Key Points
Heme activates complement alternative pathway in serum and on endothelial cell surfaces.
Heme-induced complement activation in the presence of complement mutations contributes as a secondary hit to the development of aHUS.
Abstract
Atypical hemolytic uremic syndrome (aHUS) is characterized by genetic and acquired abnormalities of the complement system leading to alternative pathway (AP) overactivation and by glomerular endothelial damage, thrombosis, and mechanical hemolysis. Mutations per se are not sufficient to induce aHUS, and nonspecific primary triggers are required for disease manifestation. We investigated whether hemolysis-derived heme contributes to aHUS pathogenesis. We confirmed that heme activates complement AP in normal human serum, releasing C3a, C5a, and sC5b9. We demonstrated that heme-exposed endothelial cells also activate the AP, resulting in cell-bound C3 and C5b9. This was exacerbated in aHUS by genetic abnormalities associated with AP overactivation. Heme interacted with C3 close to the thioester bond, induced homophilic C3 complexes, and promoted formation of an overactive C3/C5 convertase. Heme induced decreased membrane cofactor protein (MCP) and decay-accelerating factor (DAF) expression on endothelial cells, giving Factor H (FH) a major role in complement regulation. Finally, heme promoted a rapid exocytosis of Weibel-Palade bodies, with membrane expression of P-selectin known to bind C3b and trigger the AP, and the release of the prothrombotic von Willebrand factor. These results strongly suggest that hemolysis-derived heme represents a common secondary hit amplifying endothelial damage and thrombosis in aHUS.
Introduction
Atypical hemolytic uremic syndrome (aHUS) is a rare kidney-predominant thrombotic microangiopathy (TMA) associated with formation of fibrin-platelet clots in the glomerular microvasculature leading to mechanical hemolysis.1 This disease is related to a dysregulation of the complement alternative pathway (AP), as shown by the identification of genetic or acquired abnormalities in AP regulators or activators in more than 60% of patients.2 aHUS has an incomplete penetrance among mutation carriers, and a triggering event is presumably required for the disease manifestation. This primary hit can be an infection, pregnancy, drug, or other cell-activating event. Sera from aHUS patients carrying mutations deposit complement on endothelial cells activated by inflammatory cytokines,3 suggesting continuous aggression on the endothelium. However, not all infections trigger aHUS in patients. It is frequently the second pregnancy postpartum period that is associated with the disease.4 Therefore, other factors appear to be necessary to exceed the threshold of tolerable endothelial stress leading to severe TMA lesions.
In acute phase TMA, mechanical hemolysis induces the release of hemoglobin into the bloodstream.5 This hemoglobin is readily oxidized to ferric hemoglobin (methemoglobin) which, in turn, liberates heme. In physiological conditions, cell-free hemoglobin and heme are promptly scavenged by haptoglobin and hemopexin, respectively, which prevent their accumulation and limit their toxicity.6 However, at high concentrations of circulating hemoglobin, as in hemolytic diseases, these detoxification systems are overwhelmed,7 causing undesirable cell, tissue, and organ injury. The effects of heme exposure on the vascular system have been widely reported and include, in particular, heme iron–induced oxidative stress, vascular inflammation with recruitment of leukocytes, hemolysis,8-10 and thrombosis.11,12 Nevertheless, the participation of hemolysis in the development of TMA, as in aHUS, is not well studied.
Heme has been shown to activate the complement AP and to promote the deposition of C3 activation products on erythrocytes, as in malaria.13,14 Complement AP activation has also been reported during the acute phase of other hemolytic disorders, such as sickle-cell disease,15 β thalassemia major,16 and thrombotic thrombocytopenic purpura,17 but the participation of hemoglobin or its breakdown products in complement activation has not been investigated.
Here, we studied the effect of heme on complement activation and endothelial damage in the context of TMA in aHUS and potentially in other hemolysis-related diseases.
Methods
Reagents
The oxidized form of heme (hemin [ferriprotoporphyrin IX], designated as heme), hematoporphyrin IX, and hemoglobin were from Sigma. Stock solutions of 10 mM hemin and hematoporphyrin in 50 mM NaOH and 145 mM NaCl, stored at 4°C in the dark, were diluted just before use in phosphate-buffered saline (PBS) or M199 medium. All purified complement proteins and complement immunodepleted sera were from Complement Technologies. C3 from Calbiochem and Factor B (FB) from Quidel were also used. Recombinant FB was expressed in HEK293T cells as described.18 Complement enzyme-linked immunosorbent assay (ELISA) kits were from Quidel and HyCult.
aHUS patient sera
Serum samples were obtained from healthy blood donors or from aHUS patients from the French aHUS cohort.19 Approval was obtained from the Institut National de la Santé et de la Recherche Médicale (INSERM) institutional review board for these studies. Informed consent was provided according to the Declaration of Helsinki. Samples harboring six different mutations have been tested: three C3 mutations3,20 (5 patients) and three Factor H (FH) mutations.19 An AB blood group serum obtained from the French blood transfusion center Etablissement Français du Sang was used as a common standard in all experiments (normal human serum [NHS]).
Endothelial cell assays
Primary human umbilical vein endothelial cells (HUVECs) and conditionally immortalized glomerular endothelial cells (GEnCs)18,21 were cultured as described previously.3 They were incubated with heme and then serum, as described in the Figure 2, 3 and 6 legends. Cells in 24-well plates were detached, labeled, and analyzed by flow cytometry (FACSCalibur). Cells grown in 96-well plates were fixed with paraformaldehyde and analyzed by ELISA. Each condition was assayed in sixplicate wells (3 wells for the ELISA and 3 wells treated with crystal violet and analyzed by colorimetry to normalize the results with cell numbers). For fluorescence microscopy, HUVECs were grown in endothelial growth medium 2 (EGM2; Lonza) on gelatin-coated slides, stimulated, then fixed with paraformaldehyde and labeled as described in the Figure 2C legend. They were then analyzed with a Zeiss LSM700 confocal microscope, and photographs were processed with Image J64 software. Cell-free supernatants, centrifuged twice at 350g to remove residual cells, were assayed for von Willebrand factor (vWF) with a home-made sandwich ELISA.22
Surface plasmon resonance (SPR).
C3, C3b, C3a, C3d, FH, Factor D (FD), or FB were immobilized on the GLC sensor chips of ProteOn XPR36 equipment according to the manufacturer (BioRad). Six different concentrations of heme (starting with 300 nM) or heme-exposed proteins (C3, C3b, FB, or FH) were injected as analytes.
The C3/C5 convertase formation was followed by consecutive injections of native C3 (80 µg/mL), then FB (50 µg/mL) with FD (0.1 µg/mL), C3 again, and C5 (20 µg/mL) last on heme-exposed immobilized C3 and C3b. Alternatively, C3, C3b, FH, FB, or FB + FD were exposed to heme of different molar excess, diluted in cascade, and injected on a protein-coated chip.
Absorbance spectroscopy.
Absorbance spectra of hemin were recorded by using a Unicam Helios b UV-vis spectrophotometer, as described previously.23 Human C3, C3b, or C3a (Calbiochem; CompTech) was diluted to 500 nM in PBS. Aliquots of heme resulting in final concentrations of 0.01 to 20 μM were added to the optical cell–containing proteins and to a reference optical cell containing PBS only. The absorbance spectra in the wavelength range of 350 to 700 nm were recorded (300 nm/min).
Size exclusion chromatography.
The molecular composition of native and 10-fold molar excess heme-exposed C3 was analyzed by using the fast protein liquid chromatography Akta Purifier 10 system with a 30-mL Superose-6 column equilibrated with PBS. Detection was set at dual wavelengths: 280 nm for proteins and 400 nm for heme.
Molecular docking.
The atomic coordinates of C3 (PDB 2A7324 ) were used for the molecular docking. HexServer (http://hexserver.loria.fr/) was used to accommodate the heme molecule to C3, as described previously for C1q.23 Criteria for final C3-heme complex selection were based on the total energy of binding. Visualization was done by PyMol (www.pymol.org).
