A) (a) mCherry-lysenin-positive sphingomyelin (SM)-rich rafts (red), (b) fPEG-Chol-positive cholesterol-rich rafts (green), (c) phase-contrast cell morphology, (d) merged images of (a-c). (B) colocalization of fibrin (red) with green fluorescent protein–lysenin-positive SM-rich rafts (green). Scale bar, 5 μm. (C) Photoactivated localization microscopy and direct stochastic optical reconstruction microscopy (PALM/dSTORM) imaging of lysenin-positive SM-rich rafts (green) and fibrin(ogen) (red). The white line represents the shape of an adhered human platelet.
SM-depleted platelets exhibit delayed fibrin clot retraction
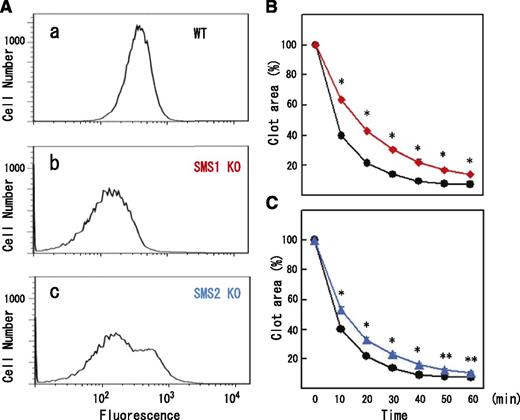
We investigated the possible involvement of SM-rich rafts in clot retraction. In mammals, 2 sphingomyelin synthase (SMS) isoforms (SMS1 and SMS2) have been shown to account for SM synthesis. SMS1 is responsible for the bulk of SM production. SMS1 or SMS2 deficiency exhibits a dysfunction of membrane rafts.24-26 Flow cytometry demonstrated that the remaining quantity of lysenin-positive SM-rich rafts in SMS1- and SMS2-deficient platelets was 43.5% (Figure 2Ab) and 73.8% (Figure 2Ac) of normal platelets, respectively. SMS1- or SMS2-deficient platelets aggregated in response to 1 U/mL thrombin (data not shown). However, clot retraction was significantly delayed in SMS1-deficient PRP (Figure 2B, red) and SMS2-deficient PRP (Figure 2C, blue). In proportion to quantity of SM-rich rafts, the average time of 50% retraction was prolonged by 2- and 1.3-fold in SMS1-deficient and SMS2-deficient PRPs, respectively. These results suggest that platelet SM-rich rafts are required for normal clot retraction.
Reduced SM-rich rafts and delayed clot retraction in SM synthase-deficient platelets. (A) Flow cytometry of SM-rich rafts of (a) wild-type, (b) SMS1-deficient, and (c) SMS2-deficient mouse platelets. (B) Time-dependent clot retraction of SMS1-deficient PRP (red diamonds) and wild-type PRP (black circles). The extent of clot retraction was assessed at the indicated times by measuring clot area. (C) Time-dependent clot retraction of SMS2-deficient PRP (blue triangles) and wild-type PRP (black circles). Data are presented as the means plus or minus SD of quadruplicates. *Statistically significant difference (P < .001). **Statistically significant difference (P < .005).
Reduced SM-rich rafts and delayed clot retraction in SM synthase-deficient platelets. (A) Flow cytometry of SM-rich rafts of (a) wild-type, (b) SMS1-deficient, and (c) SMS2-deficient mouse platelets. (B) Time-dependent clot retraction of SMS1-deficient PRP (red diamonds) and wild-type PRP (black circles). The extent of clot retraction was assessed at the indicated times by measuring clot area. (C) Time-dependent clot retraction of SMS2-deficient PRP (blue triangles) and wild-type PRP (black circles). Data are presented as the means plus or minus SD of quadruplicates. *Statistically significant difference (P < .001). **Statistically significant difference (P < .005).
