Key Points
Phagocytosis of CLL targets by neutrophils is a novel mechanism of action of the glycoengineered anti-CD20 antibody obinutuzumab.
This mechanism takes place in physiological conditions and requires CD16B and CD32A.
Abstract
Obinutuzumab (GA101) is a glycoengineered type 2 CD20 antibody with enhanced CD16A-binding and natural killer–mediated cytotoxicity. CD16B is highly homologous to CD16A and a major FcγR on human polymorphonuclear neutrophils (PMNs). We show here that glycoengineered obinutuzumab or rituximab bound CD16B with approximately sevenfold higher affinity, compared with nonglycoengineered wild-type parental antibodies. Furthermore, glycoengineered obinutuzumab activated PMNs, either purified or in chronic lymphoblastic leukemia whole blood, more efficiently than wild-type rituximab. Activation resulted in a 50% increase in CD11b expression and 70% down-modulation of CD62L on neutrophils and in release of tumor necrosis factor alpha, IL-6, and IL-8. Activation was not accompanied by generation of reactive oxygen species or antibody-dependent cellular cytotoxicity activity, but led to up to 47% phagocytosis of glycoengineered anti-CD20 opsonized chronic lymphoblastic leukemia targets by purified PMNs. Significant phagocytosis was observed in whole blood, but only in the presence of glycoengineered antibodies, and was followed by up to 50% PMN death. Finally we show, using anti-CD16B and anti-CD32A Fab and F(ab’)2 fragments, that both of these receptors are involved in PMN activation, phagocytosis, and cell death induced by glycoengineered antibodies. We conclude that phagocytosis by PMNs is an additional mechanism of action of obinutuzumab mediated through its higher binding affinity for CD16B.
Introduction
The chimeric unmodified, wild-type CD20 IgG1 monoclonal antibody rituximab (MabThera and Rituxan) has shown significant therapeutic activity in B-non–Hodgkin lymphoma (B-NHL) and chronic lymphocytic leukemia (CLL).1,2 Rituximab is thought to act largely through immune-mediated mechanisms: complement-dependent cytotoxicity (CDC), antibody-dependent cellular cytotoxicity (ADCC), and/or antibody-dependent phagocytosis (ADCP) by macrophages/monocytes.3,4 CD20 IgG1 antibodies can differ in their functional properties according to their binding mode to CD20. They are classified as type 1 when they show high CDC such as rituximab or as type 2 when they show high homotypic adhesion and direct cell death, respectively.5,6 Improved versions of rituximab have been developed with the scope of enhancing response rates and reducing relapse or resistance.7,8 One modification applied for CD20 and other therapeutic antibodies is termed “glycoengineering” and results in the decreased fucosylation of the carbohydrate attached to the Asn-297 glycosylation site of the Fc portion of the antibody.9-15 Obinutuzumab (GA101) is a glycoengineered CD20 antibody derived from the murine Bly-1 antibody and is currently in pivotal clinical trials for the treatment of B-NHL and CLL in direct comparison with rituximab.16-18 A fucosylation of IgG1 antibodies such as obinutuzumab increases their affinity for both polymorphic forms of CD16A on natural killer (NK) cells by 7- to 10-fold and therefore results in enhanced ADCC.9,10,12 Furthermore, ADCC by glycoengineered antibodies is less inhibited by complement activation or excess-free IgG, unlike that of nonglycoengineered parental antibodies.19,20 Thus, ADCC by glycoengineered antibodies takes place under physiological conditions, such as whole blood, and may therefore further enhance efficacy in vivo. Obinutuzumab is a type 2 CD20 antibody and shows enhanced direct cell death induction compared with rituximab, in addition to increased ADCC.21
Polymorphonuclear neutrophils (PMNs) are the most abundant phagocytes in the circulation. When activated, they may degranulate, release important inflammatory mediators, produce radical oxygen species, and mediate ADCC and phagocytosis.22,23 PMNs express CD32A (FcγRIIA) and CD16B (FcγRIIIB).24-26 The latter shows >97% amino acid sequence identity with CD16A in its extracellular ligand–binding domain but is GPI anchored and lacks a signaling module. CD16B is a polymorphic molecule, the NA1 and NA2 polymorphisms within the coding sequence being the most common forms in the human population. CD16B is known to be involved in PMN activation and immune complex–mediated phagocytosis by neutrophils.26-29 Still little is known about the role of PMNs in the therapeutic activity of IgG antibodies.30 Results about their role in vivo using murine models are still controversial.31-35 Therefore, we have investigated the functional activity of PMNs in response to CD20 antibodies obinutuzumab and rituximab, in both their glycoengineered or nonglycoengineered (wild-type) formats.
Materials and methods
Antibodies
Glycoengineered and nonglycoengineered wild-type rituximab, obinutuzumab, and control anti-melanoma antigen M4-3ML2 antibodies were prepared as described.9 Control IgG1κ anti-HER2 trastuzumab (TRZ; Herceptin, Roche) and anti-HER1 cetuximab (CTX, Erbitux; Bristol-Myers Squibb) were a kind gift from Dr. Carlo Tondini (Oncology Department, Ospedale Papa Giovanni XXIII, Bergamo). Anti-CD16 (clone 3G8) Fab and F(ab’)2 fragments, as well as anti-CD32 F(ab’)2 (clone 7.3), were from Adipogen AG (Liestal, Switzerland). Anti-CD32 Fab fragment (clone IV.3) was from Fusion Antibodies.36
Surface plasmon resonance
Recombinant soluble CD16B-NA2 (O75015 UniProt, amino acids 17-199) and CD32A-R131 (P12318, amino acids 34-217) ectodomains with a C-terminal hexahistidine tag were expressed in HEK293-EBNA. The CD32A-H131 isoform was produced in CHO cells.
Surface plasmon resonance experiments were performed on a Biacore T200 with HBS-EP as a running buffer. For CD16B-NA2 measurements, direct coupling of the anti-human Fab-specific antibody was performed on a CM5 chip at pH 5.0 using the standard amine coupling kit (GE Healthcare), and different antibodies at 200 nM were captured on the second flow cell. A dilution series of CD16B-NA2 (from 31.25-8000 nM) was passed on both flow cells to record the association phase (120 seconds). The dissociation phase was monitored for 120 seconds and triggered by switching from the sample solution to HBS-EP. Bulk refractive index differences were corrected by subtracting the response obtained on the reference flow cell (first flow cell). The steady-state response was used to derive the dissociation constant KD by nonlinear curve fitting of the Langmuir binding isotherm using the Biaeval software (GE Healthcare). For CD32A measurements, a dilution series of CD32A (from 15.6-8000 nM) was used. The association and the dissociation phases were monitored for both polymorphisms for 180 seconds. The analysis was performed as described for CD16B-NA2.
