Abstract
Challenges in reporting subjective hemorrhagic symptoms consistently has led to the need for standardized, quantitative Bleeding Assessment Tools (BATs), some of which assign Bleeding Scores (BSs). The ISTH-BAT (International Society on Thrombosis and Hemostasis – Bleeding Assessment Tool (Rodeghiero et al JTH 2010; 8:2063)) aimed to consolidate and optimize advances made by its predecessors, which were based on the 2005 “Vicenza Bleeding Questionnaire”. It is important to note, however, that the scoring systems differ among the BATs, with each bleeding symptom scored from 0 to +3 for the original Vicenza, -1 to +4 for the MCMDM-1VWD and Condensed MCMDM-1VWD Bleeding Questionnaires and the PBQ (Pediatric Bleeding Questionnaire), and 0 to +4 for the ISTH-BAT. As a result, the normal ranges of BSs vary among questionnaires. To date, the normal range for the ISTH-BAT has not been established; the objective of this study was to determine the normal range of bleeding scores for the ISTH-BAT for both adults and pediatric patients.
BS data from different studies, originally generated using 4 different Vicenza-based BATs, were compiled using a bioinformatics system created to facilitate the collation and analysis using different scoring systems. Demographic and BS data, along with blood group, VWF:Ag/VWF:RCo/FVIII:C (when available) were collected from all enrolled subjects. Data were derived from multiple studies; all defined normal subjects as those without a known problem with bleeding or bruising. All BATs were expert-administered. The normal range for both adults and pediatrics was determined by: 1) removing outliers > 3 SD away from the mean and then, 2) selecting the mid-95th %ile.
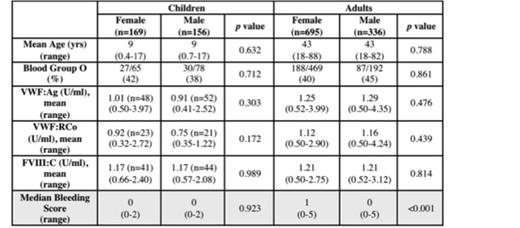
1,422 normal subjects were included (adult: n=1,079, pediatric: n=343). Adult data were collected using MCMDM-1VWD (n=294), Condensed MCMDM-1VWD (n=660), and ISTH-BAT (n=125), while pediatric data were collected using PBQ (n=324) and ISTH-BAT (n=19). 48 adults were removed from the analysis because they had BSs > 6.3, (i.e., >3 SD away from the mean), leaving n=1,031 for determination of the normal range. For children, BSs > 3.5 were judged to be outliers and therefore 18 children were removed, leaving n=325 children for determination of the normal range. The remaining adults had a mean age of 43 yrs (range 18 – 88) with 695 females and 336 males. The remaining children had a mean age of 9 yrs (range 0.4 – 17 yrs), with 169 females and 156 males. The relationship between BSs and demographic and lab data are given in Table 1.
For the ISTH-BAT, the normal range of BSs was 0 - 4 in adults (meaning that for individuals 18 yrs or older, a BS 5 or greater is positive or abnormal) and 0 - 2 in children (meaning that for individuals < 18 yrs, a BS 3 or greater is positive or abnormal).
The newly established normal BS ranges can now be used to objectively assess the bleeding symptoms of individuals by administration of the ISTH-BAT. They also highlight the strength of merging existing datasets to generate meaningful results. By making these data accessible to all investigators using the web-based ISTH-BAT system housed at Rockefeller University we hope to aid investigators initiating new studies and facilitate correlating bleeding symptoms with genotypic, molecular, and environmental data.
Mauer:CSL Behring: Honoraria. James:CSL Behring: Honoraria, Research Funding; Octapharma: Honoraria, Research Funding; Baxter: Honoraria; Bayer: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.