Results
Heme activates complement AP
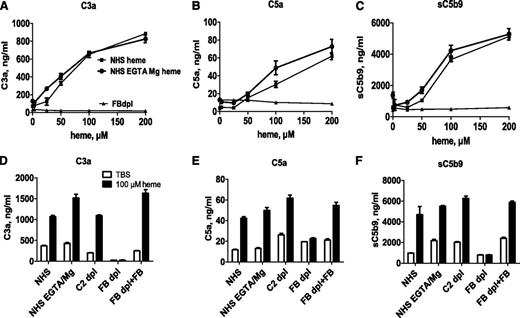
Heme induced fluid-phase complement activation in NHS, as shown by a dose-related increase of C3a, C5a, and sC5b9 levels, measured by ELISA (Figure 1). Similar levels of C3a, C5a, and C5b9 were measured in NHS, in NHS-EGTA-Mg2+, and in C2-depleted serum. The latter two conditions allow AP activation only. No activation was observed in FB-depleted serum, which is deprived of AP activation. Adding back purified FB restored the heme-induced complement activation capacity. No complement activation was observed with an iron-devoid derivative of heme, hematoporphyrin, under the same conditions (data not shown).
Heme activates the complement AP in serum. (A-C) Normal or FB-depleted sera was incubated v/v with twofold dilutions of heme in M199 medium, without or with 10 mM EGTA and 5 mM MgCl2. After a 30-minute incubation at 37°C, the levels of released C3a, C5a, and sC5b9 were measured by ELISA. (D-F) To show the involvement of the AP, these complement components were measured after a similar incubation of NHS, without or with EGTA-Mg, C2-depleted (dpl) serum, or FB-depleted serum, reconstituted or not with 150 μg/mL purified FB, with 100 μM heme. Results are expressed as mean ± standard deviation (SD) of 2 experiments with triplicate measurements.
Heme activates the complement AP in serum. (A-C) Normal or FB-depleted sera was incubated v/v with twofold dilutions of heme in M199 medium, without or with 10 mM EGTA and 5 mM MgCl2. After a 30-minute incubation at 37°C, the levels of released C3a, C5a, and sC5b9 were measured by ELISA. (D-F) To show the involvement of the AP, these complement components were measured after a similar incubation of NHS, without or with EGTA-Mg, C2-depleted (dpl) serum, or FB-depleted serum, reconstituted or not with 150 μg/mL purified FB, with 100 μM heme. Results are expressed as mean ± standard deviation (SD) of 2 experiments with triplicate measurements.
Exposure of endothelial cells to heme results in complement activation on the cell surface
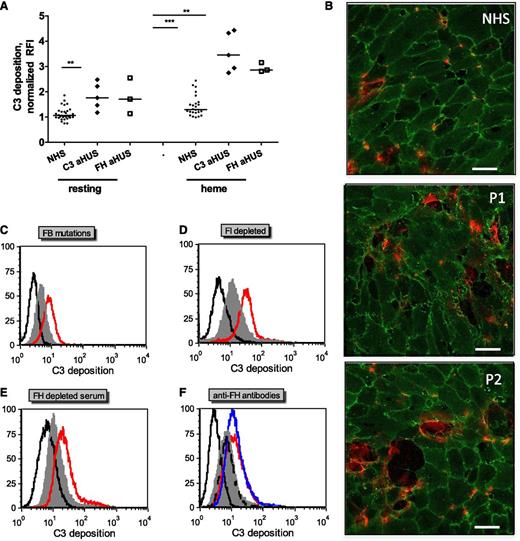
To mimic the situation of intravascular hemolysis, human endothelial cells, HUVECs, and GEnCs were pretreated with increasing heme concentrations, washed, and incubated with NHS. Cell-bound C3 fragments (markers of complement activation) were analyzed by flow cytometry after cell detachment or by ELISA and immunofluorescence on adherent cells. As shown in Figure 2A-C, the level of cell-bound C3 was minimal on resting cells when compared with the immunoglobulin G1 isotype control. Exposure to heme resulted in a concentration-dependent increase of C3 deposits on both HUVECs and GEnCs detected by flow cytometry (Figure 2A), ELISA (Figure 2B), and by immunofluorescence microscopy (Figure 2C). Complement deposits were still observed when heme-treated cells were submitted to up to 5 washes before the addition of complement (data not shown), revealing a cell-directed effect of heme. Complement activation by heme-treated cells occurred via the AP, since C3 deposits were persistent in the presence of EGTA-Mg2+ and in C2-depleted serum but strikingly diminished in FB-depleted serum (Figure 2D). The addition of purified FB to the FB-depleted serum restored C3 fragment deposition. These effects were observed with both HUVECs (Figure 2D) and GEnCs (data not shown). Importantly, C5b9 complexes were also detected by flow cytometry on heme-treated cells (Figure 2E), reflecting the formation of a C5 convertase. Exposure of endothelial cells to heme up to 200 µM for 1 hour did not induce apoptosis and necrosis as measured by Annexin V and propidium iodide labeling (data not shown).
Complement activation on heme-treated endothelial cells results in cell-bound C3 fragments and C5b9. HUVECs or GEnCs were stimulated with increasing concentrations of heme in M199 medium for 20 minutes at 37°C. If not stated otherwise, cells washed with PBS with Ca2+/Mg2+ (Gibco) were incubated for 30 minutes at 37°C in NHS diluted 1:4 in M199 medium. (A) Washed cells were detached with PBS, 1% bovine serum albumin, 10 mM EDTA, 0.1% sodium azide, without (HUVECs) or with 5 mg/mL lidocaine (GEnC) to be analyzed by flow cytometry after labeling with anti-C3c monoclonal antibody (mAb) (Quidel) and phycoerythrin-labeled secondary antibody (mean ± SD of mean fluorescent intensities relative to the mAb isotype control (RFI, n = 3). (B) Washed cells were fixed with 4% paraformaldehyde and 2% saccharose for 20 minutes at room temperature for analysis by cell-ELISA after labeling with biotin–anti-C3c (Quidel) in Tris-buffered saline–bovine serum albumin and peroxidase-extravidin (Sigma) revealed with 3,3′,5,5′-tetramethylbenzidine substrate (Pierce) (mean ± SD of triplicate wells from one of 3 similar experiments). (C) HUVECs were fixed with paraformaldehyde as in (B) to be analyzed by confocal microscopy after labeling with a mouse anti-CD31 mAb and a rabbit anti-C3 Ab revealed with Alexa555 (red)- and Alexa488 (green)-labeled secondary antibodies (Molecular Probes); scale bar, 50 µm. (D) Heme-exposure, under the experimental conditions used, did not induce cell death. HUVECs were exposed to 100 µM heme, quickly detached with trypsin, stained with Annexin V and propidium iodide, and analysed by flow cytometry. Apopto-necrotic HUVECs spontaneously detached overnight from a confluent monolayer served as a positive control for Annexin V and propidium iodide staining. Representative dot plots out of 3 independent experiments are shown. (E) Heme-induced C3 deposition on HUVECs were measured by flow cytometry with an anti-C3c mAb, under conditions favoring a fluid phase complement activation (heme added to the cells together with serum, “heme in serum”), direct effect on the cell surface (heme exposure as in panels [A], [B] and [C], “heme, wash, serum”); or both (cells pre-exposed to heme, then treated with serum with no washing step, “heme, serum”) (mean ± SD of 3 independent experiments). (F) The presence of uncleaved C3 on the surface of heme-exposed HUVECs were tested by staining with anti-C3a mAb the cells exposed with 100 µM heme and NHS as in (E). Blue, resting cells; purple, “heme, wash, serum”; green, “heme, serum”; black, unstained cells; red, isotype control IgG1. (G) Acidic wash of the uncovalently bound C3 forms from the heme-exposed cells. HUVECs were exposed to 100 µM heme, washed and incubated with NHS. Subsequently, 3 washes with PBS (purple histogram) or with a pH 2.7 PBS-25 mM Glycine buffer (green line) were performed. The cells were stained with anti-C3c, anti-C3a or anti-iC3b neoepitope antibody (Quidel) and analyzed by flow cytometry. Signal from unstained cells is presented in black and the isotype control IgG1 is in red; representative histograms of 3 independent experiments. (H) Heme-induced C3 deposition is dependent on the alternative complement pathway. HUVECs treated as in (A), with or without 100 μM heme, were incubated with 1:4 diluted NHS, with or without 10 mM EDTA or 10 mM EGTA and 5 mM MgCl2, C2-depleted or FB-depleted serum, with and without 150 mg/ml purified FB. C3 deposits were measured, as in (A), by flow cytometry (mean ± SD RFI, n = 4). (I) HUVECs treated as in (A) were labeled with a mouse anti-C5b-9 mAb kindly provided by Prof Paul Morgan (Cardiff, United Kingdom) (n=3).