Fibrin translocation to platelet DRM raft on thrombin-stimulation
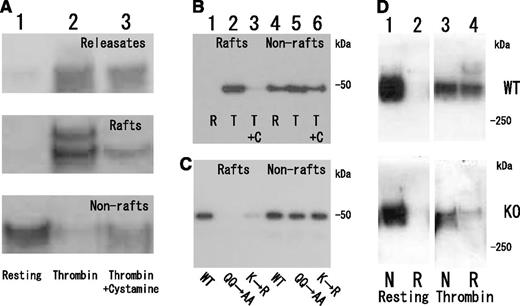
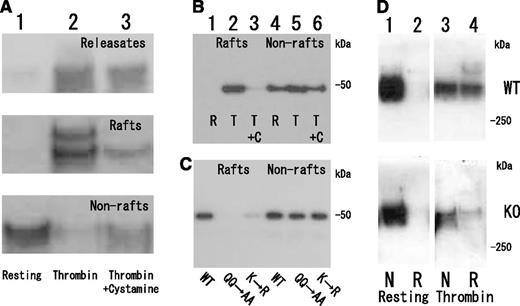
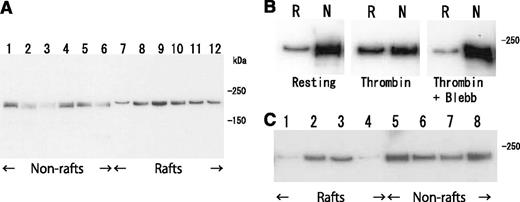
To confirm the association of fibrin(ogen) with platelet membrane rafts, we attempted to identify DRM-raft-specific proteins from activated platelets. We isolated the DRM raft fraction of thrombin-stimulated platelets by sucrose density gradient centrifugation. Several specific proteins (Figure 3Aa, right panel) were detected in the DRM fraction (lane 5) of thrombin-stimulated platelets. Three specific proteins of 65, 50, and 47 kDa were isolated by 2D-PAGE from the DRM fraction (Figure 3Bb, arrows). By mass spectrometry, they were identified as fibrins α, β, and γ, respectively, because no sequences of fibrinopeptide A (Ala1-Arg16) or fibrinopeptide B (PyroGlu1-Arg14) were detected (supplemental Figure 1, found on the Blood Web site). These results were supported by immunoblotting with an antifibrinogen antibody (Figure 3C-D) and an antifibrin antibody (Figure 3E). In resting platelets, fibrinogens Aα (67 kDa), Bβ (52 kDa), and γ (47 kDa) were detected in the nonraft fraction (Figure 3C, lanes 7-10). In contrast, fibrins α(65 kDa), β(50 kDa), and γ(47 kDa) were detected in the DRM fraction of thrombin-stimulated platelets (Figure 3C). The fibrinogen translocation to the DRM fraction was not detected in collagen- or ionophore-stimulated platelets (data not shown). Thrombin caused the dose-dependent translocation of not only 340 kDa fibrin, [αβγ]2 (nonreduced condition), but also the fibrin polymer (Figure 3Dc, asterisk) to the DRM fraction (supplemental Figure 2). Thrombin caused the rapid translocation of fibrin to the DRM fraction within 30 seconds (Figure 3D), and the time-dependent conversion of the fibrin monomer to the fibrin polymer (Figure 3Dc, asterisk). Fibrin was detected in only the DRM fraction of thrombin-stimulated platelets (Figure 3E, lane 3). Moreover, immunoelectron microscopy revealed that the fibrin fiber associated with the DRM (∼300 nm) of thrombin-stimulated platelets (Figure 3F). The immunogold-positive fibrin fiber was directly associated with the surface of DRM (Figure 3G). These data suggest that fibrinogen [AαBβγ]2 is released from α-granules of platelets, converted to fibrin [αβγ]2 by the cleavage of fibrinopeptides A and B with thrombin, and translocated to membrane rafts of thrombin-stimulated platelets.
Fibrin translocation to DRM raft fraction of human platelets by thrombin stimulation. (A) Sucrose density gradient analysis of proteins in washed human platelets. Resting platelets (left panel) and platelets stimulated for 3 minutes with 1 U/mL thrombin (right panel) were lysed in Triton X-100, and sucrose gradients (5% to 30%) were formed over them. Ten fractions were collected from top to bottom after centrifugation. The proteins were subjected to SDS-PAGE and stained with Coomassie brilliant blue (a). Immunoblotting of raft marker proteins with anti-CD36 antibody (b) and anti-Lyn antibody (c). A 40-kDa major protein was identified as actin by immunoblotting. (B) Two-dimensional PAGE analysis of DRM raft fraction (fraction 5) in resting platelets (a) and thrombin-stimulated platelets (b). The asterisk indicates actin. The arrowhead indicates integrin αIIb. (C) Immunoblotting with antifibrinogen antibody of panel A. (D) Time-dependent translocation of fibrin(ogen) by thrombin for 0 seconds (lane 1), 30 seconds (lane 2), 1 minute (lane 3), 5 minutes (lane 4), and 15 minutes (lane 5). Immunoblotting under nonreduced condition by antifibrinogen antibody of releasates (a), nonraft fraction (b), and DRM raft fraction (c). The asterisk indicates the fibrin polymer. (E) Immunoblotting with antifibrin specific antibody of DRM raft fraction (lanes 1 and 3), nonraft fraction (lanes 2 and 4) of resting platelets (lanes 1 and 2), and thrombin-stimulated platelets at 3 minutes (lanes 3 and 4). (F) Association of fibrin fiber with DRM of thrombin-stimulated platelets in immunoelectron microscopy. The DRM of thrombin-stimulated human platelets was incubated with an antifibrinogen antibody and then anti-IgG-labeled with colloidal gold. Scale bar, 200 nm. (G) Magnification of gold-attached area on DRM in panel F. The gold-positive fibrin fiber directly associated with the surface of DRM.
Fibrin translocation to DRM raft fraction of human platelets by thrombin stimulation. (A) Sucrose density gradient analysis of proteins in washed human platelets. Resting platelets (left panel) and platelets stimulated for 3 minutes with 1 U/mL thrombin (right panel) were lysed in Triton X-100, and sucrose gradients (5% to 30%) were formed over them. Ten fractions were collected from top to bottom after centrifugation. The proteins were subjected to SDS-PAGE and stained with Coomassie brilliant blue (a). Immunoblotting of raft marker proteins with anti-CD36 antibody (b) and anti-Lyn antibody (c). A 40-kDa major protein was identified as actin by immunoblotting. (B) Two-dimensional PAGE analysis of DRM raft fraction (fraction 5) in resting platelets (a) and thrombin-stimulated platelets (b). The asterisk indicates actin. The arrowhead indicates integrin αIIb. (C) Immunoblotting with antifibrinogen antibody of panel A. (D) Time-dependent translocation of fibrin(ogen) by thrombin for 0 seconds (lane 1), 30 seconds (lane 2), 1 minute (lane 3), 5 minutes (lane 4), and 15 minutes (lane 5). Immunoblotting under nonreduced condition by antifibrinogen antibody of releasates (a), nonraft fraction (b), and DRM raft fraction (c). The asterisk indicates the fibrin polymer. (E) Immunoblotting with antifibrin specific antibody of DRM raft fraction (lanes 1 and 3), nonraft fraction (lanes 2 and 4) of resting platelets (lanes 1 and 2), and thrombin-stimulated platelets at 3 minutes (lanes 3 and 4). (F) Association of fibrin fiber with DRM of thrombin-stimulated platelets in immunoelectron microscopy. The DRM of thrombin-stimulated human platelets was incubated with an antifibrinogen antibody and then anti-IgG-labeled with colloidal gold. Scale bar, 200 nm. (G) Magnification of gold-attached area on DRM in panel F. The gold-positive fibrin fiber directly associated with the surface of DRM.