Cells
Peripheral blood, drawn in 50 µg/mL of lepirudin37 (Refludan; Celgene Corporation, Summit, NJ), was obtained from patients with untreated CLL or from healthy volunteers after informed consent in accordance with the Declaration of Helsinki. The study was approved by the hospital’s Ethical Committee. In some cases, the mononucleated cell fraction (peripheral blood mononuclear cells [PBMCs]) was a purified by standard Ficoll Hypaque gradient centrifugation (Seromed, Berlin, Germany). The CD20+ Burkitt lymphoma cell line BJAB has been described previously.38
PMNs were purified from peripheral blood by sedimentation over a 50-g/L dextran solution followed by standard Ficoll Hypaque gradient centrifugation (Seromed). The washed lower phase contained >90% CD15+ PMNs.
Antibody binding to PMNs
Healthy volunteers were screened for CD16B NA1 and NA2 polymorphisms as described39 and at least 3 homozygous individuals for each polymorphism were identified. Anti-CD20 1 mg/mL was mixed with fluorescein isothiocyanate (FITC)-labeled anti-human κ light chain F(ab’)2 antibody (Dako Italia SpA, Milan, Italy) and incubated for 30 minutes at 4°C with 3 × 105 PMNs at a 10 µg/mL final concentration. Binding was analyzed by flow cytometry.
PMN activation and cell death
Experiments on PMNs were performed either with 400 μL of unmanipulated peripheral blood from patients with CLL or from healthy donors, drawn in 50 µg/mL of lepirudin, or with 5 × 105 purified PMNs from a healthy donor mixed with CLL or BJAB cells at a 1:3 ratio (PMN:CLL). In some experiments, whole blood from a healthy donor was spiked with CLL mononuclear cells, at a 1:3 PMN:CLL ratio. The samples were plated with 1 to 100 µg/mL of anti-CD20 or 0.1 µM of phorbol myristic acetate (PMA, Sigma). In some cases, 10 μg/mL of anti-CD16 or anti-CD32 F(ab’)2 antibody fragments were added 5 minutes before the anti-CD20 antibodies. Samples were incubated for 2 to 24 hours at 37°C, 5% CO2 and then stained for 20 minutes with anti-CD62L-APC and anti-CD11b-PE antibodies to measure PMN activation, or with anti-CD15-FITC and 7-aminoactinomycin-D, to measure PMN cell death. Whole blood samples were then lysed with hypotonic lysis solution (Pharm Lyse; BD Biosciences, San José, CA) to eliminate platelets and red blood cells, whereas purified PMNs were simply washed in physiological solution. All samples were analyzed on a FACSCantoII instrument (BD Biosciences).
ROS production
Whole blood or 4 × 105 purified PMNs were incubated for 30 minutes with 1 to 3 µM of 2',7'-dichlorodihydrofluorescein diacetate. CLL or BJAB cell lines at 100 µL were then added at a 1:5 effector:target (E:T) ratio, in the presence or absence of different CD20 antibody concentrations. After 40 minutes of incubation at 37°C, 5% of CO2, 20 µL of anti-CD15-FITC were added, and incubation was carried out for an additional 20 minutes. Cells were then lysed in hypotonic Pharm Lyse solution (whole blood) or washed once in physiological solution (purified PMNs), and were analyzed by flow cytometry.
Measurements of cytokines
Levels of cytokines in plasma were measured by flow cytometry using the BD Cytometric Bead Array kit, according to the manufacturer’s instructions (BD Biosciences).
Phagocytosis assays
Thawed CLL cells were stained with 2 µM of PKH26 dye (Life Technologies, Monza, Italy) and were mixed with purified PMNs or whole blood from a healthy donor in a 1:3 E:T ratio, in the presence or absence of anti-CD20. In some cases, 10 μg/mL of blocking anti-CD16 or anti-CD32 Fab fragments were added 5 minutes before anti-CD20 or 20% human serum (HS) or heat-inactivated HS were added. Cells were incubated at 37°C for 2 to 24 hours, and then stained with anti-CD15-FITC and CD19-APC (BD Biosciences), washed, and analyzed by flow cytometry. Percentage phagocytosis was defined as percentage PKH26+/CD15+/CD19APC- cells relative to total CD15+ cells. In some cases, samples were centrifuged onto glass slides at 500 rpm for 5 minutes using a Shandon centrifuge, fixed in 2% paraformaldehyde, and mounted in mounting medium containing 1.5 μg/mL of 4,6 diamidino-2-phenylindole (Vectashield; Vector Laboratories, Burlingame, CA). Slides were viewed at room temperature in an Axio Imager.Z2 microscope and 20× EC Plan-NEOFLUAR 20×/0.5 Ph2∞/0.17 lens (Zeiss Microscopy GmbH, Jena, Germany). Images were taken with a CoolCube1c camera and Isis Fluorescence Imaging System software (MetaSystems GmbH, Altlussheim, Germany) at 20× magnification.
ADCC
ADCC was performed as described previously,40 using as effectors either purified PMNs or PBMCs as the source of NK cells, at a 10:1 E:T ratio.
Statistical analysis
The data were analyzed using the paired or unpaired Student t test, as appropriate. *: P < .05; **: P < .01; ***: P < .001.