Complement activation on heme-treated endothelial cells results in cell-bound C3 fragments and C5b9. HUVECs or GEnCs were stimulated with increasing concentrations of heme in M199 medium for 20 minutes at 37°C. If not stated otherwise, cells washed with PBS with Ca2+/Mg2+ (Gibco) were incubated for 30 minutes at 37°C in NHS diluted 1:4 in M199 medium. (A) Washed cells were detached with PBS, 1% bovine serum albumin, 10 mM EDTA, 0.1% sodium azide, without (HUVECs) or with 5 mg/mL lidocaine (GEnC) to be analyzed by flow cytometry after labeling with anti-C3c monoclonal antibody (mAb) (Quidel) and phycoerythrin-labeled secondary antibody (mean ± SD of mean fluorescent intensities relative to the mAb isotype control (RFI, n = 3). (B) Washed cells were fixed with 4% paraformaldehyde and 2% saccharose for 20 minutes at room temperature for analysis by cell-ELISA after labeling with biotin–anti-C3c (Quidel) in Tris-buffered saline–bovine serum albumin and peroxidase-extravidin (Sigma) revealed with 3,3′,5,5′-tetramethylbenzidine substrate (Pierce) (mean ± SD of triplicate wells from one of 3 similar experiments). (C) HUVECs were fixed with paraformaldehyde as in (B) to be analyzed by confocal microscopy after labeling with a mouse anti-CD31 mAb and a rabbit anti-C3 Ab revealed with Alexa555 (red)- and Alexa488 (green)-labeled secondary antibodies (Molecular Probes); scale bar, 50 µm. (D) Heme-exposure, under the experimental conditions used, did not induce cell death. HUVECs were exposed to 100 µM heme, quickly detached with trypsin, stained with Annexin V and propidium iodide, and analysed by flow cytometry. Apopto-necrotic HUVECs spontaneously detached overnight from a confluent monolayer served as a positive control for Annexin V and propidium iodide staining. Representative dot plots out of 3 independent experiments are shown. (E) Heme-induced C3 deposition on HUVECs were measured by flow cytometry with an anti-C3c mAb, under conditions favoring a fluid phase complement activation (heme added to the cells together with serum, “heme in serum”), direct effect on the cell surface (heme exposure as in panels [A], [B] and [C], “heme, wash, serum”); or both (cells pre-exposed to heme, then treated with serum with no washing step, “heme, serum”) (mean ± SD of 3 independent experiments). (F) The presence of uncleaved C3 on the surface of heme-exposed HUVECs were tested by staining with anti-C3a mAb the cells exposed with 100 µM heme and NHS as in (E). Blue, resting cells; purple, “heme, wash, serum”; green, “heme, serum”; black, unstained cells; red, isotype control IgG1. (G) Acidic wash of the uncovalently bound C3 forms from the heme-exposed cells. HUVECs were exposed to 100 µM heme, washed and incubated with NHS. Subsequently, 3 washes with PBS (purple histogram) or with a pH 2.7 PBS-25 mM Glycine buffer (green line) were performed. The cells were stained with anti-C3c, anti-C3a or anti-iC3b neoepitope antibody (Quidel) and analyzed by flow cytometry. Signal from unstained cells is presented in black and the isotype control IgG1 is in red; representative histograms of 3 independent experiments. (H) Heme-induced C3 deposition is dependent on the alternative complement pathway. HUVECs treated as in (A), with or without 100 μM heme, were incubated with 1:4 diluted NHS, with or without 10 mM EDTA or 10 mM EGTA and 5 mM MgCl2, C2-depleted or FB-depleted serum, with and without 150 mg/ml purified FB. C3 deposits were measured, as in (A), by flow cytometry (mean ± SD RFI, n = 4). (I) HUVECs treated as in (A) were labeled with a mouse anti-C5b-9 mAb kindly provided by Prof Paul Morgan (Cardiff, United Kingdom) (n=3).
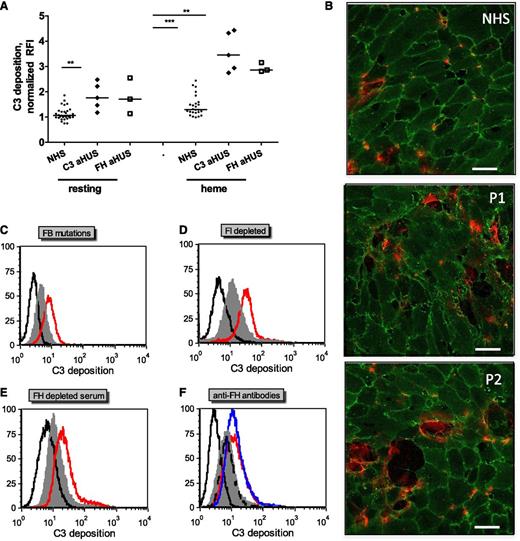
Complement activation on endothelial cells is exacerbated in the presence of sera with aHUS-related complement mutations and aHUS-relevant model conditions
To test whether complement dysregulation or overactivation in sera induces modification in C3 deposition on heme-exposed endothelial cells, sera from normal controls or from aHUS patients with previously described FH mutations (FH-aHUS)19 or C3 mutations (C3-aHUS)3,20 were used. Exacerbation of the C3 deposition was observed by flow cytometry on GEnCs (Figure 3A) and on HUVECs (data not shown) in contact with patients’ sera compared with normal controls (n = 30). C3 deposition on resting cells was enhanced in sera from about half the patients, although on heme-exposed cells, it was significantly increased with sera from all of the patients when compared with 30 normal sera. Immunofluorescence microscopy observations of heme-exposed HUVECs incubated with sera from patients with C3 mutations showed increased C3 deposition, cell retraction, and/or detachment when compared with cells incubated with normal sera (Figure 3B). C3 deposition was similarly augmented on heme-treated HUVECs incubated in conditions mimicking aHUS sera, that is, with FB-depleted serum restored with a recombinant FB with aHUS gain-of-function mutation D254G18 compared with the wild-type FB (Figure 3C); in the presence of FI-depleted (Figure 3D) or FH-depleted (Figure 3E) sera, as a model for FI and FH deficiency; or in the presence of blocking anti-FH antibody directed against the N- and C-termini of FH, as found in patients with autoimmune aHUS25,26 (Figure 3F).
C3 deposits on heme-treated endothelial cells are increased with sera from aHUS patients compared with normal sera. Endothelial cells were treated as in Figure 2A with 100 μM heme and 1:4 dilutions of various sera representing aHUS relevant conditions. (A) Resting or heme-exposed GEnCs were incubated with NHS (n = 30) or sera from aHUS patients with FH (n = 3) or C3 (n = 5) mutations, and the level of C3 deposition was measured by fluorescence-activated cell sorter. (B) HUVECs were exposed to 100 µM heme and 1:4 dilutions of NHS and sera from two aHUS patients with C3 mutations (P1 and P2) and labeled with anti-CD31 (green) and anti-C3 (red) for immunofluorescence, as in Figure 2C. Scale bar = 50 µm. (C-E) Heme-exposed HUVECs were treated with normal sera (gray) or different model conditions for aHUS (red). (C) FB-depleted serum complemented with recombinant FB either wild-type or with aHUS-related mutation D254G, (D) FI-depleted serum, (E) FH-depleted serum, and (F) normal serum complemented with blocking anti-FH antibodies against the N-terminus (Ox24, AbD Serotec; blue) or C-terminus (C18, Santa Cruz; red) or nonblocking antibody (Ox23, dashed line). The isotype control is in black. A representative histogram from 1 of at least 3 independent experiments is presented. Statistical significance, **P < .01, ***P < .001.
