Abstract
Lymphoma immunotherapy with anti-CD20 antibody (rituximab) acts primarily through Antibody Dependent Cellular Cytotoxicity (ADCC), implying a role for NK cells. The activation and inhibition of NK cells are influenced by Killer Immunoglobulin-like Receptors (KIRs) and their HLA ligands. To evaluate the involvement of NK cells in response to immunotherapy, we investigated the role of both KIR and HLA polymorphisms in response to single agent rituximab. We have analyzed the relationship between patient genotype and clinical parameters to determine if there are beneficial KIR/HLA interactions that can lead to improved clinical outcome.
204 follicular lymphoma (FL) patients participating in the Eastern Cooperative Oncology Group (ECOG) RESORT trial (E4402) were treated with rituximab. The patients who showed an initial response were randomized into two different rituximab treatment regimens. Approximately 70% of FL patients showed initial response to 4 weekly doses of rituximab, and were randomized between arm A: therapy “as needed” (4 weekly doses, only upon progression) or arm B: scheduled maintenance (1 dose every 13 weeks). Clinical outcomes analyzed included duration of response, time to rituximab failure (TTRF), time to cytotoxic therapy (TCT) and decrease in tumor size. The data presented here represent both treatment arms combined, i.e. arms A+B, for all clinical data parameters mentioned. Genomic DNA samples were used for genotyping patients for the presence of 15 KIR loci using a Real Time PCR-based method as previously published (Alves, Tissue Antigens, 2008). HLA specificities corresponding to KIR ligands (HLA-C1, C2, Bw4 and ABw4) were typed using a combination of PCR-SSP methods (Olerup and Invitrogen).
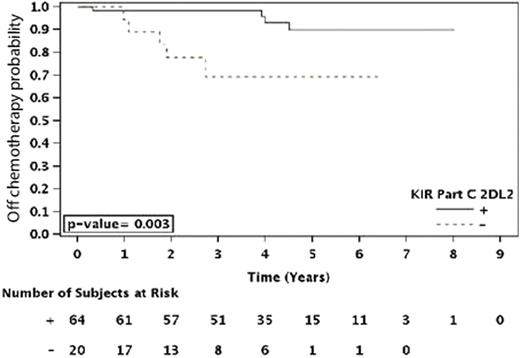
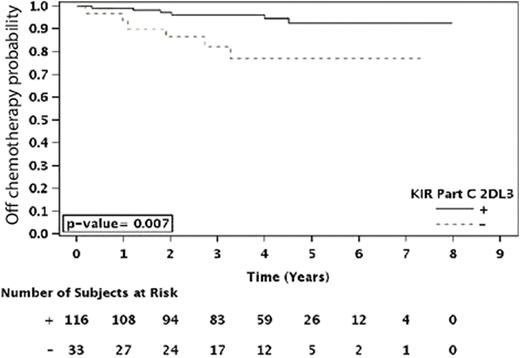
Those patients homozygous for HLA-C1 (C1C1) showed a trend towards a longer duration of response (p=0.06) as compared to those that were heterozygous (C1C2) or homozygous for HLA-C2 (C2C2). A similar difference was observed where patients that were HLA-C1 positive [C1+ (C1C1 or C1C2)] showed a trend towards longer TTRF (p=0.071), as well as a significantly longer TCT (p=0.003), compared to C2C2 patients. Further analyses revealed that the influence of these HLA C specificities on clinical outcome could be related to the presence or absence of inhibitory and/or activating KIR genes. Specifically, HLA-C1 is a ligand for the inhibitory KIR genes 2DL2 and 2DL3. Among the patients who had the KIR2DL2 inhibitory gene (KIR2DL2+), those that were C1+ showed a prolonged duration of response (p=0.049), TCT [Figure 1A, (p=0.003)] and TTRF (p<0.001)] compared to KIR2DL2+ patients who were C2C2 and thus lacked the C1 cognate ligand. Similarly, KIR2DL3+ patients that were C1+ had a significantly longer TCT (p=0.007) than patients that were C2C2, but significant effects were not observed for these KIR2DL3+/C1+ patients with respect to the three other analyzed clinical parameters. Therefore, further studies are required to confirm that KIR2DL3 has an effect independent of other KIRs.
Time to Cytotoxic Therapy
HLA-C2 alleles are ligands for an activating KIR gene, KIR2DS1. The observed HLA-C genotype effect on clinical outcome could potentially be conveyed by the interaction of HLA-C2 with KIR2DS1. Among the C2C2 patients, KIR2DS1+ patients had a significantly shorter TTRF than those without KIR2DS1 (p=0.023), as well as a significantly smaller decrease in tumor size (p=0.039), implying that the interaction of the activating KIR receptor (KIR2DS1) with its cognate ligand (HLA-C2) conveys a disadvantage to patient outcome.
Single-agent rituximab immunotherapy of FL reveals differences in outcomes based on KIR and HLA polymorphisms. The HLA-C2 homozygous individuals seem to be at a disadvantage. This may relate to 2 significant observations highlighting diverse effects of inhibitory and activating KIR-HLA interactions leading to NK cell “licensing” and “hyporesponsiveness”, respectively. Specifically, the data from this study are consistent with: A) a beneficial effect from inhibitory KIR2DL2 and KIR2DL3 interaction with HLA-C1, and B) a detrimental effect due to the contribution of activating KIR2DS1 interacting with its ligand HLA-C2.
BG, AKE and WW are co-first authors.
Horning:Genentech: Employment, Equity Ownership. Kahl:Genentech: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.