Results
CD16B has a higher affinity for the glycoengineered than the wild-type CD20 antibodies
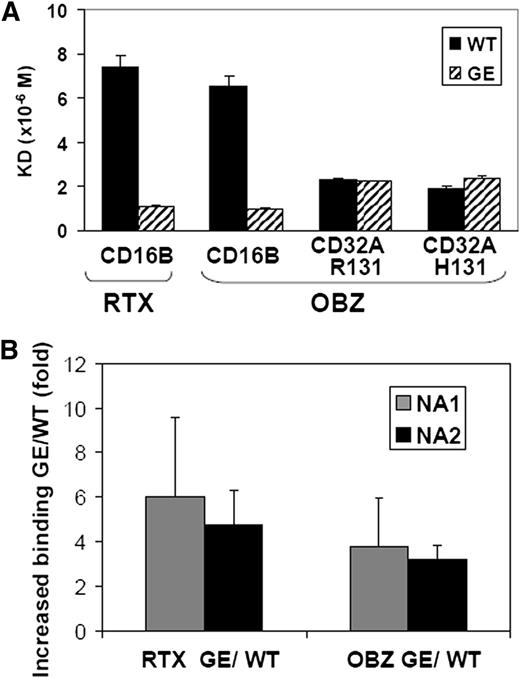
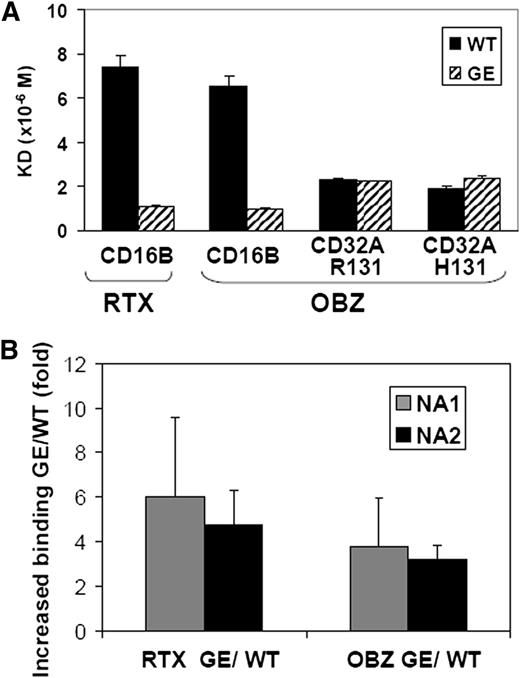
Glycoengineered obinutuzumab has a higher affinity for CD16A than nonglycoengineered (wild-type) rituximab. The different CD16B isoforms (NA1, NA2, and SH) share >97% sequence identity with CD16A in their extracellular portion (supplemental Figure 1), suggesting that glycoengineering may increase the affinity of IgG1 antibodies also for CD16B. Therefore, we measured by surface plasmon resonance the binding affinity for recombinant human CD16B of obinutuzumab and rituximab, either in their glycoengineered or wild-type forms. The NA2 isoform of CD16B was used because previous data had suggested only a minor effect of CD16B polymorphisms on IgG1 binding.41,42 Both glycoengineered antibodies showed a 6- to 7-fold higher affinity for CD16B than their wild-type counterparts (Figure 1A). We also analyzed the binding constant of glycoengineered and wild-type obinutuzumab for CD32A-R131 and CD32A-H131. We observed that glycoengineering did not affect the binding affinity for the two CD32A isoforms (Figure 1A).
Glycoengineered CD20 antibodies bind to CD16B with higher affinity than their non–glycoengineered wild-type counterparts. (A) The dissociation constants of the glycoengineered (GE; striped bars) and nonglycoengineered wild-type formats (WT, black bars) of obinutuzumab (OBZ) and rituximab (RTX) for purified soluble CD16B-NA2, CD32A-R131, or CD32A-H131 proteins were measured by surface plasmon resonance. (B) The glycoengineered (GE) and nonglycoengineered wild-type (WT) CD20 antibodies were cross-linked with FITC-labeled anti-κ light chain F(ab’)2, and binding to purified PMNs from NA1 (grey bars) and NA2 homozygous donors (black bars) was measured by flow cytometry. The results are the mean fluorescence intensity ratios of glycoengineered vs wild-type rituximab (RTX) or obinutuzumab (OBZ), obtained from 4 experiments using at least 3 different donors for each CD16B isoform.
Glycoengineered CD20 antibodies bind to CD16B with higher affinity than their non–glycoengineered wild-type counterparts. (A) The dissociation constants of the glycoengineered (GE; striped bars) and nonglycoengineered wild-type formats (WT, black bars) of obinutuzumab (OBZ) and rituximab (RTX) for purified soluble CD16B-NA2, CD32A-R131, or CD32A-H131 proteins were measured by surface plasmon resonance. (B) The glycoengineered (GE) and nonglycoengineered wild-type (WT) CD20 antibodies were cross-linked with FITC-labeled anti-κ light chain F(ab’)2, and binding to purified PMNs from NA1 (grey bars) and NA2 homozygous donors (black bars) was measured by flow cytometry. The results are the mean fluorescence intensity ratios of glycoengineered vs wild-type rituximab (RTX) or obinutuzumab (OBZ), obtained from 4 experiments using at least 3 different donors for each CD16B isoform.
Next, we performed binding assays on whole PMNs by flow cytometry. Human PMNs express CD16B at high levels, CD32A more weakly and CD64 only after activation by interferon-γ (supplemental Figure 2). Thus >95% of IgG binding to resting PMNs is mediated by CD16B, as confirmed by blocking experiments with anti-CD16 F(ab’)2 fragments (data not shown and Nagarajan et al28 ). To investigate the possible role of the most commonly expressed CD16B isoforms, we performed binding assays using PMNs isolated from either NA1 or NA2 homozygous individuals. We observed a 3.5- to 6-fold higher binding of glycoengineered compared with wild-type CD20 antibodies by human purified PMNs in vitro. There was no significant difference between NA1 or NA2 homozygous PMNs (Figure 1B).
We conclude that glycoengineering increases binding to both CD16B NA1 or NA2 isoforms, but not to CD32A.
Obinutuzumab induces PMN activation more efficiently than rituximab
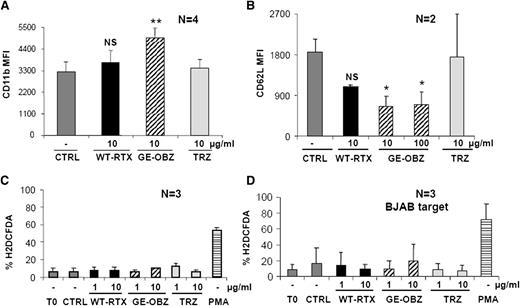
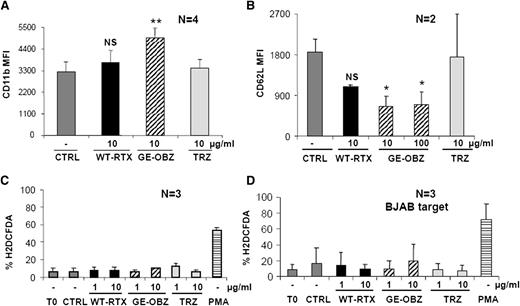
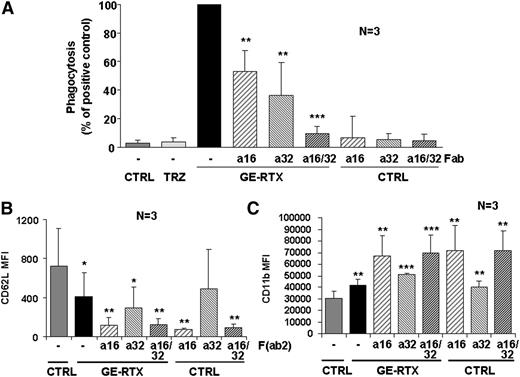
Next, we investigated whether CD20 antibodies could activate PMNs in vitro. Purified PMNs from healthy donors were incubated with CLL targets opsonized with either glycoengineered obinutuzumab or nonglycoengineered wild-type rituximab or TRZ as control and CD11b, CD11a, CD16B, and CD62L expression analyzed after 2, 6, or 24 hours. Obinutuzumab was found to reproducibly induce by 1.5- to 2-fold CD11b expression on PMNs at 6 and 24 hours (Figure 2A and data not shown) and to down modulate by ∼70% CD62L expression at the same time points (Figure 2B) (P < .01 or P < .05, respectively). The effect of wild-type rituximab was weaker and did not reach statistical significance. An induction of CD11a (lymphocyte function antigen-1) and downmodulation of CD16B on PMNs were also observed (data not shown), but only CD11b and CD62L were used as markers of PMN activation in further experiments.