C3 deposits on heme-treated endothelial cells are increased with sera from aHUS patients compared with normal sera. Endothelial cells were treated as in Figure 2A with 100 μM heme and 1:4 dilutions of various sera representing aHUS relevant conditions. (A) Resting or heme-exposed GEnCs were incubated with NHS (n = 30) or sera from aHUS patients with FH (n = 3) or C3 (n = 5) mutations, and the level of C3 deposition was measured by fluorescence-activated cell sorter. (B) HUVECs were exposed to 100 µM heme and 1:4 dilutions of NHS and sera from two aHUS patients with C3 mutations (P1 and P2) and labeled with anti-CD31 (green) and anti-C3 (red) for immunofluorescence, as in Figure 2C. Scale bar = 50 µm. (C-E) Heme-exposed HUVECs were treated with normal sera (gray) or different model conditions for aHUS (red). (C) FB-depleted serum complemented with recombinant FB either wild-type or with aHUS-related mutation D254G, (D) FI-depleted serum, (E) FH-depleted serum, and (F) normal serum complemented with blocking anti-FH antibodies against the N-terminus (Ox24, AbD Serotec; blue) or C-terminus (C18, Santa Cruz; red) or nonblocking antibody (Ox23, dashed line). The isotype control is in black. A representative histogram from 1 of at least 3 independent experiments is presented. Statistical significance, **P < .01, ***P < .001.
Heme binds to C3
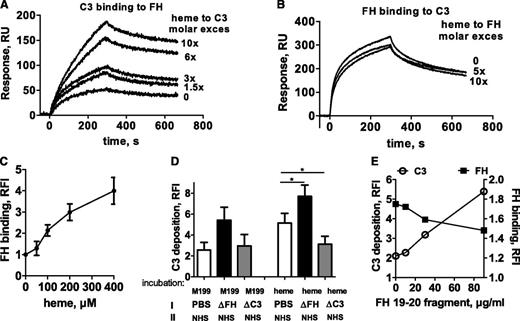
To find out whether heme activation of AP was a result of its interaction with a particular complement protein, the binding of heme to C3, FB, FH, and FD was tested by SPR. Strong, specific, and dose-dependent binding to surface-immobilized C3 (hence C3b like C3(H2O)27 ) and 50% less to FH and FB was detected (Figure 4A). No binding to FD was found. Further, heme was shown to bind the C3a fragment of C3 in contrast to C3b and C3d (Figure 4B). C3(H2O), C3b, and C3d were functionally active, since they bound FH (data not shown), excluding artifacts due to protein alterations.
Binding of heme to C3. SPR analysis of the binding of different concentrations of heme to (A) AP proteins and (B) C3 fragments immobilized on the chip (mean ± SD from 3 experiments on 2 chips). The flow rate was 30 μL/min with N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) running buffer (10 mM HEPES, 150 mM NaCl, Tween 0.005%; pH 7.3). Each interaction was tested on at least two different chips at least twice per chip. Data were double referenced and analyzed by using ProteOn manager software. (C) Absorption spectroscopy for the binding of heme at a 10-fold molar excess to C3, C3b, and C3a. Dashed gray line represents the spectrum of heme in PBS and the black line represents the spectrum of heme in the presence of protein. (D) Dose-dependence of the binding of heme to C3, C3b, and C3a. Heme was added to the protein solution and incubated for 2 minutes in the dark at 20°C before recording the spectrum. Absorbance differences between protein-bound hemin and hemin at absorbance maxima (λ = 395 nm) in the Soret region (ΔA) were used to build titration binding curves. (E) Molecular docking of heme to C3: heme (red spheres) in all 14 retrieved models docked to three regions of the molecule (I, II, and III), one being close to the ANA domain (cyan), the second being close to the thioester bond (green) in the TED domain (blue), and the third again being close to the TED domain. (F) Zoomed in view of the II cluster with the top-score heme molecule visualized using ball-and-stick art in red. The distance between the thioester bond (green) and heme is less than 10 Å. (G) SPR analysis of the binding of heme-exposed C3 to C3 immobilized on the chip. One experiment of 3 with similar results is presented. No interaction was detected in the absence of heme, although dose-dependent binding was found with increasing heme concentrations. (H) Size exclusion chromatography of native C3 and heme-exposed C3. Fast protein liquid chromatography with a Superose 6 column with dual wavelength was used (280 nm for C3, black line; 400 nm for heme, gray dashed line). C3 at 6.4 µM was incubated for 10 minutes on ice with PBS or 64 µM heme, giving 10-fold molar excess adjusted to a final volume of 1 mL and loaded to the column.
Binding of heme to C3. SPR analysis of the binding of different concentrations of heme to (A) AP proteins and (B) C3 fragments immobilized on the chip (mean ± SD from 3 experiments on 2 chips). The flow rate was 30 μL/min with N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) running buffer (10 mM HEPES, 150 mM NaCl, Tween 0.005%; pH 7.3). Each interaction was tested on at least two different chips at least twice per chip. Data were double referenced and analyzed by using ProteOn manager software. (C) Absorption spectroscopy for the binding of heme at a 10-fold molar excess to C3, C3b, and C3a. Dashed gray line represents the spectrum of heme in PBS and the black line represents the spectrum of heme in the presence of protein. (D) Dose-dependence of the binding of heme to C3, C3b, and C3a. Heme was added to the protein solution and incubated for 2 minutes in the dark at 20°C before recording the spectrum. Absorbance differences between protein-bound hemin and hemin at absorbance maxima (λ = 395 nm) in the Soret region (ΔA) were used to build titration binding curves. (E) Molecular docking of heme to C3: heme (red spheres) in all 14 retrieved models docked to three regions of the molecule (I, II, and III), one being close to the ANA domain (cyan), the second being close to the thioester bond (green) in the TED domain (blue), and the third again being close to the TED domain. (F) Zoomed in view of the II cluster with the top-score heme molecule visualized using ball-and-stick art in red. The distance between the thioester bond (green) and heme is less than 10 Å. (G) SPR analysis of the binding of heme-exposed C3 to C3 immobilized on the chip. One experiment of 3 with similar results is presented. No interaction was detected in the absence of heme, although dose-dependent binding was found with increasing heme concentrations. (H) Size exclusion chromatography of native C3 and heme-exposed C3. Fast protein liquid chromatography with a Superose 6 column with dual wavelength was used (280 nm for C3, black line; 400 nm for heme, gray dashed line). C3 at 6.4 µM was incubated for 10 minutes on ice with PBS or 64 µM heme, giving 10-fold molar excess adjusted to a final volume of 1 mL and loaded to the column.
To further examine the binding of heme to C3 and its fragments, C3, C3b, and C3a were exposed to heme, and alterations in the specific electronic configuration of heme were then studied by absorption spectroscopy. The absorbance spectrum of oxidized heme showed a ΔA increase in the molar extinction coefficient at the Soret band (around 400 nm) in the presence of C3 and C3a and a 4-nm and 8-nm red shift, respectively (Figure 4C). C3b showed only ΔA with no red shift. The titration of C3a and C3b with increasing concentrations of heme resulted in saturable ΔA (at about two- to sixfold heme excess), although titration of C3 did not reach saturation even at 20-fold molar excess of heme, suggesting C3 oligomer formation (Figure 4D).