Integrin αIIbβ3 is required for fibrin translocation to DRM rafts of thrombin-stimulated platelets
To investigate the role of integrin αIIbβ3 in fibrin translocation to the DRM fraction, we used platelets from a type I Glanzmann’s thrombasthenia patient, a disease characterized by the absence of αIIbβ3, a fibrin receptor.27 In αIIbβ3-deficient platelets, a small amount of fibrinogen is detectable by western blotting, and almost no fibrin is translocated to the DRM fraction even with thrombin stimulation (supplemental Figure 3Aa, upper panel). This result suggests that αIIbβ3 is required for the fibrin translocation to the DRM fraction. The HHLGGAKQAGDV sequence at the carboxyl termini of the γ-chains of human fibrin provides recognition sites for the binding of fibrin protofibril to αIIbβ3 on activated human platelets. The dodecapeptide, γ 400-411, inhibited the fibrin translocation to the DRM fraction (supplemental Figure 3B). To confirm the possible involvement of γ-chains in fibrin translocation to the DRM fraction, we used the fibrinogen γ-chain C-terminal (residues 144-411) fusion protein with a human growth hormone (supplemental Figure 3C). The fibrinogen γ-chain C-terminal (144-411) fusion protein, but not the C-terminal deletion mutant (144-399) in the fibrinogen γ-chain, bound to the DRM of thrombin-stimulated platelets (supplemental Figure 3Cd, lane R). These results suggest that the carboxyl termini of human fibrin γ-chains are involved in fibrin translocation to the DRM fraction. We observed that a small amount of αIIbβ3 is present in DRM rafts, and a large amount of αIIbβ3 is present in the nonraft fraction (supplemental Figure 4). These data suggest that αIIbβ3 is necessary for the initial binding to fibrinogen on a thrombin-activated platelet surface, but not sufficient for the fibrin translocation to DRM rafts.
Requirement of FXIII crosslinking activity in fibrin translocation to DRM raft of thrombin-stimulated platelets
We found that the thrombin receptor-activating peptide (TRAP) did not cause fibrinogen translocation to the DRM fraction (supplemental Figure 5), suggesting that the protease activity of thrombin is necessary for fibrin translocation to the DRM fraction. To investigate the possible involvement of FXIII in that fibrin translocation to DRM fraction, we investigated whether the fibrin translocated to the DRM fraction is a substrate for FXIII. 5-(Biotinamido) pentylamine (5BAPA)–incorporated proteins induced by transglutaminase with molecular weights of 65 and 47 kDa are predominantly present in the DRM of platelets stimulated with thrombin for 15 minutes (supplemental Figure 6A, lane 4), but not TRAP or collagen. The 5BAPA-incorporated 65-kDa and 47-kDa proteins (supplemental Figure 6B, lane 3) comigrated with fibrin in the DRM fraction (supplemental Figure 6B, lane 7), suggesting that they are fibrin α- and γ-chains, respectively. Another major 5BAPA-incorporated 95-kDa protein was identified as a cytosolic protein (data not shown). Cell-surface 5BAPA incorporation was analyzed by flow cytometry (supplemental Figure 6C). 5BAPA was incorporated to the surface of thrombin-stimulated human platelets (supplemental Figure 6Cb). Pretreatment with cystamine, a transglutaminase inhibitor, just before thrombin stimulation completely inhibited the 5BAPA incorporation (supplemental Figure 6Cc). 5BAPA was not incorporated to the surface of TRAP-stimulated human platelets (supplemental Figure 6Cd). Fibrin γ 400-411, the HHLGGAKQAGDV dodecapeptide, inhibited the 5BAPA incorporation to fibrin α- and γ-chains in thrombin-activated platelets (supplemental Figure 6D, lane 3). Confocal imaging showed that cell-surface 5BAPA incorporation to fibrin was colocalized with lysenin-positive SM-rich rafts (supplemental Figure 6E). Furthermore, 5BAPA incorporation was impaired in FXIII A subunit-deficient mouse platelets (supplemental Figure 6F, lane 2) but not in tissue transglutaminase-deficient mouse platelets (supplemental Figure 6F, lane 3). A substantial amount of FXIII was identified in the raft fraction of thrombin-stimulated platelets (supplemental Figure 7) but not in resting platelets, suggesting that platelet-derived FXIII was expressed on the surface. These results suggest that fibrin is a specific substrate for FXIII in SM-rich rafts on thrombin-stimulated platelet surface. However, it is a question whether FXIII is involved in fibrin translocation to rafts.