Glycoengineered obinutuzumab activates purified PMNs more efficiently than wild-type rituximab. CLL (panels A-C) or BJAB targets (panel D) were opsonized with 1 to 100 µg/mL of nonglycoengineered wild-type rituximab (WT-RTX), glycoengineered obinutuzumab (GE-OBZ), or control TRZ antibodies and incubated with purified PMNs from healthy donors at a 1:3 E:T ratio. PMA was used as a control. CD11b (panel A), CD62L (panel B), or ROS expression (panels C-D) on PMNs was analyzed by flow cytometry after 24 hours, 6 hours, or 1 hour, respectively. The data are the means and standard deviations of 2 to 4 experiments.
Glycoengineered obinutuzumab activates purified PMNs more efficiently than wild-type rituximab. CLL (panels A-C) or BJAB targets (panel D) were opsonized with 1 to 100 µg/mL of nonglycoengineered wild-type rituximab (WT-RTX), glycoengineered obinutuzumab (GE-OBZ), or control TRZ antibodies and incubated with purified PMNs from healthy donors at a 1:3 E:T ratio. PMA was used as a control. CD11b (panel A), CD62L (panel B), or ROS expression (panels C-D) on PMNs was analyzed by flow cytometry after 24 hours, 6 hours, or 1 hour, respectively. The data are the means and standard deviations of 2 to 4 experiments.
Reactive oxygen species (ROS) production by PMNs was then measured. Neutrophils were cocultured with CLL cells (Figure 2C) or the BJAB cell line opsonized with glycoengineered obinutuzumab or wild-type rituximab (Figure 2D). In neither case could significant ROS production be measured, whereas PMA used as a control was clearly active (Figure 2C-D).
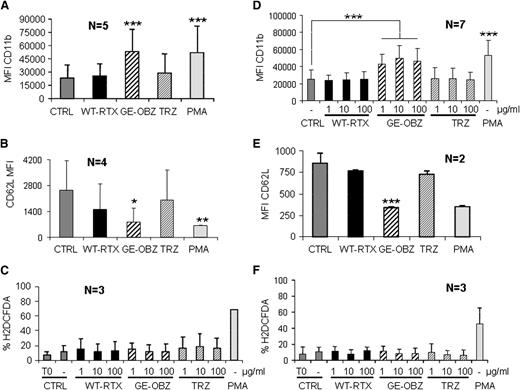
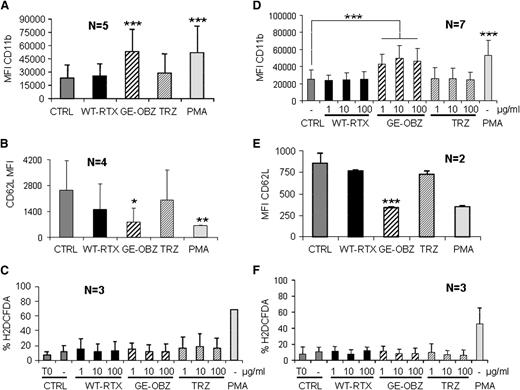
Next, we wished to establish whether PMN activation took place under physiological conditions (ie, in whole blood). We first verified that PMN activation was unaffected by the anticoagulant lepirudin (supplemental Figure 3). We then performed PMN activation experiments using whole blood from both healthy donors or patients with CLL drawn in lepirudin. As shown in Figure 3, glycoengineered obinutuzumab increased CD11b on PMNs by 2.5-fold (Figure 3A,D) and decreased CD62L expression by 60% to 70% (Figure 3B,E), whereas wild-type rituximab was not significantly active. This was true for both healthy donors (panels A-B) and CLL samples (panels D-E). As expected, similar PMN activation was not observed in presence of an irrelevant glycoengineered or wild-type melanoma antibody (data not shown). ROS was induced by PMA at 30 or 60 minutes, but not by any of the antibodies tested in whole blood assays (Figure 3C,F), similarly to what had been observed with purified PMNs (Figure 2C-D), both from healthy donors or CLL samples (Figure 3C,F).
Glycoengineered obinutuzumab activates PMNs in whole blood more efficiently than wild-type rituximab. Nonglycoengineered wild-type rituximab (WT-RTX) or glycoengineered obinutuzumab (GE-OBZ) at 10 µg/mL (panels A,B,E); 1, 10, or 100 µg/mL (panels D,C,F); or 0.1 µM of PMA were added to whole blood from healthy donors (panels A-C) or from patients with CLL (panels D-F). CD11b (panels A,D), CD62L (panels B,E), and ROS expression by PMNs (panels C,F) were analyzed after 24 hours, 6 hours, and 1 hour, respectively. The data are the means and standard deviations of 2 to 7 independent experiments, as indicated in each panel.
Glycoengineered obinutuzumab activates PMNs in whole blood more efficiently than wild-type rituximab. Nonglycoengineered wild-type rituximab (WT-RTX) or glycoengineered obinutuzumab (GE-OBZ) at 10 µg/mL (panels A,B,E); 1, 10, or 100 µg/mL (panels D,C,F); or 0.1 µM of PMA were added to whole blood from healthy donors (panels A-C) or from patients with CLL (panels D-F). CD11b (panels A,D), CD62L (panels B,E), and ROS expression by PMNs (panels C,F) were analyzed after 24 hours, 6 hours, and 1 hour, respectively. The data are the means and standard deviations of 2 to 7 independent experiments, as indicated in each panel.
We conclude that glycoengineered obinutuzumab activates PMNs, both purified and in whole blood, more effectively than wild-type rituximab, but it does not induce significant ROS production.