To gain further insight into the possible location of the heme binding site(s) in the C3 molecule, molecular docking was performed. HexServer returned 14 top-scored models with total calculated energy of the system between −397.61 kJ/mol and −306.06 kJ/mol (Figure 4E). The first binding site (4 models) was located near the anaphylatoxin (ANA) domain (C3a after cleavage by the C3 convertase). The second site (8 models) was less than 10 Å from the thioester bond (Cys988-Gln991) in the thiol ester-containing domain (TED) at the interface with macroglobulin domain 2 (MG2) and macroglobulin domain 8 (MG8) (Figure 4F). The last site (2 models) is located in close proximity to the second one at the interface between the TED and MG2 domains.
Heme binding to C3 favors C3/C3 interactions
The possibility that heme induces homophilic C3 interactions was assessed by SPR. Heme-exposed C3 injected in the fluid phase was able to bind in a dose-dependent manner to C3 (Figure 4G), but not to C3b (data not shown) immobilized on the chip. Heme-exposed C3b on the chip did not interact with either C3b or C3 (data not shown). C3 on the chip, exposed to increasing heme concentrations, bound native C3 injected in the fluid phase (first step of the SPR profile in Figure 5A) in a heme dose-dependent manner.
Heme interaction with C3 favors the formation of the AP C3/C5 convertases. C3 convertase was formed on an SPR chip coated with (A) C3 and (B) C3b. MgCl2 (1 mM final) was added in HEPES running buffer for interactions involving FB. C3 or C3b on the chip was exposed to increasing concentrations of heme as indicated. Subsequently, a series of injections of C3, FB + FD, C3, and C5 allowed formation of a C3/C5 convertase on the chip. One representative experiment of 4 is shown. The influence of heme on the capacity of FB and FD to bind to (C) C3 and (D) C3b was measured by SPR. C3 and C3b were immobilized on the chip and FB and FD, either native or exposed to five- and 10-fold molar excess of heme, were injected. One representative sensorogram set of 3 experiments is shown. Each interaction was tested on at least two different chips at least twice per chip, and the data were double referenced and analyzed by using ProteOn manager software.
Heme interaction with C3 favors the formation of the AP C3/C5 convertases. C3 convertase was formed on an SPR chip coated with (A) C3 and (B) C3b. MgCl2 (1 mM final) was added in HEPES running buffer for interactions involving FB. C3 or C3b on the chip was exposed to increasing concentrations of heme as indicated. Subsequently, a series of injections of C3, FB + FD, C3, and C5 allowed formation of a C3/C5 convertase on the chip. One representative experiment of 4 is shown. The influence of heme on the capacity of FB and FD to bind to (C) C3 and (D) C3b was measured by SPR. C3 and C3b were immobilized on the chip and FB and FD, either native or exposed to five- and 10-fold molar excess of heme, were injected. One representative sensorogram set of 3 experiments is shown. Each interaction was tested on at least two different chips at least twice per chip, and the data were double referenced and analyzed by using ProteOn manager software.
To assess the stability of heme-induced C3-C3 complexes, the molecular composition of C3 after exposure to heme was analyzed by size exclusion chromatography. A fraction of heme-exposed C3 was eluted earlier than native C3, corresponding to homophilic C3 interactions and formation of dimers and higher-order oligomers (Figure 4H). These oligomers showed stable heme binding, since some heme, detected by its specific absorbance at 400 nm, co-eluted with the oligomeric protein detected by the 280-nm absorbance. Free heme has a low molecular weight and is retained by the column.
Heme interaction with C3 favors the formation of a C3/C5 convertase
To explain the observed heme-induced AP activation and in view of the heme-induced homophilic C3 interaction described earlier, the effect of heme on the C3 convertase formation was investigated by SPR (Figure 5). C3 (Figure 5A) and C3b (Figure 5B) were immobilized on the sensor chip and exposed to increasing concentrations of heme. As in Figure 4, a dose-dependent binding of heme to C3, but not to C3b, was detected. A fixed amount of C3 was then injected, which bound strongly to heme-exposed C3 but only minimally to C3b. Further fixed concentrations of FB and FD were flowed onto the chip, to form the C3 convertase. A dose-dependent increase of the signal was detected on heme-exposed C3-coated flow cells but there was little or no effect on C3b-coated flow cells. Subsequent injections of fixed concentrations of C3 followed by C5 resulted again in a dose-dependent increase of binding to the heme-exposed C3 surface. Since a slight heme binding to FB was detected (as shown in Figure 4A), the effect of heme on the ability of FB to interact with C3 and to form a C3 convertase was tested. No difference in the interaction of FB with C3 and C3b in the presence of FD was detected after FB exposure to up to 10-fold molar excess of heme (Figure 5C-D).
Heme enhances FH binding to C3 and to endothelial cell surfaces
Since heme binding to FH was detected in Figure 4A, the influence of heme on the binding of FH to its C3 and C3b ligands was studied. Heme-exposed C3 bound FH more strongly compared with native C3, reaching more than a threefold increase at 10-fold molar excess of heme (Figure 6A). In contrast, heme-exposed C3b bound FH similarly to native C3b binding (data not shown). The increased C3-FH binding was a consequence of C3 exposure to heme, since FH exposure to up to 10-fold molar excess of heme did not alter its interaction with C3 and C3b (Figure 6B).
FH binds to heme-treated C3 and is the major serum factor regulating C3 deposition on heme-treated cells. (A) Heme-exposed C3 bound more strongly to immobilized FH, as measured by SPR but (B) heme-exposed FH bound immobilized C3 in the same manner as did native FH. (C) HUVECs were treated with increasing doses of heme and 1:4 NHS, detached as in Figure 2A, and labeled with anti-FH mAb Ox23. Results are expressed as RFI mean ± SD (n = 3). (D) Resting HUVECs and cells treated with 100 μM heme for 20 minutes, were sequentially incubated for 30 minutes at 37°C, first with either PBS, FH-depleted serum, or C3-depleted serum (source of FH), washed, and incubated as a second step with NHS (*P < .05, paired Student t test; n = 3). (E) HUVECs treated with 100 μM heme for 20 minutes were incubated with increasing concentrations of Short Consensus Repeat (SCR) 19-20 FH peptide for 15 minutes at 37°C, then with 1:4 NHS for 30 minutes. (D-E) C3 deposits were analyzed by flow cytometry and expressed as mean ± SD RFI (n = 3).
FH binds to heme-treated C3 and is the major serum factor regulating C3 deposition on heme-treated cells. (A) Heme-exposed C3 bound more strongly to immobilized FH, as measured by SPR but (B) heme-exposed FH bound immobilized C3 in the same manner as did native FH. (C) HUVECs were treated with increasing doses of heme and 1:4 NHS, detached as in Figure 2A, and labeled with anti-FH mAb Ox23. Results are expressed as RFI mean ± SD (n = 3). (D) Resting HUVECs and cells treated with 100 μM heme for 20 minutes, were sequentially incubated for 30 minutes at 37°C, first with either PBS, FH-depleted serum, or C3-depleted serum (source of FH), washed, and incubated as a second step with NHS (*P < .05, paired Student t test; n = 3). (E) HUVECs treated with 100 μM heme for 20 minutes were incubated with increasing concentrations of Short Consensus Repeat (SCR) 19-20 FH peptide for 15 minutes at 37°C, then with 1:4 NHS for 30 minutes. (D-E) C3 deposits were analyzed by flow cytometry and expressed as mean ± SD RFI (n = 3).
Heme treatment of HUVECs resulted in a dose-dependent increase of FH binding to the cell surface upon incubation with NHS (Figure 6C). Of note, no FH was detected on HUVECs in the absence of serum by using a mixture of three anti-FH monoclonal antibodies (mAbs) (data not shown). In order to test whether FH bound to heme-treated cells was functionally active and able to control C3 deposition, cells were incubated with PBS, with C3-depleted serum (as a source of FH without C3 deposition), or with FH-depleted serum (allowing C3 deposition with no FH), followed by a second step of NHS incubation (Figure 6D). Preincubation with FH contained in the C3-depleted serum resulted in a 40% lower C3 deposition than the control preincubated in PBS. By contrast, preincubation with FH-depleted serum resulted in 20% higher C3 deposition than with the NHS control. Moreover, inhibition of FH-mediated control by cell preincubation with an FH 19-20 construct able to interact with the cell membrane and to compete with FH28 resulted in a dose-dependent decrease of FH binding and concomitant increase of C3 deposition on the cell surface (Figure 6E).