Next we examined the effect of cystamine on fibrin translocation to DRM fraction of thrombin-stimulated platelets (Figure 4A). Pretreatment with cystamine just before thrombin stimulation inhibited fibrin translocation to DRM fraction and polymer formation (Figure 4A, lane 3), binding the fibrinogen γ-chain C-terminal fragment (144-411) fusion protein to DRM fraction (Figure 4B, lane 3). Furthermore, the fibrinogen γ-chain C-terminal fragment (144-411) fusion protein mutants of FXIII-crosslinking site Q398Q399 or K406 hardly bound to the DRM (Figure 4C, lanes 2 and 3) but still bound to the nonraft fraction of thrombin-stimulated platelets (Figure 4C, lanes 5 and 6). Finally, the thrombin-induced fibrin translocation to DRM fraction was impaired in FXIII A subunit-deficient platelets (Figure 4D, lane 4). These results suggest that transglutaminase activity of FXIII is required for the fibrin translocation to DRM fraction of thrombin-stimulated platelets. A precise role of FXIII in fibrin translocation to rafts remains to be explored.
Requirement of FXIII transglutaminase for fibrin translocation to DRM raft fraction of thrombin-stimulated platelets. (A) Inhibition of fibrin translocation to DRM raft fraction by transglutaminase inhibitor cystamine. Immunoblotting under nonreduced condition by antifibrinogen antibody of releasates (upper panel), DRM raft fraction (middle panel), and nonraft fraction (lower panel) in resting (lane 1) and thrombin-stimulated human platelets in the absence (lane 2) or presence (lane 3) of cystamine. Total amount of fibrin in releasate, raft, and nonraft fraction of thrombin-stimulated human platelets in the presence of cystamine (lane 3) is comparable to that of fibrinogen in nonraft fraction of resting platelets (lane 1). (B) Transglutaminase-dependent association of fibrinogen γ-chain fusion protein with DRM raft fraction in thrombin-stimulated platelets. Immunoblotting of DRM raft fraction (lanes 1, 2, and 3) and nonraft fraction (lanes 4, 5, and 6) in resting (lanes 1 and 4) and thrombin-stimulated platelets in the absence (lanes 2 and 5) or presence (lanes 3 and 6) of cystamine. (C) FXIII-dependent association of fibrinogen γ-chain fusion protein with DRM raft fraction in thrombin-stimulated platelets. Immunoblotting by anti-His tag antibody of DRM raft fraction (lanes 1, 2, and 3) and nonraft fraction (lanes 4, 5, and 6) using fibrinogen γ-chain fusion protein (lanes 1 and 4), fibrinogen γ-chain fusion protein mutants of FXIII-crosslinking site Q398Q399 (lanes 2 and 5), or K406 (lanes 3 and 6). (D) Impaired fibrin translocation to DRM raft fraction of thrombin-stimulated platelets in FXIIIA subunit-deficient mice. Immunoblotting under nonreduced condition by antifibrinogen antibody of DRM raft fraction (lanes 2 and 4) and nonraft fraction (lanes 1 and 3) in resting platelets (lanes 1 and 2) and thrombin-stimulated platelets (lanes 3 and 4) of wild-type mice (upper panel) and FXIIIA-deficient mice (lower panel).
Requirement of FXIII transglutaminase for fibrin translocation to DRM raft fraction of thrombin-stimulated platelets. (A) Inhibition of fibrin translocation to DRM raft fraction by transglutaminase inhibitor cystamine. Immunoblotting under nonreduced condition by antifibrinogen antibody of releasates (upper panel), DRM raft fraction (middle panel), and nonraft fraction (lower panel) in resting (lane 1) and thrombin-stimulated human platelets in the absence (lane 2) or presence (lane 3) of cystamine. Total amount of fibrin in releasate, raft, and nonraft fraction of thrombin-stimulated human platelets in the presence of cystamine (lane 3) is comparable to that of fibrinogen in nonraft fraction of resting platelets (lane 1). (B) Transglutaminase-dependent association of fibrinogen γ-chain fusion protein with DRM raft fraction in thrombin-stimulated platelets. Immunoblotting of DRM raft fraction (lanes 1, 2, and 3) and nonraft fraction (lanes 4, 5, and 6) in resting (lanes 1 and 4) and thrombin-stimulated platelets in the absence (lanes 2 and 5) or presence (lanes 3 and 6) of cystamine. (C) FXIII-dependent association of fibrinogen γ-chain fusion protein with DRM raft fraction in thrombin-stimulated platelets. Immunoblotting by anti-His tag antibody of DRM raft fraction (lanes 1, 2, and 3) and nonraft fraction (lanes 4, 5, and 6) using fibrinogen γ-chain fusion protein (lanes 1 and 4), fibrinogen γ-chain fusion protein mutants of FXIII-crosslinking site Q398Q399 (lanes 2 and 5), or K406 (lanes 3 and 6). (D) Impaired fibrin translocation to DRM raft fraction of thrombin-stimulated platelets in FXIIIA subunit-deficient mice. Immunoblotting under nonreduced condition by antifibrinogen antibody of DRM raft fraction (lanes 2 and 4) and nonraft fraction (lanes 1 and 3) in resting platelets (lanes 1 and 2) and thrombin-stimulated platelets (lanes 3 and 4) of wild-type mice (upper panel) and FXIIIA-deficient mice (lower panel).