Obinutuzumab induces cytokine production
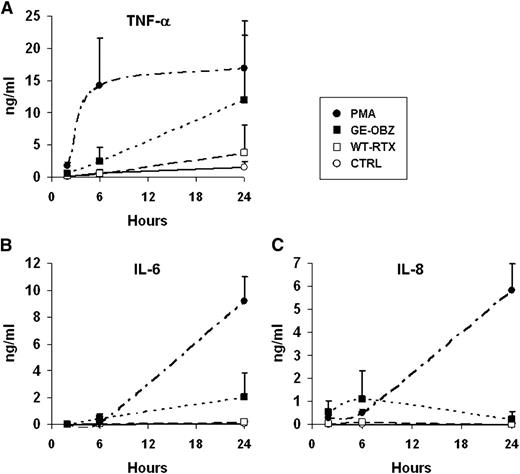
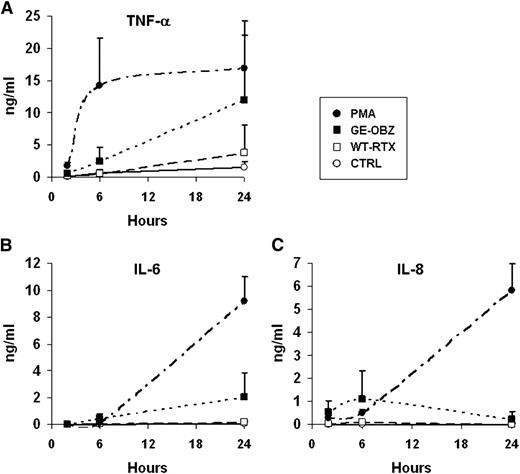
Given that CD16A and CD16B are expressed by different immune cells, we also measured the levels of tumor necrosis factor-alpha (TNF-α), IL-1β, IL-6, IL-8, IL-10, and IL-12p70 released in CLL whole blood after stimulation with glycoengineered obinutuzumab vs wild-type rituximab. Significant amounts of IL-8, TNF-α, and IL-6, but not other cytokines, were detected after stimulation with CD20 antibodies. Obinutuzumab was, in all cases, more active than rituximab. TNF-α was induced more rapidly with a peak of ∼1 ng/mL at 6 hours (Figure 4A), whereas IL-8 and IL-6 were induced more slowly, reaching means of ∼10 ng/mL and 2 ng/mL, respectively (Figure 4B-C). Maximal levels were, in all cases, well below those observed with PMA.
Glycoengineered obinutuzumab induces higher levels of TNF-α, IL-6, and IL-8 than wild-type rituximab. CLL whole blood was incubated for 2, 6, or 24 hours in the presence of 10 µg/mL of nonglycoengineered wild-type rituximab (WT-RTX, open squares), glycoengineered obinutuzumab (GE-OBZ, closed squares), control antibody cetuximab (CTRL, open circles), or 0.1 µM of PMA (closed circles). TNF-α (A), IL-6 (B), and IL-8 (C) in plasma were measured by flow cytometry using calibrated beads. The results are the means and standard deviations of 3 independent experiments.
Glycoengineered obinutuzumab induces higher levels of TNF-α, IL-6, and IL-8 than wild-type rituximab. CLL whole blood was incubated for 2, 6, or 24 hours in the presence of 10 µg/mL of nonglycoengineered wild-type rituximab (WT-RTX, open squares), glycoengineered obinutuzumab (GE-OBZ, closed squares), control antibody cetuximab (CTRL, open circles), or 0.1 µM of PMA (closed circles). TNF-α (A), IL-6 (B), and IL-8 (C) in plasma were measured by flow cytometry using calibrated beads. The results are the means and standard deviations of 3 independent experiments.
We conclude that higher-affinity binding to CD16A and CD16B by glycoengineering leads to increased production of inflammatory cytokines in whole blood.
Activated PMNs mediate phagocytosis but not ADCC
Next, we investigated whether activation of PMNs by glycoengineered obinutuzumab could lead to ADCC. We performed assays using target BJAB cells and either purified PMNs as effectors or PBMCs as a source of NK cells. PBMCs mediated ∼35% lysis of BJAB targets in the presence of obinutuzumab, whereas PMNs were inactive in the same conditions (supplemental Figure 4A-B, respectively). These data suggest that PMNs do not mediate significant ADCC with obinutuzumab.
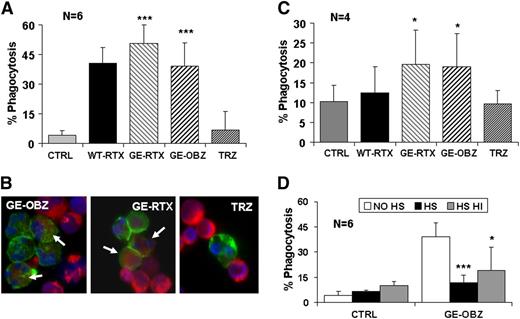
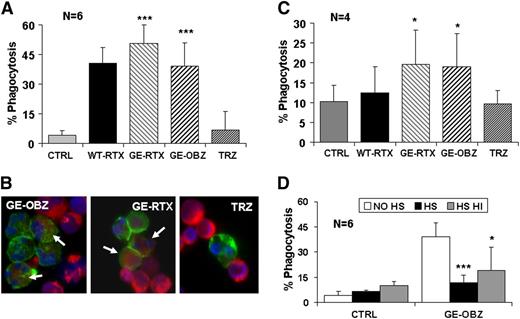
Next, we asked whether PMNs could phagocytose CLL targets opsonized by CD20 antibodies. We first performed experiments using purified PMNs from healthy donors and used triple fluorescence analysis by flow cytometry to measure phagocytosis. All CD20 antibodies were observed to mediate significant phagocytosis, ranging from 38% to 47% at 24 hours (Figure 5A). Glycoengineered rituximab was somewhat more efficient than its parental antibody, as expected by its higher capacity to activate PMNs (data not shown), similar to glycoengineered obinutuzumab. A time course of phagocytosis showed that significant phagocytosis (12%-33%) was observed already at 6 hours and was maximal at 24 hours (28%-42%) and could not be detected with an irrelevant glycoengineered or wild-type melanoma antibody (M3-4ML2, supplemental Figure 5). Finally, we performed cytospin at the end of the assay and observed green (CD15+) PMNs having engulfed red (PKH26+) CLL targets (Figure 5B).
Glycoengineered CD20 antibodies mediate phagocytosis of CLL targets by PMNs. CLL targets were labeled with PKH26 and incubated for 24 hours with either purified PMNs (panels A,B,D) or whole blood from healthy donors (panel C), in the presence or absence of 10 µg/mL of the indicated antibodies. Phagocytosis was measured as the percentage of CD15+/PKH26+/CD19- cells with respect to total CD15+ cells. The data are the means and standard deviations of 4 to 6 experiments. In some experiments with purified PMNs, cytospins were prepared to visualize CD15-FITC–labeled PMNs (green) having engulfed PKH26+ CLL targets (red). Slides were mounted in medium containing 4,6 diamidino-2-phenylindole to visualize the nuclei (blue) under a fluorescence microscope (original magnification ×20). Statistical significance was calculated for antibody treated vs control. In panel D, the statistical significance was calculated for samples treated with HS or heat-inactivated HS with respect to no serum (no HS).