Heme decreases the membrane control of complement activation
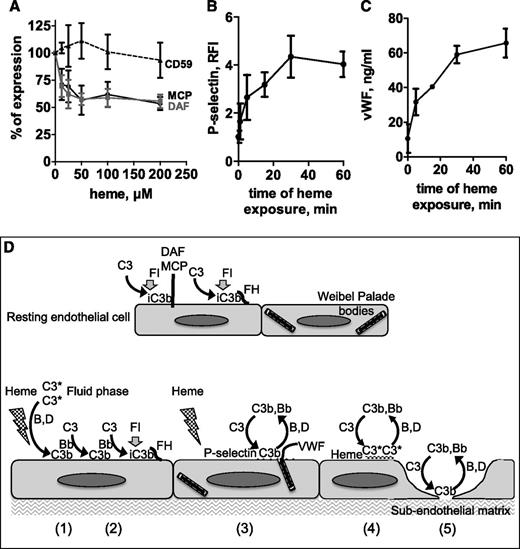
Heme-induced complement activation on endothelial cells could be the result of either defective control or local overactivation or both. To study the role of complement regulators, expression levels of CD59, decay-accelerating factor (DAF), and membrane cofactor protein (MCP) were measured on resting or heme-treated HUVECs by flow cytometry. As shown in Figure 7A, heme treatment decreased the membrane expression of DAF and MCP in a dose-dependent manner, with no modification of CD59 levels. Heme at 50 µM resulted in about 50% loss of MCP and DAF when compared with untreated cells.
Effects of heme treatment of endothelial cells. (A) HUVECs treated with increasing doses of heme for 20 minutes and detached were labeled with anti-CD55, phycoerythrin-labeled anti-MCP, or anti-CD59 and analyzed by flow cytometry. Results are expressed as the percentage of the initial mean fluorescence intensity measured on untreated cells (mean ± SD; n = 3). (B) HUVECs treated with 100 µM heme for different times were detached at 4°C and labeled with anti–P-selectin (R&D Systems) (mean ± SD; n = 3). (C) Supernatants of cells were tested in a vWF-specific ELISA. Results are expressed as mean ± SD of triplicate wells from one of 3 similar experiments. (D) Schematic view of different nonexclusive mechanisms leading to complement activation on heme-treated cells. (1) Heme intercalates into the C3 molecule (C3*), activates complement in the fluid phase, and deposits C3b on nearby endothelial cells. (2) This C3b initiates the formation of a cell-bound C3 convertase, favored by the decrease of DAF and MCP membrane expressions on heme-treated cells, FH remaining as the major AP control protein. (3) Heme induces the mobilization of Weibel-Palade bodies with the secretion of vWF and the appearance of P-selectin able to bind C3b and to focus the AP amplification cycle. (4) Heme can also bind directly to the cell surface, where it activates complement as in the fluid phase. (5) Heme induces cell retraction, exposing the subendothelial matrix known to efficiently activate complement.
Effects of heme treatment of endothelial cells. (A) HUVECs treated with increasing doses of heme for 20 minutes and detached were labeled with anti-CD55, phycoerythrin-labeled anti-MCP, or anti-CD59 and analyzed by flow cytometry. Results are expressed as the percentage of the initial mean fluorescence intensity measured on untreated cells (mean ± SD; n = 3). (B) HUVECs treated with 100 µM heme for different times were detached at 4°C and labeled with anti–P-selectin (R&D Systems) (mean ± SD; n = 3). (C) Supernatants of cells were tested in a vWF-specific ELISA. Results are expressed as mean ± SD of triplicate wells from one of 3 similar experiments. (D) Schematic view of different nonexclusive mechanisms leading to complement activation on heme-treated cells. (1) Heme intercalates into the C3 molecule (C3*), activates complement in the fluid phase, and deposits C3b on nearby endothelial cells. (2) This C3b initiates the formation of a cell-bound C3 convertase, favored by the decrease of DAF and MCP membrane expressions on heme-treated cells, FH remaining as the major AP control protein. (3) Heme induces the mobilization of Weibel-Palade bodies with the secretion of vWF and the appearance of P-selectin able to bind C3b and to focus the AP amplification cycle. (4) Heme can also bind directly to the cell surface, where it activates complement as in the fluid phase. (5) Heme induces cell retraction, exposing the subendothelial matrix known to efficiently activate complement.
Heme induces the membrane expression of P-selectin on endothelial cells
P-selectin has been described as a C3b-binding protein, capable of initiating complement activation.29 Thus, we analyzed the membrane expression of P-selectin on endothelial cells and observed a time-dependent increase of this expression during heme treatment (Figure 7B). The rapid expression, in less than 30 minutes, excluded a de novo synthesis but suggested a mobilization of Weibel-Palade bodies. Indeed, a secretion of vWF was observed, as measured by ELISA, in parallel with P-selectin membrane expression (Figure 7C).
Discussion
We report that heme activates complement in the fluid phase and on endothelial cells and induces a prothrombotic phenotype by a set of five probably co-existing mechanisms (summarized in Figure 7D). Our data allow us to propose a novel hypothesis in which hemolysis would serve as a secondary hit for aHUS manifestation. A triggering event preceding the acute phase of aHUS may lead to formation of an initial thrombus responsible for erythrocyte rupture and heme release. We suggest that heme amplifies complement activation and creates a positive feedback loop that sustains and perpetuates the histologic TMA lesions. Upon exceeding a threshold, this heme-induced complement overactivation becomes a second hit for the manifestation of a full-blown aHUS. This may shed new light on aHUS pathogenesis and explain, at least in part, the incomplete penetrance of the disease, which would develop only after such an amplification of primary pathogenic events. First, we found that the presence of heme in NHS is sufficient to trigger fluid phase complement AP activation with release of anaphylotoxins C3a and C5a, as well as sC5b9, in agreement with previous reports.13,30 Heme has a dual role in complement, activating AP but inhibiting the binding of C1q to its ligands.23 We investigated the mechanism of AP complement activation and showed that it results from the interaction of heme with the C3 molecule, which favors C3 homophilic interactions and leads to the formation of an active AP C3/C5 convertase. Molecular docking revealed that one of the most likely heme-binding sites on C3 is adjacent to the thioester bond. The insertion of the redox active heme at this site may favor the thioester bond hydrolysis and the transition of native C3 to hydrolyzed C3*. Importantly, hydrolyzed C3* is able to bind FB and form a C3 convertase, contrary to native C3. Therefore, heme-induced generation of C3* could explain the fluid phase AP activation in heme-treated serum (Figure 7D, step 1). Heme is known to mediate protein covalent cross-linking and oligomer formation31 and indeed induces the formation of C3/C3 complexes. Those are particularly critical for the formation of a fluid phase C5 convertase, which requires C3b/C3b covalent linkage for an efficient affinity for C5.32 Iron-devoid heme derivative hematoporphyrin did not activate complement, strongly suggesting that iron redox activity is involved.5 This is reminiscent of previous data showing that H2O2 and neutrophil-derived oxidants trigger the complement AP and may also activate C5 directly.33
Circulating free heme concentrations are about 6 to 30 μM in sickle-cell disease and thalassemia7 but have not been reported in aHUS. Mechanical hemolysis in the renal microvasculature in aHUS probably results in local heme concentration, at the site of hemolysis and before dilution in the blood flow, much higher than concentrations measured in the peripheral blood. The concentration-dependent effects of heme on complement and endothelium could, at least in part, explain why vascular lesions are mainly restricted to microvessels in TMA. The 100 μM heme concentration used in these in vitro experiments, which corresponds to the destruction of less than 0.05% of bloodstream erythrocytes,13 thus seems physiologically reasonable.