Involvement of fibrin and myosin translocation to SM-rich rafts in clot retraction
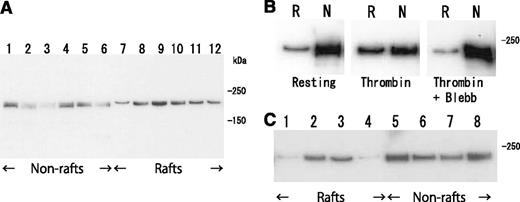
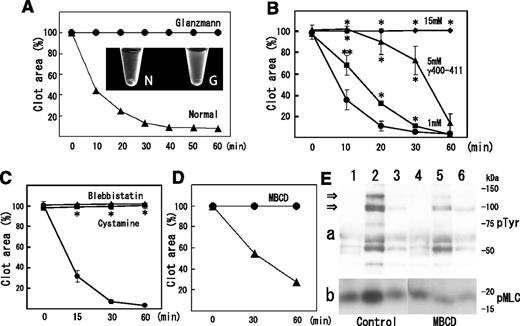
Clot retraction is mediated by the interaction of the fibrin fiber and actomyosin via the integrin αIIbβ3, together with the activation of the platelet contractile apparatus. Consistent with this idea, clot retraction was impaired in type I Glanzmann’s thrombasthenia (Figure 5A). Therefore, we investigated the distribution of myosin in thrombin-stimulated platelets on sucrose density gradient. Thrombin caused a transient (within 5 minutes) increase in myosin level in the DRM fraction (from 3.4% to 26.5%) and a transient decrease in the nonraft fraction (from 51.0% to 26.8%) (Figure 6A). The increase in myosin level in the DRM fraction was inhibited by blebbistatin, a myosin adenosine triphosphatase (ATPase) inhibitor (Figure 6B). Furthermore, TRAP also caused an increase in myosin in the DRM fraction (Figure 6C, lane 3). These results suggest that the activation of thrombin receptors induced the ATPase activity-dependent translocation of a significant amount of myosin to the DRM raft fraction.
Implication of fibrin and myosin translocation to DRM raft fraction in clot retraction. (A) Impairment of clot retraction in type I Glanzmann’s thrombasthenia. Time-dependent clot retraction of Glanzmann’s thrombasthenia PRP (circles) and normal PRP (triangles). PRP was incubated with 1 U/mL thrombin and 2 mM CaCl2. The extent of clot retraction was assessed at the indicated times by measuring clot area. Photo image of clot retraction assay after 120 minutes: N, normal PRP; G, Glanzmann’s thrombasthenia PRP (inset). (B) Inhibition of clot retraction by fibrinogen γ-chain 400-411 dodecapeptide. PRP was incubated with 1 U/mL thrombin, 2 mM CaCl2 with no peptide (circles), and 1 mM (squares), 5 mM (triangles), and 15 mM (diamonds) fibrinogen γ-chain 400-411 dodecapeptide. Data are presented as means plus or minus SD of quadruplicates. *Statistically significant difference (P < .01). **Statistically significant difference (P < .05). (C) Inhibition of clot retraction by cystamine or blebbistatin. PRP was incubated with 1 U/mL thrombin, 2 mM CaCl2 with buffer (circles), 10 mM cystamine (squares), or 100 μM blebbistatin (triangles). Data are presented as means plus or minus SD of triplicates. *Statistically significant difference (P < .001). (D) Inhibition of clot retraction by raft disruption by MBCD. Mixture of washed platelets and purified fibrinogen was incubated with 1U/ml thrombin in the presence (circle) and absence (triangle) of 2% MBCD. The photograph was taken after 30 and 60 minutes. (E) Raft disruption by MBCD inhibits transient increase in degree of tyrosine phosphorylation (a) and phosphorylation of myosin light chain at serine residue 19 (b). A mixture of washed platelets and purified fibrinogen was incubated with 1 U/mL thrombin for 0 minutes (lanes 1 and 4), 5 minutes (lanes 2 and 5), and 60 minutes (lanes 3 and 6) in the presence (lanes 4-6) and absence (lanes 1-3) of 2% MBCD. Arrows indicate the tyrosine phosphorylation of 125-kDa and 100-kDa proteins.
Implication of fibrin and myosin translocation to DRM raft fraction in clot retraction. (A) Impairment of clot retraction in type I Glanzmann’s thrombasthenia. Time-dependent clot retraction of Glanzmann’s thrombasthenia PRP (circles) and normal PRP (triangles). PRP was incubated with 1 U/mL thrombin and 2 mM CaCl2. The extent of clot retraction was assessed at the indicated times by measuring clot area. Photo image of clot retraction assay after 120 minutes: N, normal PRP; G, Glanzmann’s thrombasthenia PRP (inset). (B) Inhibition of clot retraction by fibrinogen γ-chain 400-411 dodecapeptide. PRP was incubated with 1 U/mL thrombin, 2 mM CaCl2 with no peptide (circles), and 1 mM (squares), 5 mM (triangles), and 15 mM (diamonds) fibrinogen γ-chain 400-411 dodecapeptide. Data are presented as means plus or minus SD of quadruplicates. *Statistically significant difference (P < .01). **Statistically significant difference (P < .05). (C) Inhibition of clot retraction by cystamine or blebbistatin. PRP was incubated with 1 U/mL thrombin, 2 mM CaCl2 with buffer (circles), 10 mM cystamine (squares), or 100 μM blebbistatin (triangles). Data are presented as means plus or minus SD of triplicates. *Statistically significant difference (P < .001). (D) Inhibition of clot retraction by raft disruption by MBCD. Mixture of washed platelets and purified fibrinogen was incubated with 1U/ml thrombin in the presence (circle) and absence (triangle) of 2% MBCD. The photograph was taken after 30 and 60 minutes. (E) Raft disruption by MBCD inhibits transient increase in degree of tyrosine phosphorylation (a) and phosphorylation of myosin light chain at serine residue 19 (b). A mixture of washed platelets and purified fibrinogen was incubated with 1 U/mL thrombin for 0 minutes (lanes 1 and 4), 5 minutes (lanes 2 and 5), and 60 minutes (lanes 3 and 6) in the presence (lanes 4-6) and absence (lanes 1-3) of 2% MBCD. Arrows indicate the tyrosine phosphorylation of 125-kDa and 100-kDa proteins.