Glycoengineered CD20 antibodies mediate phagocytosis of CLL targets by PMNs. CLL targets were labeled with PKH26 and incubated for 24 hours with either purified PMNs (panels A,B,D) or whole blood from healthy donors (panel C), in the presence or absence of 10 µg/mL of the indicated antibodies. Phagocytosis was measured as the percentage of CD15+/PKH26+/CD19- cells with respect to total CD15+ cells. The data are the means and standard deviations of 4 to 6 experiments. In some experiments with purified PMNs, cytospins were prepared to visualize CD15-FITC–labeled PMNs (green) having engulfed PKH26+ CLL targets (red). Slides were mounted in medium containing 4,6 diamidino-2-phenylindole to visualize the nuclei (blue) under a fluorescence microscope (original magnification ×20). Statistical significance was calculated for antibody treated vs control. In panel D, the statistical significance was calculated for samples treated with HS or heat-inactivated HS with respect to no serum (no HS).
We then performed phagocytosis assays in whole blood. Phagocytosis did indeed take place in whole blood, although to a lower extent than with purified PMNs (Figure 5C). Glycoengineered CD20 antibodies were significantly more efficient that wild-type rituximab, with 12% to 13% phagocytic PMNs observed above background. In contrast wild-type rituximab was not significantly active in these conditions. The lower phagocytosis observed in whole blood is likely the result of competition by free IgGs and complement activation in plasma, similarly to what has been observed previously for NK-mediated ADCC and macrophage-mediated phagocytosis.19,37,43 Indeed, the addition of 50% untreated or heat-inactivated HS induced ∼50% to 75% inhibition of phagocytosis by purified PMNs, suggesting an effect of competing IgGs in plasma (Figure 5D).
We conclude that glycoengineered CD20 antibodies mediate significant phagocytosis by both purified PMNs and PMNs in whole blood and are more efficient in this activity than wild-type rituximab.
Phagocytosis of CLL targets by PMNs requires CD16B and CD32A
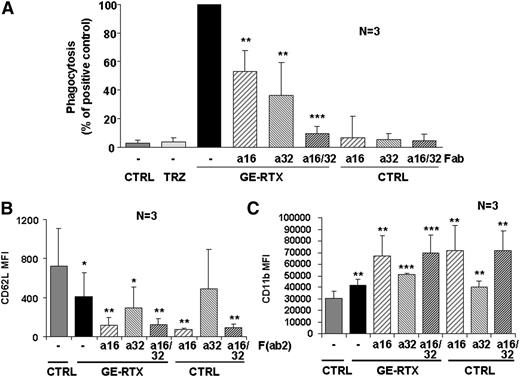
We next investigated the role of FcγRs in phagocytosis using Fab fragments of blocking anti-CD16 and anti-CD32 antibodies. We chose glycoengineered rituximab as a positive control because obinutuzumab induces stronger homotypic adhesion, a phenomenon that may cause artifacts by flow cytometry.19,44 We observed that phagocytosis of glycoengineered rituximab-opsonized targets by purified PMNs in whole blood was partially blocked by either Fab fragment, but fully inhibited when both were present (Figure 6A). These data suggest that both CD16B and CD32A are involved in phagocytosis by glycoengineered CD20 antibodies.
CD16B and CD32A mediate PMN activation and phagocytosis. (A) Phagocytosis. CLL targets were labeled with PKH26 and incubated for 24 hours in the presence or absence of 10 µg/mL of glycoengineered rituximab (GE-RTX) and/or 10 µg/mL of blocking anti-CD16 (a16) or anti-CD32 (a32) Fab fragments. Phagocytosis was measured by flow cytometry. The statistical significance of adding anti-CD16 and/or anti-CD32 Fab fragments with respect to adding CD20 antibody alone is shown. (B-C) PMN activation. CLL targets were opsonized with 10 µg/mL of glycoengineered rituximab antibody and added to whole blood from healthy donors in the presence or absence of 10 µg/mL of anti-CD16 (a16) or anti-CD32 (a32) F(ab’)2 fragments. CD62L down-modulation on PMNs was measured at 6 hours (panel B), whereas CD11b induction was measured at 24 hours (panel C). The statistical significance of treated vs control (CTRL) samples is shown. All data are the means and standard deviations of 3 independent experiments.
CD16B and CD32A mediate PMN activation and phagocytosis. (A) Phagocytosis. CLL targets were labeled with PKH26 and incubated for 24 hours in the presence or absence of 10 µg/mL of glycoengineered rituximab (GE-RTX) and/or 10 µg/mL of blocking anti-CD16 (a16) or anti-CD32 (a32) Fab fragments. Phagocytosis was measured by flow cytometry. The statistical significance of adding anti-CD16 and/or anti-CD32 Fab fragments with respect to adding CD20 antibody alone is shown. (B-C) PMN activation. CLL targets were opsonized with 10 µg/mL of glycoengineered rituximab antibody and added to whole blood from healthy donors in the presence or absence of 10 µg/mL of anti-CD16 (a16) or anti-CD32 (a32) F(ab’)2 fragments. CD62L down-modulation on PMNs was measured at 6 hours (panel B), whereas CD11b induction was measured at 24 hours (panel C). The statistical significance of treated vs control (CTRL) samples is shown. All data are the means and standard deviations of 3 independent experiments.
In confirmation of the role of CD16B and CD32A in PMN activation, we observed that anti-CD16 F(ab’)2 alone and, to a lesser extent, anti-CD32A F(ab’)2 induced PMN activation, even in the absence of glycoengineered rituximab antibody (Figure 6B-C).