This work is the first demonstration that free heme triggers complement AP activation on endothelial cells. Although little or no C3 deposits were observed on resting cells, they reached significant levels on heme-treated HUVECs and GEnCs. Moreover, cell-bound C5b9 was detected, reflecting the formation of a C5 convertase and the release of C5 fragments C5a and C5b9 known to activate endothelial cells and to promote endothelium permeability.34
Imbalance between complement activation and regulation may explain the observed AP activation on endothelial cells (Figure 7D, step 2). Indeed, a decrease of DAF (CD55) and MCP (CD46) expression was detected after exposure of HUVECs to heme, similar to previous observations of downregulated complement regulators on retinal epithelial cells submitted to brief oxidative stress.35 DAF and MCP are responsible for the protection of endothelium against autologous complement attack. Their downregulation has been reported on apoptotic and necrotic cells,36 but we did not detect significant increase of apoptosis with heme under the experimental conditions used.
In contrast to the decreased expression of MCP and DAF, exposure of endothelial cells to heme resulted in enhancement of FH binding to the cell membrane. FH thus appears to compensate for the loss of membrane-bound complement inhibitors DAF and MCP, as has been described on apoptotic cells.37 Heme-treated cells would then be particularly sensitive to complement attack in the presence of defective FH, as is found in more than 30% of aHUS patients presenting with FH mutations or anti-FH autoantibodies.1,19,38 Indeed, levels of C3 deposits on heme-treated cells increased in FH-depleted serum or in the presence of the FH SCR 19-20 fragment known to compete with FH28 or with anti-FH blocking antibodies.
FH enhanced binding to heme-treated cells could result from FH interaction with oxidized epitopes on the heme-exposed membrane39 or with heme itself, bound to the lipid bilayer.40,41 Indeed, a direct binding of heme to FH was detected by SPR in this study. Heme-exposed C3 also bound more strongly to FH compared with native C3. Heme-exposed FH interacted normally with C3 and C3b and, once bound to the cell membrane, it maintained its functional activity.
Complement activation on endothelial cells could also result from the expression of P-selectin (Figure 7D, step 3). Indeed, P-selectin has been identified as a C3b-binding protein, and on cells expressing P-selectin, this expression alone was sufficient to activate the complement system marked by C3b deposition, C3a generation, and C5b9 formation.29 We observed that heme treatment of endothelial cells induced membrane expression of P-selectin concomitantly with the secretion of vWF. This probably results from iron-induced oxidative stress, since oxygen radicals were shown to induce exocytosis of Weibel-Palade bodies and membrane P-selectin.42 The exposure of P-selectin could thus focus the AP activation on the surface of heme-challenged endothelial cells. Of note, the main function of P-selectin is the recruitment of leukocytes expressing its ligand PSGL-1, and heme treatment could thus favor inflammation at the surface of endothelial cells. vWF secretion could recruit platelets and contribute to a prothrombotic state of heme-exposed endothelial cells. The overall effects of heme reported here are reminiscent of those of Shiga toxin, a primary trigger of HUS, which induces P-selectin expression on endothelial cells, resulting in C3 binding and alternative pathway activation together with a vWF-mediated thrombus formation.43
Heme also binds directly to the cell membrane phospholipids,40,44 where it could activate complement as in the fluid phase (Figure 7D, step 4). In addition, the endothelial cell retraction, which occurs after treatment with heme45 and exposes the complement activating subendothelial matrix46 may lead to heme-dependent C3 deposition on the endothelium (Figure 7D, step 5). We indeed observed a preferential localization of C3 deposits along intercellular junctions.
In HUS, we hypothesize that multiple potential triggers such as viral infection, pregnancy, or drugs lead to initial microthrombi formation. Erythrocyte fractionation on these microthrombi would result locally in microvessels in concentrations of heme sufficient to promote the release of vWF and the activation of complement and the release of C5a and C5b9, thus amplifying the initial thrombosis and inflammation. This amplification would normally be limited by the homeostatic control of complement and by the cleavage of vWF by the 13th member of a disintegrin-like and metalloprotease with thrombospondin type 1 motif, 13 (ADAMTS-13). Genetic or antibody-induced alterations of complement proteins in aHUS or of ADAMTS-13 in HUS would synergize with hemolysis, thus decreasing the threshold of the initial triggers to overcome homeostatic control and trigger an explosive endothelial injury and thrombosis. Moreover, heme-induced complement activation and endothelial injury may not be restricted to HUS and could contribute to the pathogenesis of thrombotic events observed in hemolytic episodes of different etiologies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Prof P. Mathieson and Dr S. Satchel (Bristol, United Kingdom) for providing the GEnC cell line, Prof Paul Morgan (Cardiff, United Kingdom) for his gift of the anti-C5b9 mAb, Dr Sakari Jokiranta (Helsinki, Finland) for giving us the FH SCR 19-20 construct, Dr Julie Rayes for her help with the vWF analysis, and Prof P. Lesavre (Paris, France) for the fruitful discussions. Part of the cytometric analysis was done at the Centre d’Imagerie Cellulaire et de Cytomètrie, Centre de Recherche des Cordeliers UMRS 872 (Paris, France). Centre d'Imagerie Cellulaire et de Cytomètrie is a member of the Université Pierre et Marie Curie Flow Cytometry network.
This work was supported by grants from Agence Nationale de la Recherche (ANR Genopath 2009-2012 09geno03101I), Assistance Publique-Hôpitaux de Paris (Programme Hospitalier de Recherche Clinique (AOM08198), European EU FP7 grant 2012-305608 (EURenOmics Consortium), by Association pour l'Information et la Recherche sur les maladies Rénales Génétiques France, and by INSERM.
Authorship
Contribution: L.T.R., L.H.-M., J.D.D., and V.F.-B. designed the project and initiated the research; M.F., L.T.R., L.H.-M., and J.D.D. designed and performed the experiments, did the analysis, and prepared the figures; F.T. and C.P. performed experiments; M.F., L.T.R., L.H.-M., J.D.D., and V.F.-B. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lubka T. Roumenina, Cordeliers Research Center, INSERM UMRS 872, team 13; 15 rue de l’Ecole de Medecine, entrance E, floor 3; 75006 Paris, France; e-mail: lubka.roumenina@crc.jussieu.fr.