Transient translocation of myosin to DRM raft fraction. (A) Time-dependent translocation of myosin from nonraft fraction (lanes 1-6) to DRM raft fraction (lanes 7-12) in platelets by thrombin stimulation for 0 seconds (lanes 1 and 7), 30 seconds (lanes 2 and 8), 1 minute (lanes 3 and 9), 5 minutes (lanes 4 and 10), 15 minutes (lanes 5 and 11), and 60 minutes (lanes 6 and 12). Immunoblotting was performed with a myosin heavy-chain antibody. (B) ATPase-activity-dependent translocation of myosin to DRM raft fraction. Immunoblotting with antimyosin antibody of DRM raft fraction (lane R) and nonraft fraction (lane N) in resting platelets (left panel) and thrombin-stimulated platelets in the absence (middle panel) or presence (right panel) of 100 μM myosin ATPase inhibitor blebbistatin. (C) PAR-dependent translocation of myosin to DRM raft fraction. Immunoblotting with antimyosin antibody of DRM raft fraction (lanes 1-4) and nonraft fraction (lanes 5-8) in resting platelets (lanes 1 and 5) and platelets stimulated with 0.2 U/mL thrombin (lanes 2 and 6), 25 μM TRAP (lanes 3 and 7), and 20 μM adenosine 5′-diphosphate (lanes 4 and 8) for 3 minutes.
Transient translocation of myosin to DRM raft fraction. (A) Time-dependent translocation of myosin from nonraft fraction (lanes 1-6) to DRM raft fraction (lanes 7-12) in platelets by thrombin stimulation for 0 seconds (lanes 1 and 7), 30 seconds (lanes 2 and 8), 1 minute (lanes 3 and 9), 5 minutes (lanes 4 and 10), 15 minutes (lanes 5 and 11), and 60 minutes (lanes 6 and 12). Immunoblotting was performed with a myosin heavy-chain antibody. (B) ATPase-activity-dependent translocation of myosin to DRM raft fraction. Immunoblotting with antimyosin antibody of DRM raft fraction (lane R) and nonraft fraction (lane N) in resting platelets (left panel) and thrombin-stimulated platelets in the absence (middle panel) or presence (right panel) of 100 μM myosin ATPase inhibitor blebbistatin. (C) PAR-dependent translocation of myosin to DRM raft fraction. Immunoblotting with antimyosin antibody of DRM raft fraction (lanes 1-4) and nonraft fraction (lanes 5-8) in resting platelets (lanes 1 and 5) and platelets stimulated with 0.2 U/mL thrombin (lanes 2 and 6), 25 μM TRAP (lanes 3 and 7), and 20 μM adenosine 5′-diphosphate (lanes 4 and 8) for 3 minutes.
Next, we confirmed the involvement of fibrin and myosin translocation to the DRM fraction in clot retraction. The γ 400-411 dodecapeptide inhibited not only fibrin translocation to the DRM fraction (supplemental Figure 3B) but also clot retraction in a dose-dependent manner (Figure 5B). Furthermore, cytamine or blebbistatin inhibited not only fibrin (Figure 4A) or myosin (Figure 6B) translocation to the DRM fraction but also clot retraction (Figure 5C). These results suggest that the translocation of fibrin and myosin to SM-rich rafts is involved in clot retraction. We investigated the effect of cholesterol depletion with methyl-β-cyclodextrin (MBCD) on the disruption of SM-rich rafts, the tyrosine phosphorylation of cellular proteins, and the phosphorylation at serine 19 of the myosin light chain (which increases actin-activated myosin ATPase activity) using a plasma-free clot retraction system with washed platelets in purified fibrinogen. Flow cytometry demonstrated that both lysenin-positive SM-rich and BCθ-positive cholesterol-rich rafts were prerequisitely present on resting platelets, that the amounts of both rafts were unchanged with thrombin stimulation (supplemental Figure 9), and that treatment with MBCD reduced the amount of SM-rich rafts to 28.6%, suggesting that SM-rich rafts are cholesterol-dependent (supplemental Figure 9A). Pretreatment with MBCD inhibited clot retraction (Figure 5D). Thrombin caused a rapid (within 5 minutes) tyrosine phosphorylation of the 125-kDa and 100-kDa proteins, and such phosphorylation returned to the control level at 60 minutes (Figure 5Ea). A previous study demonstrated a close relationship between clot retraction and a transient tyrosine phosphorylation.28 The level of phosphorylation of the myosin light chain (MLC) at Ser19 was increased by thrombin treatment of 5 minutes, which returned to control level at 60 minutes (Figure 5Eb). MBCD inhibited the transient phosphorylation of the 125-kDa and 100-kDa proteins and MLC. These results suggested that SM-rich rafts act as platforms of fibrin-mediated outside-in signal, leading to clot retraction.