Phagocytosis is followed by PMN cell death
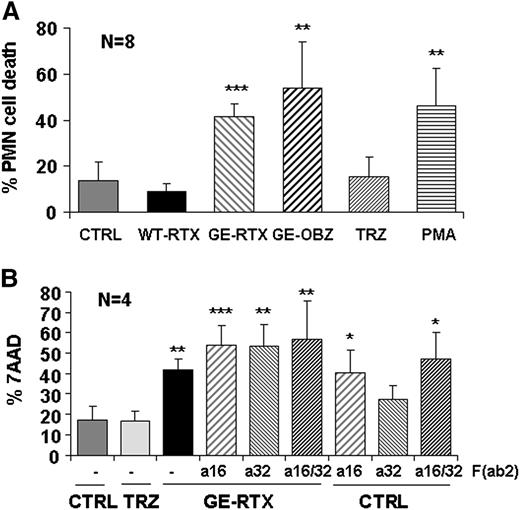
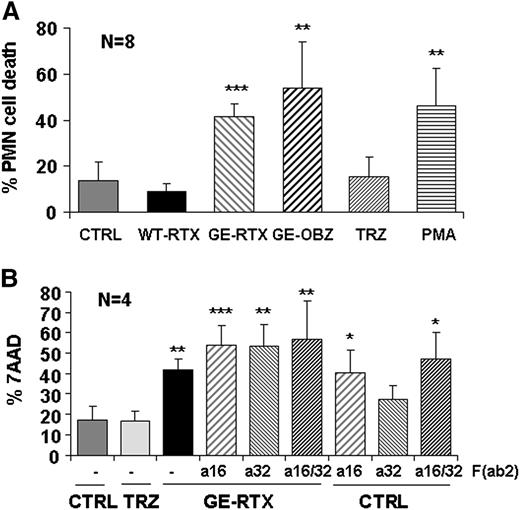
PMN activation is known to be often followed by effector cell death.23 We indeed observed that obinutuzumab and glycoengineered rituximab, similar to PMA, induced up to 50% PMN cell death over background PMNs at 24 hours in whole blood, whereas wild-type rituximab had little effect (Figure 7A). Also, in this case we observed that anti-CD16 F(ab’)2 fragments alone induced cell death to a similar extent to that observed in the presence of glycoengineered CD20 antibodies (Figure 7B). There was also a small effect of anti-CD32A F(ab’)2 alone, but this trend was not statistically significant. We conclude that PMN cell death follows PMN activation via CD16B and, more weakly, CD32A and, like phagocytosis, requires glycoengineered antibodies bound to their target antigen.
Glycoengineered CD20 antibodies induce PMN cell death. CLL cells were labeled with PKH26 and incubated in whole blood from a healthy donor in the presence or absence of 10 µg/mL of nonglycoengineered wild-type (WT) or glycoengineered (GE) CD20 antibodies, control TRZ, or 0.1 µM of PMA (panel A). In some experiments, 10 µg/mL of anti-CD16 or anti-CD32 F(ab’)2 fragments were added alone or in combination with each other or with glycoengineered rituximab (panel B). PMN cell death was measured by flow cytometry (7-AAD+/CD15+) after 24-hour incubation. The results are the mean and standard deviations of 4 to 8 experiments. In both panels, the statistical significance is analyzed for antibody-treated samples with respect to untreated control.
Glycoengineered CD20 antibodies induce PMN cell death. CLL cells were labeled with PKH26 and incubated in whole blood from a healthy donor in the presence or absence of 10 µg/mL of nonglycoengineered wild-type (WT) or glycoengineered (GE) CD20 antibodies, control TRZ, or 0.1 µM of PMA (panel A). In some experiments, 10 µg/mL of anti-CD16 or anti-CD32 F(ab’)2 fragments were added alone or in combination with each other or with glycoengineered rituximab (panel B). PMN cell death was measured by flow cytometry (7-AAD+/CD15+) after 24-hour incubation. The results are the mean and standard deviations of 4 to 8 experiments. In both panels, the statistical significance is analyzed for antibody-treated samples with respect to untreated control.
Discussion
In this report, we show that glycoengineered CD20 antibodies activate PMNs in whole blood more effectively than nonglycoengineered parental antibodies and induce phagocytosis, but not ADCC, of target CLL cells leading to PMN cell death. We also demonstrate that these effects are due to their higher binding affinity to the CD16B receptor.
Glycoengineered rituximab or obinutuzumab bound the purified CD16B NA2 isoform with an approximately sevenfold increased affinity with respect to their nonglycoengineered wild-type antibodies. This is approximately the same order of magnitude observed for binding of the same antibodies to CD16A10 and is not surprising in view of the high homology between CD16B and CD16A. In contrast, the glycoengineered CD20 antibodies did not show any significant difference in binding to the CD32A-H131 or CD32A-R131 isoforms, similar to previous data on CD32B.14 We cannot completely exclude that different glycosylation patterns of CD16B and CD32A isoforms expressed in different cell types may affect binding affinity to glycoengineered or wild-type IgG1 antibodies.15,45,46 However, the crystal structure of the CD16A/Fc complex15 suggests that the interaction between Fc and CD16 N162-carbohydrate mainly involves the first 2 monosaccharides of the glycan tree, which are conserved among N-linked glycans produced in either HEK293 or CHO cells.45,46 In addition, CD32A N-linked carbohydrates are relatively distant from the Fc-binding interface, although some interaction may take place in the R131 isoform.47 Our data are in reasonable agreement with previous data, where only a minimal difference in binding for the CD32A-R131, but not the CD32A-H131, isoform was observed, compared with a much wider difference observed for both CD16A polymorphic forms.48
We also investigated binding of glycoengineered or wild-type CD20 antibodies to whole PMNs derived from either CD16B NA1 or NA2 homozygous carriers. We could show a similar increase in binding of the glycoengineered vs the nonglycoengineered wild-type antibodies in both cases, which was completely blocked by anti-CD16 F(ab’)2. The fact that we detected mostly CD16B and not CD32A binding by glycoengineered antibodies on whole PMNs was presumably because of the much higher expression levels of CD16B on PMNs and its higher affinity for IgG1 compared with CD32A, as already demonstrated by other authors.28,41,49 A similar binding of glycoengineered or wild-type CD20 antibodies to both NA1 and NA2 isoforms of CD16B has been shown previously.42,50 In contrast, other authors have suggested a higher phagocytic capacity of NA1 compared with NA2 homozygous PMNs, but this was suggested to be the result of the higher capacity of NA1 to prime CD32A rather than through a significant different binding affinity for IgG1.42,51 Nonetheless, a more detailed investigation of the effects of all CD16B allelic variants, including copy number variants, on the functional activity of glycoengineered CD20 antibodies is warranted but is beyond the scope of this article.52
Weak but measurable activation of purified PMNs, seen as CD11b induction and CD62L down-modulation, was observed in the presence of wild-type rituximab. However, activation of these cells was significantly stronger when glycoengineered antibodies were used. Furthermore, significant PMN activation in whole blood was observed only with glycoengineered antibodies and not with rituximab. This shows that, similar to NK-mediated ADCC, the results obtained in whole-blood assays only in part reflect those observed using purified cell populations, because of the presence of high concentrations of free IgGs, as well as the intact complement cascade, both of which can inhibit the effector mechanisms mediated by low-medium affinity FcγRs such as CD16 and CD32.19,25,43,53 Therefore, glycoengineered antibodies, unlike parent rituximab, are competent for PMN activation in physiological conditions.
