Abstract
Despite improved survival with new molecules, allogeneic hematopoietic stem cell transplantation (alloHSCT) remains the only curative option for multiple myeloma (MM). Myeloablation with autologous HSCT (autoHSCT) followed by nonmyeloablative (NMA) alloHSCT has reduced the high nonrelapse mortality (NRM) rates associated with myeloablative alloHSCT, but randomized trials comparing NMA alloHSCT with standard therapy have yielded conflicting results. Factors affecting long-term outcomes have not been clearly defined. We sought to identify factors associated with outcomes in a large single-center cohort of 93 tandem transplant recipients.
We conducted a prospective phase II trial in Durie-Salmon (DS) stage II/III newly diagnosed MM patients <65 years with a 6/6 sibling donor, characterized by outpatient alloHSCT and complete immunosuppression withdrawal by day +100. Following induction therapy, patients received autoHSCT followed 3 months later by an outpatient NMA alloHSCT. Conditioning regimen consisted of fludarabine (30 mg/m2 x 5 days) and cyclophosphamide (300 mg/m2 x 5 days), followed by donor G-CSF mobilized stem cells (target dose: ≥4 x 10^6 CD34+/kg). GVHD prophylaxis included tacrolimus (day -10 to +50, taper by +100) and mycophenolate mofetil 1000 mg BID (day +1 to +50). Probabilities of overall and progression-free survival (OS and PFS), relapse, NRM and graft-versus-host disease (GVHD) incidences were estimated taking competing risks into account when appropriate. Regression analysis using Cox and Fine & Gray models were used to determine factors influencing outcomes. Severity of chronic GVHD was assessed by the proportion of alive patients on systemic immunosuppression.
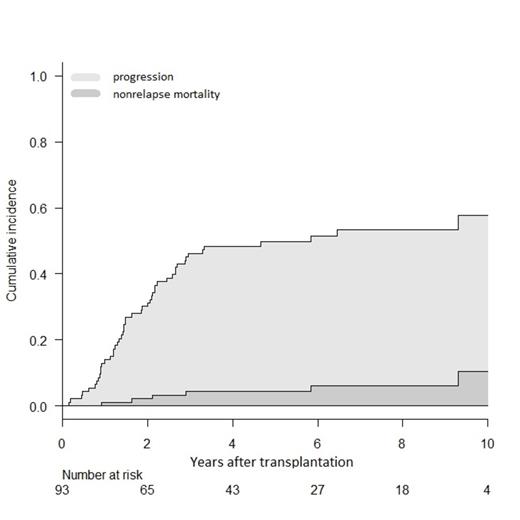
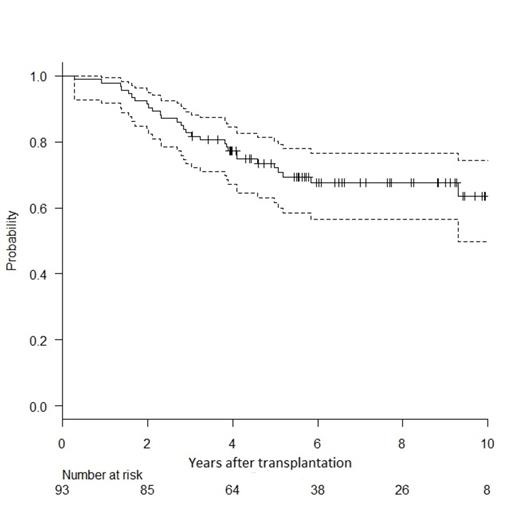
From 2001 to 2010, 93 patients received tandem HSCT from a matched sibling donor; median age was 52 years (range 39-64) and DS stage III present in 76%. All but 6 patients received a single line of induction therapy. Median time from diagnosis to autoHSCT and from auto- to alloHSCT were 7 (range 3-57) and 4 (range 2-13) months, respectively. At least partial remission (PR) status was obtained in 84% patients before tandem HSCT. After a median follow-up of 7 years, cumulative incidences of grade II-IV acute and extensive chronic GVHD were 9% (95%CI: 4-15) and 85% (75-91) respectively. Cumulative incidences of NRM and progression were 10% (95%CI: 3-22) and 47% (37-58; Fig. 1). Probability of 10-year OS and PFS were 64% (50-74; Fig. 2) and 44% (31-56; Fig. 1) respectively. In multivariate analysis, at least stable disease (SD) status before alloHSCT was a significant protective factor for progression incidence and PFS (respectively, HR 0.18, CI: 0.05-0.64, and HR 0.12, CI: 0.04-0.36). Extensive chronic GVHD as time-dependent variable was also protective for PFS (HR 0.39, CI: 0.19-0.80). The most significant protective factor for OS was at least SD status before alloHSCT (HR 0.15, CI: 0.04-0.56). The association between chronic GVHD and OS did not reach statistical significance. In survivors, the probability of being on systemic immunosuppressive treatment for GVHD was 39% (CI: 27-52) at 5 years and 15% (CI: 4-42) at 10 years.
Figure 1(left) shows stacked cumulative incidence of transplant failure by either progression (light grey) or nonrelapse mortality (dark grey).
Figure 1(left) shows stacked cumulative incidence of transplant failure by either progression (light grey) or nonrelapse mortality (dark grey).
Figure 2 (right) shows the probability of overall survival with 95% confidence interval.
Figure 2 (right) shows the probability of overall survival with 95% confidence interval.
We report a very high PFS of 44% at 10 years in a large cohort of 93 NMA alloHSCT recipients. Despite the high incidence of chronic GVHD, we observed a low NRM of 10% and an acceptable prevalence of immunosuppressive therapy of 15% at 10 years. Chronic extensive GVHD and achievement of disease control before HSCT were both associated with better PFS. Patients with progressive disease do not seem to benefit from NMA alloHSCT and efforts to reduce chronic
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.