Colocalization of activated myosin with fibrin in SM-rich rafts of adhered platelets by thrombin
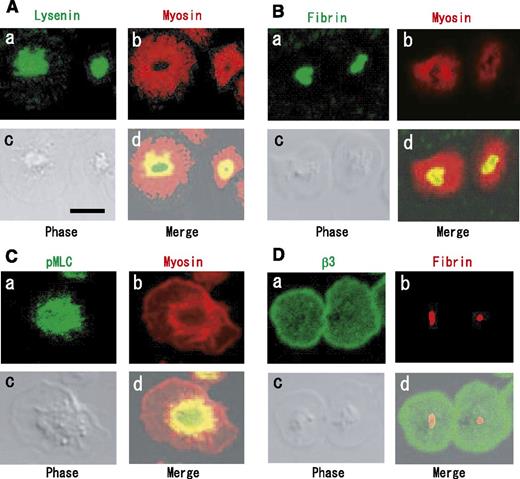
We investigated the distributions of fibrin, myosin, αIIbβ3, and SM-rich rafts by immunofluorescence analysis. Myosin was localized around a central region of spreading platelets (Figure 7A, red). This is consistent with previous findings of a myosin-containing spherical structure surrounding a central zone in spreading platelets.29 A subpopulation of myosin was shown to colocalize in part with SM-rich rafts (Figure 7Ad) and fibrin (Figure 7Bd) inside the myosin-containing spherical structure. Interestingly, Ser19-phosphorylated MLC (activated myosin) was mostly localized in the central region (Figure 7Ca) and colocalized in part with myosin (Figure 7Cd). These data suggest that myosin is activated beneath SM-rich rafts and that activated myosin is colocalized with fibrin. In contrast to fibrin, integrin β3 was localized evenly on the membrane (Figure 7Da), suggesting the selective association of fibrin with a subpopulation of αIIbβ3 in SM-rich rafts. In conclusion, we propose that fibrin is translocated to SM-rich rafts in combination with FXIII and that platelet SM-rich rafts act as platforms where extracellular fibrin and the intracellular actomyosin efficiently join via integrin αIIbβ3 to promote clot retraction.
Immunocytochemical colocalization of myosin and Ser19-phosphorylated myosin light chain with fibrin on SM-rich rafts. (A) Localization of SM-rich rafts (green) and myosin (red). Scale bar, 5 μm. (B) Localization of fibrin (green) and myosin (red). (C) Localization of Ser19-phosphorylated MLC (green) and myosin (red). (D) Integrin β3 (green) and fibrin (red).
Immunocytochemical colocalization of myosin and Ser19-phosphorylated myosin light chain with fibrin on SM-rich rafts. (A) Localization of SM-rich rafts (green) and myosin (red). Scale bar, 5 μm. (B) Localization of fibrin (green) and myosin (red). (C) Localization of Ser19-phosphorylated MLC (green) and myosin (red). (D) Integrin β3 (green) and fibrin (red).
Discussion
In this study, we demonstrated that fibrin translocation in platelet DRM rafts is a specific event in platelet activation by thrombin. This is because (1) fibrin translocation did not occur in type I Glanzmann’s thrombasthenic platelets, (2) the fibrinogen γ-chain dodecapeptide (residues 400-411) or cystamine significantly inhibited fibrin translocation, (3) the fibrinogen γ-chain C-terminal fusion protein, which has no ability to form fibers, also bound to the DRM of thrombin-stimulated platelets, and (4) immunocytochemical study showed the colocalization of fibrin with raft markers. In PALM image, fibrin and SM-rich rafts were not exactly colocalized (Figure 1C). When we stained SM with Dronpa-lysenin and Alexa 647-lysenin simultaneously, SM-rich domains stained with Dronpa-lysenin and Alexa 647-labeled lysenin were adjacent to one another, as described previously.30 Therefore, this result implies that the problem is caused by the chromatic aberration and suggests that fibrin and SM-rich rafts are indeed in very close proximity.
Membrane rafts are spatially and functionally heterogenous in the cell membrane.10,11 In a migrating T cell, a ganglioside GM3-rich raft containing a chemokine receptor is present at the leading edge, whereas a GM1-rich raft containing integrin β1 is present at the uropod.31 In Jurkat T cells, SM-rich rafts are spatially distinct from GM1-rich rafts.32 SMS1 deficiency impairs T-cell receptor signaling through the dysfunction of membrane rafts24 and the raft-dependent apoptosis of lymphoma.25 SMS2 deficiency impairs receptor clustering to membrane rafts.26 SM-rich rafts are required for cytokinesis.30 In this study, we demonstrated that SM-rich rafts were colocalized with fibrin in the central region of adhered platelets by thrombin. SMS1- and SMS2-deficient platelets exhibit a reduced fraction of SM-rich rafts and delayed fibrin clot retraction, suggesting that SM-rich rafts are involved in clot retraction.