CD11b activation and CD62L down-modulation on PMNs are events triggered by a variety of agents.23 CD11b associates with CD18, forming the β integrin αMβ molecule (also called MAC1), a pattern recognition receptor that binds iC3b fragment and intercellular adhesion molecule 1 on endothelial and other cells. MAC1 activation is associated with PMN adhesion, rolling, and trans-endothelial extravasation.23,54,55 CD62L (l-selectin) binds several ligands including GlyCAM-1 on endothelial cells and is also implicated in PMN rolling, adhesion to endothelial cells, and transmigration into tissues. CD62L down-modulation is mostly regulated by protease cleavage, in particular by the disintegrin and metalloprotease-17, a feedback mechanism to avoid excess PMN migration into tissues and tissue damage.56,57
TNF-α, IL-8, and IL-6 were also induced more effectively in the presence of glycoengineered compared with wild-type antibodies, indicating again a role for CD16A/B. Significant IL-8, but not IL-6 or TNF-α, was secreted by purified PMNs stimulated by glycoengineered obinutuzumab-opsonized BJAB cells (L.B., unpublished observations), suggesting that at least part of the IL-8 was neutrophil derived. In contrast, it is likely that TNF and IL-6 were mostly produced by CD16A+ monocytes. The levels of cytokines induced by glycoengineered obinutuzumab were similar to those previously reported for alemtuzumab or OKT3 in vivo or in whole blood.58 Because TNF-α and IL-6 release have been associated with cytokine release syndrome, premedication is especially important for patients treated with glycoengineered antibodies.59,60 Interestingly, it has been recently suggested that IL-8 may have a beneficial effect, by favoring NK cell activation and CLL depletion by CD20 antibodies.61
Glycoengineered CD20 antibodies also induced phagocytosis of CLL targets by PMNs, but not ADCC. PMNs have been reported previously to mediate ADCC with IgG1 antibodies, but in some cases, PMNs were preactivated with granulocyte colony-stimulating factor.62-64 Furthermore ADCC by PMNs was weaker than that induced by NK cells, detectable at high effector target ratios and dependent on CD32A rather than CD16B, explaining the reported lack of effect of defucosylation on PMN-mediated ADCC.63,64 Finally, ADCC by PMNs has been shown to be triggered less efficiently by CD20 compared with other antibodies.62 Our data are therefore altogether consistent with these findings. In contrast to ADCC, we observed strong phagocytosis of opsonized CLL targets by purified PMNs. This phagocytosis was more effective in the presence of glycoengineered compared with wild-type CD20 antibodies. In whole blood, however, phagocytosis was lower, observed only in the presence of glycoengineered antibodies and was not induced significantly by wild-type rituximab. This finding is similar to what has been observed for NK-mediated ADCC, which in serum or whole blood can be observed only in the presence of glycoengineered antibodies, because of inhibition by complement activation and excess IgGs.19,43 These data are also in agreement with previous data using other CD20 antibodies.50
Analysis of the role of CD16 and CD32 using blocking Fab fragments specific for these receptors revealed that both of these molecules are involved and cooperate in PMN-mediated phagocytosis by glycoengineered CD20 antibodies, because both blocking antibodies were required for full inhibition of phagocytosis. Furthermore, we observed that F(ab’)2 fragments against both of these FcγRs, but more significantly CD16B, induced PMN activation and cell death, confirming the known signaling capacity of these molecules. The stronger activation induced by anti-CD16B is in agreement with other studies,65 although we cannot exclude that the weaker effect observed with our CD32A antibody may have been due to the specific clone used. CD16B and CD32A are known to be independent signaling molecules that cooperate with each other.65-70 Indeed, after immune complex binding, CD16B induces Ca+ mobilization, actin assembly, as well as CD32A priming and its mobilization into lipid rafts, followed by PMN degranulation. Activation of CD32A then cooperates with CD16B to mediate phagocytosis.27,29,30,68
The data presented are important because they suggest that phagocytosis by PMNs may be an additional mechanism of action of CD20 antibodies in patients, and that it can be significantly enhanced by glycoengineering, particularly in physiological conditions. This is particularly significant in view of the abundance of these cells in the circulation and the facility with which they can be mobilized into the blood and tissues.30 It will be important to investigate whether these effects contribute to the efficacy of obinutuzumab in vivo in patients. PMN activation by glycoengineered antibodies may also induce indirect effects, for example, activation of macrophages by cytokines and granule contents released by PMNs or induction of target cell death through antibody cross-linking by CD16B.71,72
Finally, it is interesting to note that neutropenia grade 3 to 4 appears to be higher in patients with CLL with a high peripheral tumor load treated with obinutuzumab compared with rituximab, whereas the incidence of neutropenia in patients with B-NHL appears similar with either antibody.59,73-75 It is tempting to speculate that this neutropenia may be related to PMN activation and that phagocytosis induced by this antibody in vivo reflects the specific mechanism of action of this glycoengineered antibody.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank patients and healthy donors who have generously agreed on multiple occasions to provide blood samples for these studies. We also thank the physicians and nurses of the Haematology division for their constant and kind help. We thank Tilman Schlothauer and Petra Rüger (Roche Diagnostics GmbH, Penzberg, Germany) for providing purified CD32A (H131 and R131 polymorphic forms).
This work was supported in part by a grant from the Associazione Italiana Ricerca contro il Cancro (J.G.), the Associazione Italiana Lotta alle Leucemie, Linfomi e Mieloma, and by Roche Glycart AG.
Authorship
Contribution: J.G., M.I., and C.K. have designed and supervised the experiments; F.D.R. and L.B. have performed PMN activation and binding, ADCC, phagocytosis, and cytokine-release experiments; C.F. has performed binding experiments with soluble recombinant CD16B and CD32A; A.R. and J.H.L. have provided reagents and critically read the manuscript; and J.G. has written the manuscript.
Conflict-of interest disclosure: C.K. and C.F. are employees of Roche Glycart AG, the company developing obinutuzumab. J.G. has received research grants from Roche Glycart AG. A.R. and J.G. have received honoraria from Roche Italia, the firm developing obinutuzumab. The remaining authors declare no competing financial interests.
Correspondence: Josee Golay, Center of Cellular Therapy “G. Lanzani,” Azienda Ospedaliera Papa Giovanni XXIII, c/o Presidio Matteo Rota, Via Garibaldi 11-13, 24128 Bergamo, Italy; e-mail: jgolay@hpg23.it.
References
Author notes
F.D.R. and L.B. have contributed equally to this study.