To the editor:
Transformation of polycythemia vera (PV) into post-PV myelofibrosis (MF) occurs at a rate of 5.1 per 1000 person-years.1 The reverse of this transformation, from MF to PV, has been described2-5 but is rare, and these cases predate testing for the JAK2V617F mutation. Reverse transformation has not been described in 1000 cases of patients with primary myelofibrosis (PMF) seen at the Mayo Clinic.6 We wish to report a case of a patient who presented with PMF that subsequently evolved to PV. This report represents, to the best of our knowledge, the first published case report of a patient with “reversed” transformation that has undergone JAK2V617F confirmation.
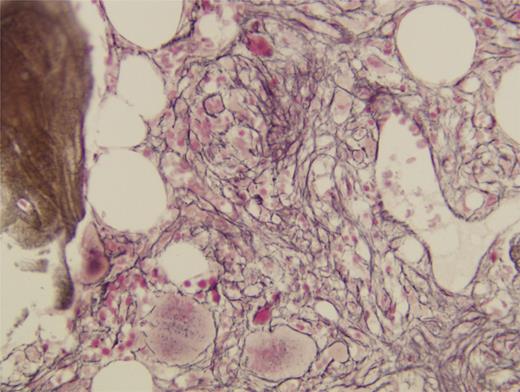
A 51-year-old woman presented with weakness, fatigue, and progressive dyspnea. Examination revealed tachycardia, tachypnea, and massive splenomegaly. Initial laboratory studies showed normocytic anemia with a hemoglobin concentration of 8.8 g/dL, as well as profound leukopenia with repeated absolute neutrophil counts of less than 1000 K/uL. The platelet count was normal, and no circulating blasts were detected. Renal and hepatic function were within normal limits. Serum erythropoietin level was elevated at 80.5 mU/mL. A peripheral blood smear showed many teardrop red cells and rare nucleated erythrocytes. Ferritin level was with normal range (65 ng/ml). Bone marrow aspiration was acellular, and a bone marrow biopsy result revealed a hypocellular marrow with grade 3 to 4 fibrosis demonstrated by reticulum staining (Figures 1 and 2). There were no findings to suggest secondary fibrosis, and the patient was diagnosed with PMF, for which she received red blood cell transfusions.
At 1 year later, the patient remained transfusion dependent, and results on repeated bone marrow biopsy showed progressive fibrosis. During the course of the next year, cell counts for all 3 lineages were noted to spontaneously increase; therefore, transfusions were discontinued. The slow increase in cell counts continued without treatment, and after 4 years, both hemoglobin and platelet counts exceeded the normal range.
In 2007, testing for the JAK2V617F mutation revealed the presence of mutant alleles (not quantified). Total body red cell volume was 77 mL/kg, twice the laboratory’s top normal range in women (28 mL/kg). Serum erythropoietin level was repeated and was found to have decreased below normal at 3.0 mU/mL. A diagnosis of PV was made, and a therapeutic phlebotomy was initiated.
To date, the patient has undergone 38 therapeutic phlebotomies during a 6-year period, and the patient continues with elevated hemoglobin concentrations and platelet counts. Her symptoms are well controlled, although she continues to have splenomegaly according to both physical examination and radiographic imaging results. She has never had a thrombotic event, nor has she ever received a cytotoxic agent.
Result of a repeated bone marrow biopsy, performed 12 years after the initial diagnosis of PMF and 6 years after the diagnosis of PV, was a markedly hypercellular presence, without reticulum or fibrosis. Genetic study results showed a normal karyotype, and fluorescence in situ hybridization testing results did not reveal abnormalities. A quantitative JAK2 assay result showed an allele burden of 54%. At this point in the course of the disease, there is no evidence of prior MF in this patient.
The case of this patient demonstrates the evolution of transfusion-dependent PMF into phlebotomy-dependent PV, a reversal of the usual progression. According to the DPSS-plus risk system, the patient had 3 adverse features on presentation, placing her in the Intermediate-2 group with a median survival time of 3.6 years. Despite these adverse features on presentation, she has now survived 13 years after the intial presentation and is behaving in an indolent fashion, typical of PV and not PMF.
Authorship
Contributions: M.J.B. and P.I.R. wrote the paper, and P.D. provided pathologic review and photomicrographs.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paul I. Roda, Geisinger-Hazleton Cancer Center, 1740 E. Broad St, Hazleton, PA 18201; e-mail: proda1@geisinger.edu.