What is the mechanism of SM-rich raft-mediated clot retraction? The retraction results from the actomyosin contraction of a platelet pseudopod attached to a fibrin strand.33 The thrombin-induced phosphorylation of tyrosines 747 and 759 in an integrin β3 results in the linkage of integrin αIIbβ3 to myosin to mediate the transmission of force to the fibrin clot during clot retraction.34 Activated αIIbβ3 connects with actin through talin and vinculin. In this study, we demonstrated the translocation of fibrin and myosin to the platelet DRM raft fraction and the microscopic colocalization of fibrin and myosin with SM-rich rafts in thrombin-stimulated platelets. We observed that the fibrinogen γ-chain 400-411 dodecapeptide and blebbistatin inhibited not only the fibrin and myosin translocation to the DRM fraction, respectively, but also clot retraction. Furthermore, we also demonstrated that αIIbβ3 is required for fibrin translocation to the DRM raft fraction and that αIIbβ3 (Figure 3B, arrowhead, and supplemental Figure 4) and actin (Figure 3A, 40 kDa protein, and Figure 3B, asterisk) are partially present in the platelet DRM raft fraction. These observations suggest that platelet SM-rich rafts, restricted areas on the platelet membrane, act as platforms where extracellular fibrin and the intracellular actomyosin system efficiently join via αIIbβ3 for clot retraction. To support this idea, a previous study demonstrated that DRM rafts specifically associate with the actin cytoskeleton upon platelet activation in an αIIbβ3-dependent manner.35
Clot retraction is regulated through multiple signaling pathways. Thrombin receptors, coupled to Gq and G13 heterotrimeric G proteins, can regulate clot retraction through the activation of phospholipase C (PLC)β and Rho kinase, which activates MLC kinase and inhibits MLC phosphatase, respectively.36 Integrin αIIbβ3 outside-in signal is also required for optimal clot retraction. The engagement of αIIbβ3 is known to activate the c-src and tyrosine phosphorylation of PLCγ2.37 The src-dependent activation of PLCγ2 induces calcium mobilization, MLC kinase activation, MLC phosphorylation, and actomyosin contraction. The src-dependent actomyosin contraction mediates clot retraction.36 During clot retraction, the level of tyrosine phosphorylation of the 125-kDa and 100-kDa proteins increased to its peak after 5 minutes and returned to its control level after 60 minutes, in parallel to retraction.28 Thus, clot retraction is associated with a transient tyrosine phosphorylation. In this study, MBCD treatment reduced the fraction of SM-rich rafts and inhibited the transient increase in the tyrosine phosphorylation of the 125-kDa and 100-kDa proteins and the transient phosphorylation of MLC at Ser 19. Immunofluorescence study showed the colocalization of Ser19-phosphorylated MLC with fibrin in SM-rich rafts of thrombin-stimulated platelets. Therefore, fibrin translocation to SM-rich rafts by thrombin may help to cluster αIIbβ3 and enhance outside-in signaling pathway, contributing to efficient clot retraction.
We have considerable evidence to support that FXIII is required for the fibrin translocation to SM-rich rafts, leading to clot retraction: (i) cystamine inhibited the 5BAPA incorporation to fibrin, translocation of fibrin, and the fibrinogen γ-chain C-terminal (144-411) fusion protein to membrane rafts and clot retraction; (ii) the fibrinogen γ-chain C-terminal (144-411) fusion protein translocated to platelet rafts on thrombin activation, but its mutant that was replaced by A398A399 at FXIII-crosslinking sites (Q398Q399) was inhibited; and finally (iii) fibrin translocation to membrane rafts and clot retraction were impaired in FXIII-A-subunit-deficient mouse platelets.19 Furthermore, we found that batroxobin, a thrombin-like enzyme (0.25 μg/mL), caused fibrin clot formation but not clot retraction (data not shown). FXIII is activated by the cleavage of FXIIIA with thrombin but not batroxobin. Therefore, this result supports that transglutaminase activity of FXIII is required for clot retraction.
In summary, our results suggest that translocation of fibrin and myosin into platelet SM-rich rafts via integrin αIIbβ3 is an important process for clot retraction signaling. We propose a working hypothesis that the FXIII-dependent fibrin-αIIbβ3-myosin axis in platelet SM-rich rafts is required for efficient clot retraction.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are indebted to Dr J. Takagi (Osaka University) for providing expression vector of the fibrinogen γ-chain C-terminal (residues 144-411) fusion protein. We are grateful to Dr Y. Tomiyama (Osaka University) for diagnosis of Glanzmann’s thrombasthenia. We thank Dr Y. Saito (Tokyo Institute of Technology), Dr Y. Hirabayashi (RIKEN), and Dr K. Hitomi (Nagoya University) for helpful discussion.
This work was supported by Japanese Society for the Promotion of Science KAKENHI grant numbers 23570182 (to K.K.) and 22591058 (to A.I.) for scientific research (C) and by SENSHIN Medical Research Foundation (to K.K.).
Authorship
Contribution: K.K. designed and performed research, analyzed data, performed statistical analysis, and wrote the paper; M.K., T.M., K.I., N.S.-S., H.S., I.K., M. Shimonaka, and M. Abe performed research; M. Arai, Y.O.-I., S.K., T.K., T.O., and M. Souri contributed analytical tools; and A.I. and N.Y. designed research, contributed analytical tools, analyzed data, wrote the paper, and worked as senior authors.
Conflict-of-interest disclosure: M. Arai is an employee of Novo Nordisk Pharma, Ltd. The remaining authors declare no competing financial interest.
Correspondence: Kohji Kasahara, Laboratory of Biomembrane, Tokyo Metropolitan Institute of Medical Science, Kamikitazawa, Setagaya-ku, Tokyo 156-8506, Japan; e-mail: kasahara-kj@igakuken.or.jp.