![Figure 2. Complement activation on heme-treated endothelial cells results in cell-bound C3 fragments and C5b9. HUVECs or GEnCs were stimulated with increasing concentrations of heme in M199 medium for 20 minutes at 37°C. If not stated otherwise, cells washed with PBS with Ca2+/Mg2+ (Gibco) were incubated for 30 minutes at 37°C in NHS diluted 1:4 in M199 medium. (A) Washed cells were detached with PBS, 1% bovine serum albumin, 10 mM EDTA, 0.1% sodium azide, without (HUVECs) or with 5 mg/mL lidocaine (GEnC) to be analyzed by flow cytometry after labeling with anti-C3c monoclonal antibody (mAb) (Quidel) and phycoerythrin-labeled secondary antibody (mean ± SD of mean fluorescent intensities relative to the mAb isotype control (RFI, n = 3). (B) Washed cells were fixed with 4% paraformaldehyde and 2% saccharose for 20 minutes at room temperature for analysis by cell-ELISA after labeling with biotin–anti-C3c (Quidel) in Tris-buffered saline–bovine serum albumin and peroxidase-extravidin (Sigma) revealed with 3,3′,5,5′-tetramethylbenzidine substrate (Pierce) (mean ± SD of triplicate wells from one of 3 similar experiments). (C) HUVECs were fixed with paraformaldehyde as in (B) to be analyzed by confocal microscopy after labeling with a mouse anti-CD31 mAb and a rabbit anti-C3 Ab revealed with Alexa555 (red)- and Alexa488 (green)-labeled secondary antibodies (Molecular Probes); scale bar, 50 µm. (D) Heme-exposure, under the experimental conditions used, did not induce cell death. HUVECs were exposed to 100 µM heme, quickly detached with trypsin, stained with Annexin V and propidium iodide, and analysed by flow cytometry. Apopto-necrotic HUVECs spontaneously detached overnight from a confluent monolayer served as a positive control for Annexin V and propidium iodide staining. Representative dot plots out of 3 independent experiments are shown. (E) Heme-induced C3 deposition on HUVECs were measured by flow cytometry with an anti-C3c mAb, under conditions favoring a fluid phase complement activation (heme added to the cells together with serum, “heme in serum”), direct effect on the cell surface (heme exposure as in panels [A], [B] and [C], “heme, wash, serum”); or both (cells pre-exposed to heme, then treated with serum with no washing step, “heme, serum”) (mean ± SD of 3 independent experiments). (F) The presence of uncleaved C3 on the surface of heme-exposed HUVECs were tested by staining with anti-C3a mAb the cells exposed with 100 µM heme and NHS as in (E). Blue, resting cells; purple, “heme, wash, serum”; green, “heme, serum”; black, unstained cells; red, isotype control IgG1. (G) Acidic wash of the uncovalently bound C3 forms from the heme-exposed cells. HUVECs were exposed to 100 µM heme, washed and incubated with NHS. Subsequently, 3 washes with PBS (purple histogram) or with a pH 2.7 PBS-25 mM Glycine buffer (green line) were performed. The cells were stained with anti-C3c, anti-C3a or anti-iC3b neoepitope antibody (Quidel) and analyzed by flow cytometry. Signal from unstained cells is presented in black and the isotype control IgG1 is in red; representative histograms of 3 independent experiments. (H) Heme-induced C3 deposition is dependent on the alternative complement pathway. HUVECs treated as in (A), with or without 100 μM heme, were incubated with 1:4 diluted NHS, with or without 10 mM EDTA or 10 mM EGTA and 5 mM MgCl2, C2-depleted or FB-depleted serum, with and without 150 mg/ml purified FB. C3 deposits were measured, as in (A), by flow cytometry (mean ± SD RFI, n = 4). (I) HUVECs treated as in (A) were labeled with a mouse anti-C5b-9 mAb kindly provided by Prof Paul Morgan (Cardiff, United Kingdom) (n=3).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/2/10.1182_blood-2013-03-489245/4/m_282f2.jpeg?Expires=1769206518&Signature=rRcVCjp5w1Bas566ZzZrSYRilMKO93gUycGZsjpzNmnHv21NogVom8MvZTfhEyfGKaAh4InLFDMWv~rcZ~-8zEK4F5O~qg4wT36e8vmIM-hYgIwkYM10EcfbqmqGFt4m0vwenHmBIa6Dw8vQ3RxlOWI5KHZ1M0Xwcaj9kn1z1a-coDQOiMMcxawTfqFYTO~FocJmS2Anmu9wCOkIXXXN8CqfTHEaKjIsjJqnoQF4ot9Ue99JFcWTsjzEACAEiAxCAZAaU1L1L-suUczZlo6xRCxYXgwTL-sfccte~ebm~2WXrKC6KMLHrmkfEgwhcdKrDAW1ioj~lmMxAWGf-8at1g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![Figure 2. Complement activation on heme-treated endothelial cells results in cell-bound C3 fragments and C5b9. HUVECs or GEnCs were stimulated with increasing concentrations of heme in M199 medium for 20 minutes at 37°C. If not stated otherwise, cells washed with PBS with Ca2+/Mg2+ (Gibco) were incubated for 30 minutes at 37°C in NHS diluted 1:4 in M199 medium. (A) Washed cells were detached with PBS, 1% bovine serum albumin, 10 mM EDTA, 0.1% sodium azide, without (HUVECs) or with 5 mg/mL lidocaine (GEnC) to be analyzed by flow cytometry after labeling with anti-C3c monoclonal antibody (mAb) (Quidel) and phycoerythrin-labeled secondary antibody (mean ± SD of mean fluorescent intensities relative to the mAb isotype control (RFI, n = 3). (B) Washed cells were fixed with 4% paraformaldehyde and 2% saccharose for 20 minutes at room temperature for analysis by cell-ELISA after labeling with biotin–anti-C3c (Quidel) in Tris-buffered saline–bovine serum albumin and peroxidase-extravidin (Sigma) revealed with 3,3′,5,5′-tetramethylbenzidine substrate (Pierce) (mean ± SD of triplicate wells from one of 3 similar experiments). (C) HUVECs were fixed with paraformaldehyde as in (B) to be analyzed by confocal microscopy after labeling with a mouse anti-CD31 mAb and a rabbit anti-C3 Ab revealed with Alexa555 (red)- and Alexa488 (green)-labeled secondary antibodies (Molecular Probes); scale bar, 50 µm. (D) Heme-exposure, under the experimental conditions used, did not induce cell death. HUVECs were exposed to 100 µM heme, quickly detached with trypsin, stained with Annexin V and propidium iodide, and analysed by flow cytometry. Apopto-necrotic HUVECs spontaneously detached overnight from a confluent monolayer served as a positive control for Annexin V and propidium iodide staining. Representative dot plots out of 3 independent experiments are shown. (E) Heme-induced C3 deposition on HUVECs were measured by flow cytometry with an anti-C3c mAb, under conditions favoring a fluid phase complement activation (heme added to the cells together with serum, “heme in serum”), direct effect on the cell surface (heme exposure as in panels [A], [B] and [C], “heme, wash, serum”); or both (cells pre-exposed to heme, then treated with serum with no washing step, “heme, serum”) (mean ± SD of 3 independent experiments). (F) The presence of uncleaved C3 on the surface of heme-exposed HUVECs were tested by staining with anti-C3a mAb the cells exposed with 100 µM heme and NHS as in (E). Blue, resting cells; purple, “heme, wash, serum”; green, “heme, serum”; black, unstained cells; red, isotype control IgG1. (G) Acidic wash of the uncovalently bound C3 forms from the heme-exposed cells. HUVECs were exposed to 100 µM heme, washed and incubated with NHS. Subsequently, 3 washes with PBS (purple histogram) or with a pH 2.7 PBS-25 mM Glycine buffer (green line) were performed. The cells were stained with anti-C3c, anti-C3a or anti-iC3b neoepitope antibody (Quidel) and analyzed by flow cytometry. Signal from unstained cells is presented in black and the isotype control IgG1 is in red; representative histograms of 3 independent experiments. (H) Heme-induced C3 deposition is dependent on the alternative complement pathway. HUVECs treated as in (A), with or without 100 μM heme, were incubated with 1:4 diluted NHS, with or without 10 mM EDTA or 10 mM EGTA and 5 mM MgCl2, C2-depleted or FB-depleted serum, with and without 150 mg/ml purified FB. C3 deposits were measured, as in (A), by flow cytometry (mean ± SD RFI, n = 4). (I) HUVECs treated as in (A) were labeled with a mouse anti-C5b-9 mAb kindly provided by Prof Paul Morgan (Cardiff, United Kingdom) (n=3).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/2/10.1182_blood-2013-03-489245/4/m_282f2.jpeg?Expires=1769206519&Signature=0ILTeyNvrMC38EthdrnJopbMZ3jkGnEjZP8GC72fZep50VN7oUj8SOC7injCqbvDyzXpEcQC1VSty70-EHdRttFbscZgrres1yIRO~t88WmSb5dveTEEclVL7qouERFlEWQ383cUNxHEvlwrLzEWPEd0Q2HRTlVdH26luxTHu95r2AFVm~SI~NvR-9br~ldffI3cJQmb8Iv1BakyScbEKj0hZxoqJcwbKbHIe6P6zmIKvmiD6EFO9gbhUKrgoQaqkdpUoCw~UXRRRTd7DKOJs8T-l2bINQ9D3I0FvWs6bwIRz7ipoYzYktBvtCK2m7gTQs3L8Px44tzpwTwt-ysqlg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)